Abstract
Objective:
Exosome microRNAs (miRNAs) have great research outlook in clinical therapy and biomarkers, they have been found to have a close to multiple diseases. A growing number of studies have attempted to alleviate or treat diseases through exosomes. It indicates that miRNAs in exosomes have great significance in preventing and controlling diseases in clinical research. We summarise these studies below to better understand their implications.
Methods:
We screened and analyzed more than 100 articles from PubMed, Web of Science, and other databases from 1987 to 2022. Data of the clinical trials are collected from clinicaltrials.gov.
Results:
In this review, we introduce the source, type, and characteristics of several exosomes, summarising current research on their role in cardiovascular, nervous system disease, tumour, and other diseases. Further, we discuss their mechanism of action and future directions for development of treatments in several diseases, and highlight the significant research value and potential use of exosomes in clinical diagnosis and treatment.
Conclusions:
An increasing number of researchers have begun to explore the link between exosomal miRNAs and diseases. More exosome therapeutics will be used in future clinical trials, which may bring new hope for the diagnosis and treatment of several diseases.
Exosomes have unique advantages in molecular transport and cell signal transduction.
miRNAs play an essential role in the formation of multiple diseases.
Research on the clinical application and potential value of exosomes is growing.
KEY MESSAGES
1. Introduction
In 1983, a special vesicle component was discovered in sheep reticulocytes – the first one that scientists had ever observed floating outside the cell – and was named an ‘exosome’ by Johnstone in 1987. However, since the structure of this vesicle was uncomplicated it did not attract much attention at the time. With the development of life science research, researchers have found that many cells can secrete extracellular vesicles (EVs). Exosomes are a type of EV. Today, the term ‘exosome’ refers specifically to vesicles between 30 and 100 nm in diameter [Citation1,Citation2]. The volume is insignificant for this type of vesicle since it has such strong permeability, which can quickly distribute it through the whole-body tissue by fusing with cell membranes to participate in the body’s metabolism and function and play a role in intercellular communication.
Exosomes have a double-membrane structure and are stimulated by normal or abnormal signals and engulfed by invagination of the plasma membrane to form primitive intracellular vesicles [Citation3]. Many intracellular vesicles fuse and gradually mature into intraluminal vesicles (ILVs) [Citation2,Citation4]. ILVs contain many types of vesicular structures, and their mature bodies are called multivesicular bodies (MVBs). MVBs can be degraded and self-cleared through lysosomes and undergo apoptosis to ensure homeostasis. Additionally, undegraded MVBs secrete ILVs through exocytosis, distributing their contents to the whole body; once the ILVs exit the cell they become exosomes [Citation5,Citation6]. The content of the exosome is not necessarily the same as in the original secretion and can have different functions. MicroRNAs (miRNAs) can be sorted into exosomes by selectively binding to the protein heterogeneous nuclear ribonucleoprotein A2B1 and KRAS, indicating that secretion and release are complex processes, and the content will be processed and selected [Citation7]. Exosomes enter the tissue after cell recognition and exert their effects through receptor interaction, fusion-absorption, endocytosis–exocytosis and other mechanisms [Citation8] ().
Figure 1. The growth and formation of exosomes. Exosomes are small vesicle structures with an inner diameter of 30–100 nm. The cell membrane invaginates to form early endosomes, which further mature and eventually form multivesicular bodies (MVBs). Some of the MVBs are degraded by lysosomes while the other MVBs fuse with the cell membrane and release the exosomes. Exosomes contain DNA, RNA, phospholipids and proteins and enter the body to play a role through receptor interaction, fusion-absorption and endocytosis–exocytosis.
