Abstract
Purpose
To assess the prognostic significance of skin involvement in breast cancer patients with chest wall recurrence (CWR).
Methods
We retrospectively analyzed the clinicopathological data of breast cancer patients with CWR who were diagnosed pathologically between January 2000 and April 2020. Disease-free survival (DFS) was the time from radical resection for CWR to disease recurrence. Progression-free survival (PFS) was defined as the time from the diagnosis of locally unresectable CWR to the first sign of disease progression. Persistent chest wall progression was defined as three consecutive chest wall progressions with no distant organ involvement.
Results
A total of 476 patients with CWR were included in this study. Skin involvement was confirmed in 345 patients. Skin involvement was significantly correlated with a high T stage (p = 0.003), more positive nodes at initial examination (p < 0.001) and lymphovascular invasion (p < 0.001). Kaplan–Meier analysis showed that skin involvement was a predictor of shorter DFS (p < 0.001), including both local disease progression (p < 0.001) and distant disease progression (p = 0.022). Multivariate analysis showed that skin involvement was an independent biomarker for DFS (p = 0.043). Patients with skin involvement were more likely to experience persistent chest wall progression (p = 0.040). After eliminating the potential deviation caused by an insufficient follow-up time, persistent chest wall progression was more likely to be associated with a high N stage (p = 0.002), negative progesterone receptor (PR; p = 0.001) and positive human epidermal growth factor receptor 2 (HER2; p = 0.046) of the primary site, and negative oestrogen receptor (ER; p = 0.027) and PR (p = 0.013) of the chest wall lesion and skin involvement (p = 0.020).
Conclusion
Skin involvement was a predictor of poor disease control in patients with CWR and was closely related to persistent chest wall progression. We stratified the prognosis of individualized treatment for breast cancer patients with CWR to provide new insights into the biological behaviours of the disease.
KEY MESSAGES
Skin involvement is a predictor of poor local disease control in breast cancer patients with CWR and a factor contributing to persistent chest wall progression after CWR. We stratified the prognosis of individualized treatment for breast cancer patients with CWR.
Introduction
In 2020, breast cancer is the leading cause of cancer morbidity among women worldwide, accounting for 24.5% of all cancer-related morbidities [Citation1]. Recurrent breast cancer after optimal therapy is disconcerting [Citation2,Citation3]. Chest wall recurrence (CWR) can occur in 5–40% of breast cancer patients [Citation4–7], and this disease is traditionally viewed as a harbinger of systemic disease and poor prognosis [Citation8]. However, two-thirds of patients with recurrences involving the chest wall have no other sites of metastases and can therefore achieve favourable outcomes [Citation9–11]. Several studies have illustrated that patients with CWR represent a heterogeneous population [Citation12]. The disease occurs with varying frequency depending on initial tumour stage, tumour grade, lymphovascular invasion and therapy strategy [Citation5,Citation13–15]. Survival after CWR is also influenced by a multitude of factors, such as initial nodal status, hormone receptor (HR) status and human epidermal growth factor receptor 2 (HER2) status, the length of the disease-free interval (DFI) and therapy for chest wall lesions [Citation6,Citation16,Citation17].
Some patients with CWR experience persistent chest wall progression but have no signs of distant metastasis. Although these patients do not have fatal visceral metastases, compression, ulceration and pain associated with having a chest wall disease are notable. Unfortunately, little attention has been given to persistent chest wall progression. The definition of persistent chest wall progression is unclear, and the biomarkers for subsequent recurrence or disease progression are unknown.
Chest wall diseases typically present as one or more asymptomatic nodules in or near the mastectomy or lumpectomy scar. Skin, as the largest organ of the body, plays an important role in several vital processes, including the growth and progression of tumours. Skin involvement most commonly originates from melanoma and breast cancer in females, and other common origins include the lung and gastrointestinal tract. The pattern of skin involvement is different for different types of tumours. Skin involvement in patients with lung cancer usually occurs early, whereas skin involvement in patients with breast cancer often occurs several years after tumour excision [Citation18]. Compared with most tumours, skin involvement and visceral metastases are weakly correlated in breast cancer patients. Skin involvement is known to frequently occur in anatomical areas close to the primary tumour [Citation19]. However, little is known about the impact of skin involvement on the prognosis of breast cancer in patients with CWR. In this study, we aimed to identify the association of skin involvement and the patients’ clinicopathological features with survival outcomes in breast cancer patients with CWR, especially those with persistent chest wall progression, to provide suggestions for the management of recurrent breast cancer.
Patients and methods
Patients
We reviewed Asian female breast cancer patients with CWR who were diagnosed pathologically at Sun Yat-sen University Cancer Center between January 2000 and April 2020. Only patients who met all of the following criteria were included: (1) patients with a histologically confirmed diagnosis of breast cancer, (2) patients who underwent curative surgery for a primary disease, (3) patients with no signs of distant organ (including distant lymph node) metastasis at the time of the initial diagnosis, (4) patients with histologically confirmed CWR and (5) patients whose chest wall was the only site of disease progression after curative therapy was performed for the treatment of a primary disease that was identified by imaging (including magnetic resonance imaging, computed tomography, positron emission tomography/computed tomography). Patients were excluded from the study if they were male and had a serious systemic disorder, a second primary malignancy or simultaneous distant metastasis (presence of distant metastasis was defined as any metastasis in internal organs, bones, soft tissue and nonregional lymph nodes). The clinical-pathological data of the patients, including age at the initial diagnosis, TNM stage, skin involvement, as well as the pathological parameters and treatment strategy for the primary and recurrent sites, were recorded and analyzed for this study. Skin involvement referred to a tumour involving the dermis, epidermis, or both. Disease-free survival (DFS) was the time from radical resection for CWR to disease recurrence. Progression-free survival (PFS) was defined as the time from the diagnosis of locally unresectable CWR to the first sign of disease progression. Overall survival (OS) was defined as the time from the diagnosis of CWR to death (all causes). Patients who were still alive and who did not have a progressive disease were censored at the date of their last clinical visit. Persistent chest wall progression was defined as three consecutive chest wall progressions with no sign of new distant organ involvement. All the included patients were followed up regularly until death or study completion (11 February 2021). The study was approved by the Ethics Committee of Sun Yat-sen University Cancer Center (protocol code, B2020-051-01 and date of approval, 10 February 2021).
Statistics
Clinical-pathological parameters of the included patients are shown using descriptive statistics. The patients’ characteristics were compared using the chi-squared test or Kruskal–Wallis one-way ANOVA. Survival analyses were conducted using the Kaplan–Meier method and Cox proportional hazards model. p Values < 0.05 were considered statistically significant. All statistical analyses were performed using SPSS version 25.0 (SPSS Inc., Chicago, IL, USA), and all p values were two-sided.
Results
Patient characteristics
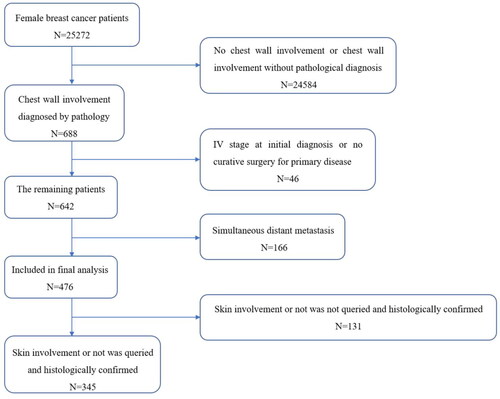
We reviewed, collected and organized all eligible cases between February and June 2021. A total of 25,272 female breast cancer patients visited Sun Yat-sen University Cancer Center between January 2000 and April 2020, and 476 (1.9%) of them met the inclusion criteria and were enrolled in this retrospective study (). The clinicopathological characteristics of the 476 patients are summarized in . All the patients underwent radical resection of the primary lesions. Twenty-six patients had a previous lumpectomy with surgical axillary staging, and the remaining patients had undergone total mastectomy with surgical axillary staging ± reconstruction. The postoperative pathological reports of all the patients showed a negative margin ≥1 mm. Invasive ductal carcinoma (IDC) was the main pathological subtype of the primary site in these patients. Almost half of the patients were T1–2 or N positive according to the TNM stage. A total of 24.2% of patients had lymphovascular invasion of the primary tumour. Compared with the primary lesion, the proportion of Ki-67 ≥ 15% increased significantly in recurrent lesions, while the positive proportion of oestrogen receptor (ER), progesterone receptor (PR) and HER2 did not change significantly (Supplementary Table). The median intervals between primary treatment and CWR and OS after CWR were 26.15 and 103.76 months, respectively. The median follow-up time after the initial breast cancer diagnosis was 63.7 months, and the median follow-up time after the CWR diagnosis was 37.4 months.
Table 1. Clinical-pathological characteristics of 476 patients with CWR.
Prognostic value of skin involvement
We further investigated the impact of skin involvement on the clinical outcome of CWR in breast cancer patients. Of these 476 patients, skin involvement was suspected and histologically confirmed in 345 patients. shows representative images of non-skin involvement, tumours involving the dermis and tumours involving the epidermis. In all the patients, the tissue was biopsied by local lesion excision. The patho-clinical characteristics of these 345 patients are shown in and . There were 198 patients with skin involvement and 147 without skin involvement. Skin involvement in patients with CWR was significantly correlated with T3–4 stage (p = 0.003), more positive lymph nodes (p < 0.001) and lymphovascular invasion (p < 0.001) of the primary tumour in breast cancer patients. Of them, 200 patients underwent radical resection of chest wall lesions and had a negative surgical margin, and the remaining 145 patients had locally unresectable disease. In addition, there was no significant bias in systematic therapy or radiotherapy for CWR between cohorts with skin involvement and those without skin involvement.
Figure 2. Representative images of non-skin involvement, tumours involving the dermis and tumours involving the epidermis. Representative images of non-skin involvement (A, 100× magnification; B, 400× magnification). Representative images of tumours involving the dermis (C, 100× magnification; D, 400× magnification). Representative images of tumours involving the epidermis (E, 100× magnification; F, 400× magnification).
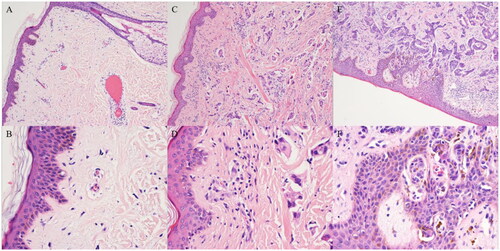
Table 2. Pathological characteristics of primary tumour in 345 patients with/without skin involvement.
Table 3. Clinical-pathological characteristics of chest wall lesions in 345 patients with/without skin involvement.
Because of the difference in treatment strategies for chest wall lesions, we divided the included 345 patients into a radical resection group and a locally unresectable disease group. The Kaplan–Meier analysis showed that although skin involvement was a predictor of a shorter DFS (p < 0.001) after radical resection for CWR, it had no significant prognostic value for OS (). Skin involvement seemed to predict poor PFS (p = 0.068) in patients with locally unresectable CWR (). Factors that were significant (p < 0.10) in the univariate analysis examining radical resection for the CWR group, including pathology subtype (hazard ratio (HR)=0.585, 95% confidence interval (CI) 0.337–1.016, p = 0.057), N stage of the primary (HR = 1.544, 95% CI 0.984–2.424, p = 0.059), Ki-67 (HR = 1.763, 95% CI 0.972–3.200, p = 0.062) and skin involvement (HR = 2.211, 95% CI 1.443–3.388, p < 0.001) were subjected to multivariate analysis. Skin involvement (HR = 1.809, 95% CI 1.018–3.215, p = 0.043) was an independent impact factor for DFS in multivariate analyses ().
Figure 3. Kaplan–Meier curve for DFS and OS of 200 patients treated with radical resection stratified by skin involvement. (A) Kaplan–Meier curve for DFS stratified by skin involvement in 200 patients treated with radical resection. (B) Kaplan–Meier curve for OS stratified by skin involvement in these patients.
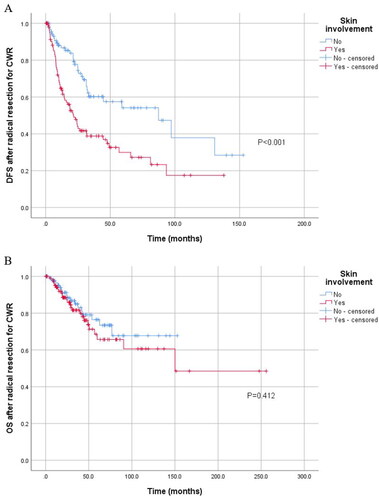
Figure 4. Kaplan–Meier curve for PFS and OS of 145 patients with locally unresectable CWR stratified by skin involvement. (A) Kaplan–Meier curve for PFS stratified by skin involvement in 145 patients with locally unresectable CWR. (B) Kaplan–Meier curve for OS stratified by skin involvement in these patients.
Table 4. Cox proportional hazard regression analysis of DFS in 200 patients treated with radical resection.
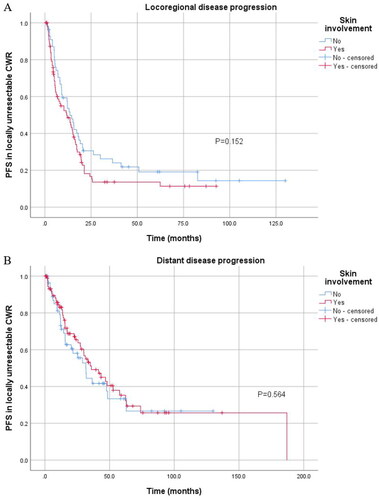
As we observed the value of skin involvement in predicting DFS and PFS, we further divided disease progression patterns into local disease progression and distant disease progression. Patients with skin involvement after radical resection for CWR were more likely to suffer from both local disease progression (p < 0.001) and distant disease progression (p = 0.022) (), while skin involvement had no significant association with disease progression in patients with locally unresectable CWR ().
Figure 5. Kaplan–Meier curve for local and distant disease progression of 200 patients treated with radical resection stratified by skin involvement. (A) Kaplan–Meier curve for local disease progression stratified by skin involvement in 200 patients treated with radical resection. (B) Kaplan–Meier curve for distant disease progression stratified by skin involvement in these patients.
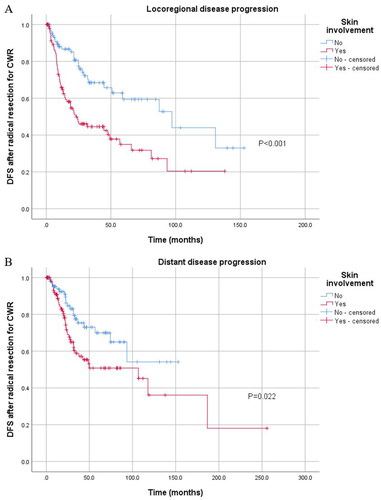
Figure 6. Kaplan–Meier curve for local and distant disease progression of 145 patients with locally unresectable CWR stratified by skin involvement. (A) Kaplan–Meier curve for local disease progression stratified by skin involvement in 145 patients with locally unresectable CWR. (B) Kaplan–Meier curve for distant disease progression stratified by skin involvement in these patients.
Skin involvement and persistent chest wall progression
Forty out of 345 (11.6%) patients experienced persistent chest wall progression. Of the 40 patients, 29 were from the skin involvement group and the remaining 11 were from the non-skin involvement group, accounting for 14.6 and 7.5% (p = 0.040), respectively. To eliminate the potential bias caused by an insufficient follow-up time, 114 patients who were followed up for <24 months after CWR were excluded from this analysis. and show the characteristics of the patients with and without persistent CWR. The following variables were significantly correlated with persistent CWR: N positive status (p = 0.002), negative PR (p = 0.001) and positive HER2 (p = 0.046) of primary site, negative ER (p = 0.027) and negative PR (p = 0.013) of chest wall and skin involvement (p = 0.020).
Table 5. Comparison of pathological characteristics of primary tumour in patients with/without persistent chest wall progression.
Table 6. Comparison of clinical-pathological characteristics of chest wall lesions in patients with/without persistent chest wall progression.
Discussion
The unpleasant symptoms of CWR, such as lymphoedema, ulceration, pain, fungal infection and persistent disease progression, pose challenges in clinical practice. However, these patients represent a heterogeneous population, and some of them may achieve long-term survival after CWR. Here, we hope to summarize the characteristics of breast cancer patients with CWR and provide novel insights for patient stratification and further individualized treatment. In the present study, we found that skin involvement predicted poor DFS in breast cancer patients who underwent radical resection for CWR, including both local disease progression and distant disease progression. In addition, patients with skin involvement tended to experience persistent chest wall progression after CWR compared with those without skin involvement.
The mechanisms that predispose certain patients to internal malignancies invading the skin have rarely been discussed in the previous literature. The skin is composed of an epidermis and dermis. A complex network of a multitude of cell types and the flexibility of the dermal vessels and the lymph nodes in the skin maintain several vital processes, such as inflammation, immune response, wound healing and angiogenesis [Citation20–24]. The levels of tumour-infiltrating lymphocytes (TILs) in patients with skin metastases are the lowest, with a higher TILs level, a higher FOXP3 level and a lower CD8/FOXP3 ratio than those of other metastatic lesions of primary breast cancer [Citation25]. This suggests that cutaneous tissue may harbour a more permissive immune microenvironment for tumour growth. A physiological mitigation of cytotoxic immune activity in skin tissue through different immunosuppressive mechanisms, a process known as ‘immune privilege’, has been described [Citation26,Citation27]. In addition, the dermis or epidermis secretes certain factors that interact with tumour cells and participate in the skin homing mechanism of malignant cells. Chemokines recognize and bind their receptors to play a role in the occurrence and metastasis of tumours. In patients with cutaneous metastasis of a melanoma, the chemokine receptor CCR10 mediates the survival of a melanoma in the skin [Citation28,Citation29]. Breast cancer, which has a similar metastatic pattern as malignant melanoma but also a high incidence of skin metastases, shows high expression levels of CXCR4 and CCR7 rather than CCR10 [Citation30]. Structurally, skin involvement in female breast cancer patients occurs mainly by haematogenous and lymphatic routes [Citation18,Citation31]. The regional distribution of dermal lymph vessels, from the perspective of tumour invasion, constitutes a functional unit [Citation32]. Joan et al. [Citation33] found that more superficially located tumours may be more likely than deeper tumours to metastasize via the lymphatic system to the axillary nodes due to the rich lymphatic system in the breast dermis, although the size of the primary site is <5 cm. In addition, cutaneous deposits as a result of breast adenocarcinomas with distinct angiogenic profiles in the skin have different growth patterns [Citation34]. The suppressive immune microenvironment in the skin cannot kill tumours, and the abundant vascular and lymphatic system in the dermis provides nutrition and spreading channels, which contribute to the growth, invasion and metastasis of tumours. This may partly explain why skin involvement is an independent factor that can predict disease progression in breast cancer patients with CWR, especially local disease progression.
The treatment of recurrent chest wall disease is complex, particularly when the chest wall has been previously subjected to radiotherapy. Full-thickness chest wall resection, as one of the most common therapeutic approaches, is not curative, but is rather disfiguring and associated with significant morbidities [Citation35,Citation36]. Previous studies have suggested that multiple sites or large areas of skin involvement are not suitable for local surgical treatment, although systemic chemotherapy is also difficult. Second- and third-line salvage chemotherapy results in overall response rates of 20–30%, at best [Citation37–40]. In our study, skin involvement predicted poorer disease control in patients with CWR, while similar OS was observed between these patients with and without skin involvement. Our results suggested that skin involvement should not be considered a contraindication for local therapy in breast cancer patients with recurrent chest wall diseases. In contrast, more effective treatment strategies and more clinical studies are needed to improve the clinical outcomes and quality of life of these patients, especially in the relatively early stage of skin invasion.
A certain proportion of patients with CWR after local or systemic treatment have second recurrence or disease progression in a shorter time. However, biomarkers for subsequent recurrence or disease progression were not consistent. Anees et al. [Citation16] found that primary tumour characteristics and treatment factors, time to recurrence, characteristics and treatment of recurrent chest wall were not significantly predictive for an additional CWR in their study. Carmen et al. [Citation14] showed that the DFI between the primary lesion and the incidence of CWR remained highly correlated with disease progression after CWR. Bruce G et al. [Citation6] demonstrated that PR status is a significant factor for distant metastasis and that HER-2/neu status is related to local-regional disease progression after local recurrence. The varying lengths of follow-up, differences in patient selection criteria and a variety of treatment strategies can explain the differences among various studies. Although some studies explore the second CWR after the first CWR, that is, repeat recurrence, there are few studies on persistent chest wall progression. To our knowledge, this is the first study on the characteristics of patients with persistent chest wall progression and the prognosis of skin involvement in these patients. In our study, initial nodal status, ER, PR and HER2 statuses and skin involvement were significantly different between the patients with persistent chest wall progression and those without persistent chest wall progression. Persistent chest wall progression was more likely associated with positive lymph node status, negative PR and positive HER2 in primary lesions, negative ER and PR in chest wall lesions and skin involvement of the chest wall. Positive lymph node status, negative HR status and positive HER2 status were generally markers of poor prognosis in breast cancer. Ki-67, as a nuclear protein expressed in proliferating cells, is associated with a poor prognosis of breast cancer. Consistent with our results, Ki-67 expression was also elevated in recurrent chest wall lesions and was associated with higher aggressiveness.
The current study had several limitations. The study will be made more persuasive by the involvement of multiple institutions. The fact that many patients were excluded for various reasons was a weakness of the study. As with any retrospective analysis of this nature, treatment strategies for primary breast cancer and recurrent disease have changed over the past 20 years in accordance with the new guidelines, even if there is no significant deviation between systemic treatment and local treatment of included patients. CWR patients without skin involvement will enjoy high local disease control and long-term PFS. We believe that the results of the present study will contribute to individualized treatment decision-making and provide a strong incentive for future research.
Increasing attention has been given to recurrent chest wall in breast cancer due to the clinical commonness and complexity of treatment. We stratified the prognoses of these patients by skin involvement and found that skin involvement was markedly related to disease progression. In addition, we described the pattern of persistent chest wall progression for the first time and demonstrated that skin involvement was a predictor of persistent chest wall progression in breast cancer patients with CWR. We hope that the biological behaviour and prognostic stratification factors revealed in this study might shed light on individualized therapies for these patients.
Ethical approval
Informed consent was obtained as part of usual clinical practice from all subjects involved in the retrospective study. For patients who continued to visit our hospital, the follow-up department checked the medical record system to know their disease progression and survival. For patients who did not continue to visit our hospital, the follow-up by telephone was performed every 3–6 months to check the disease progression and survival of the patients until these patients lost follow-up or died.
Author contributions
Conception and design: Danyang Zhou, Mei Li, Fei Xu, Qiufan Zheng, Qianyi Lu, Ruoxi Hong and Shusen Wang; analysis and interpretation: Danyang Zhou, Mei Li, Fei Xu, Qiufan Zheng and Qianyi Lu; original draft preparation: Danyang Zhou and Mei Li; review and editing: Fei Xu, Qiufan Zheng, Qianyi Lu, Ruoxi Hong and Shusen Wang; final approval of the version to be published: Ruoxi Hong and Shusen Wang; All authors agree to be accountable for all aspects of the work.
Supplemental Material
Download MS Word (13.2 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data presented in this study are available on request from the corresponding author.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):1–13. doi: 10.3322/caac.21660.
- Brackstone M, Fletcher GG, Dayes IS, et al. Locoregional therapy of locally advanced breast cancer: a clinical practice guideline. Curr Oncol. 2015;22(Suppl 1):S54–S66. doi: 10.3747/co.22.2316.
- Wakeam E, Acuna SA, Keshavjee S. Chest wall resection for recurrent breast cancer in the modern era: a systematic review and meta-analysis. Ann Surg. 2018;267(4):646–655. doi: 10.1097/SLA.0000000000002310.
- Elder EE, Kennedy CW, Gluch L, et al. Patterns of breast cancer relapse. Eur J Surg Oncol. 2006;32(9):922–927. doi: 10.1016/j.ejso.2006.06.001.
- Maishman T, Cutress RI, Hernandez A, et al. Local recurrence and breast oncological surgery in young women with breast cancer: the POSH Observational Cohort Study. Ann Surg. 2017;266(1):165–172. doi: 10.1097/SLA.0000000000001930.
- Haffty BG, Hauser A, Choi DH, et al. Molecular markers for prognosis after isolated postmastectomy chest wall recurrence. Cancer. 2004;100(2):252–263. doi: 10.1002/cncr.11915.
- Hsi RA, Antell A, Schultz DJ, et al. Radiation therapy for chest wall recurrence of breast cancer after mastectomy in a favorable subgroup of patients. Int J Radiat Oncol Biol Phys. 1998;42(3):495–499. doi: 10.1016/s0360-3016(98)00254-5.
- D’Aiuto M, Cicalese M, D’Aiuto G, et al. Surgery of the chest wall for involvement by breast cancer. Thorac Surg Clin. 2010;20(4):509–517. doi: 10.1016/j.thorsurg.2010.09.001.
- van Tienhoven G, Voogd AC, Peterse JL, et al. Prognosis after treatment for loco-regional recurrence after mastectomy or breast conserving therapy in two randomised trials (EORTC 10801 and DBCG-82TM). EORTC Breast Cancer Cooperative Group and the Danish Breast Cancer Cooperative Group. Eur J Cancer. 1999;35(1):32–38. doi: 10.1016/s0959-8049(98)00301-3.
- Recht A, Silen W, Schnitt SJ, et al. Time-course of local recurrence following conservative surgery and radiotherapy for early stage breast cancer. Int J Radiat Oncol Biol Phys. 1988;15(2):255–261. doi: 10.1016/s0360-3016(98)90002-5.
- Elfgen C, Schmid SM, Tausch CJ, et al. Radiological staging for distant metastases in breast cancer patients with confirmed local and/or locoregional recurrence: how useful are current guideline recommendations? Ann Surg Oncol. 2019;26(11):3455–3461. doi: 10.1245/s10434-019-07629-9.
- Chagpar A, Langstein HN, Kronowitz SJ, et al. Treatment and outcome of patients with chest wall recurrence after mastectomy and breast reconstruction. Am J Surg. 2004;187(2):164–169. doi: 10.1016/j.amjsurg.2003.11.006.
- Nielsen HM, Overgaard M, Grau C, et al. Study of failure pattern among high-risk breast cancer patients with or without postmastectomy radiotherapy in addition to adjuvant systemic therapy: long-term results from the Danish Breast Cancer Cooperative Group DBCG 82 b and c randomized studies. J Clin Oncol. 2006;24(15):2268–2275. doi: 10.1200/JCO.2005.02.8738.
- van der Pol CC, van Geel AN, Menke-Pluymers MBE, et al. Prognostic factors in 77 curative chest wall resections for isolated breast cancer recurrence. Ann Surg Oncol. 2009;16(12):3414–3421. doi: 10.1245/s10434-009-0662-7.
- Ragaz J, Olivotto IA, Spinelli JJ, et al. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the British Columbia randomized trial. J Natl Cancer Inst. 2005;97(2):116–126. doi: 10.1093/jnci/djh297.
- Chagpar A, Meric-Bernstam F, Hunt KK, et al. Chest wall recurrence after mastectomy does not always portend a dismal outcome. Ann Surg Oncol. 2003;10(6):628–634. doi: 10.1245/aso.2003.01.004.
- Miyauchi K, Koyama H, Noguchi S, et al. Surgical treatment for chest wall recurrence of breast cancer. Eur J Cancer. 1992;28A(6–7):1059–1062. doi: 10.1016/0959-8049(92)90456-c.
- Hu SCS, Chen GS, Lu YW, et al. Cutaneous metastases from different internal malignancies: a clinical and prognostic appraisal. J Eur Acad Dermatol Venereol. 2008;22(6):735–740. doi: 10.1111/j.1468-3083.2008.02590.x.
- Saeed S, Keehn CA, Morgan MB. Cutaneous metastasis: a clinical, pathological, and immunohistochemical appraisal. J Cutan Pathol. 2004;31(6):419–430. doi: 10.1111/j.0303-6987.2004.00207.x.
- Matejuk A. Skin immunity. Arch Immunol Ther Exp. 2018;66(1):45–54. doi: 10.1007/s00005-017-0477-3.
- Agius E, Lacy KE, Vukmanovic-Stejic M, et al. Decreased TNF-alpha synthesis by macrophages restricts cutaneous immunosurveillance by memory CD4+ T cells during aging. J Exp Med. 2009;206(9):1929–1940. doi: 10.1084/jem.20090896.
- Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557.
- Bedoui S, Whitney PG, Waithman J, et al. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat Immunol. 2009;10(5):488–495. doi: 10.1038/ni.1724.
- Ginhoux F, Collin MP, Bogunovic M, et al. Blood-derived dermal langerin + dendritic cells survey the skin in the steady state. J Exp Med. 2007;204(13):3133–3146. doi: 10.1084/jem.20071733.
- Dieci MV, Tsvetkova V, Orvieto E, et al. Immune characterization of breast cancer metastases: prognostic implications. Breast Cancer Res. 2018;20(1):62. doi: 10.1186/s13058-018-1003-1.]
- Gilhar A, Paus R, Kalish RS. Lymphocytes, neuropeptides, and genes involved in alopecia areata. J Clin Invest. 2007;117(8):2019–2027. doi: 10.1172/JCI131942.
- Wang X, Marr AK, Breitkopf T, et al. Hair follicle mesenchyme-associated PD-L1 regulates T-cell activation induced apoptosis: a potential mechanism of immune privilege. J Invest Dermatol. 2014;134(3):736–745. doi: 10.1038/jid.2013.368.
- Murakami T, Cardones AR, Hwang ST. Chemokine receptors and melanoma metastasis. J Dermatol Sci. 2004;36(2):71–78. doi: 10.1016/j.jdermsci.2004.03.002.
- Kakinuma T, Hwang ST. Chemokines, chemokine receptors, and cancer metastasis. J Leukoc Biol. 2006;79(4):639–651. doi: 10.1189/jlb.1105633.
- Müller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410(6824):50–56. doi: 10.1038/35065016.
- Marneros AG, Blanco F, Husain S, et al. Classification of cutaneous intravascular breast cancer metastases based on immunolabeling for blood and lymph vessels. J Am Acad Dermatol. 2009;60(4):633–638. doi: 10.1016/j.jaad.2008.11.008.
- Güth U, Wight E, Schötzau A, et al. Correlation and significance of histopathological and clinical features in breast cancer with skin involvement (T4b). Hum Pathol. 2006;37(3):264–271. doi: 10.1016/j.humpath.2005.11.009.
- Cunningham JE, Jurj AL, Oman L, et al. Is risk of axillary lymph node metastasis associated with proximity of breast cancer to the skin? Breast Cancer Res Treat. 2006;100(3):319–328. doi: 10.1007/s10549-006-9256-2.
- Colpaert CG, Vermeulen PB, Van Beest P, et al. Cutaneous breast cancer deposits show distinct growth patterns with different degrees of angiogenesis, hypoxia and fibrin deposition. Histopathology. 2003;42(6):530–540. doi: 10.1046/j.1365-2559.2003.01629.x.
- Elmi M, Wakeam E, Azin A, et al. Surgical morbidity of full-thickness chest wall resection for breast cancer: a retrospective study of a national database. J Surg Res. 2021;257:161–166. doi: 10.1016/j.jss.2020.07.061.
- Merritt RE. Chest wall reconstruction without prosthetic material. Thorac Surg Clin. 2017;27(2):165–169. doi: 10.1016/j.thorsurg.2017.01.010.
- Ibrahim N, Samuels B, Page R, et al. Multicenter phase II trial of ABI-007, an albumin-bound paclitaxel, in women with metastatic breast cancer. J Clin Oncol. 2005;23(25):6019–6026. doi: 10.1200/JCO.2005.11.013.
- Martín M, Ruiz A, Muñoz M, et al. Gemcitabine plus vinorelbine versus vinorelbine monotherapy in patients with metastatic breast cancer previously treated with anthracyclines and taxanes: final results of the phase III Spanish Breast Cancer Research Group (GEICAM) trial. Lancet Oncol. 2007;8(3):219–225. doi: 10.1016/S1470-2045(07)70041-4.
- Saunders Y, Stebbing J, Broadley K, et al. Recurrent locally advanced breast cancer: the treatment of chest wall disease with further chemotherapy. Clin Oncol. 2001;13(3):195–199. doi: 10.1053/clon.2001.9252.
- Vassilomanolakis M, Koumakis G, Demiri M, et al. Vinorelbine and cisplatin for metastatic breast cancer: a salvage regimen in patients progressing after docetaxel and anthracycline treatment. Cancer Invest. 2003;21(4):497–504. doi: 10.1081/cnv-120022358.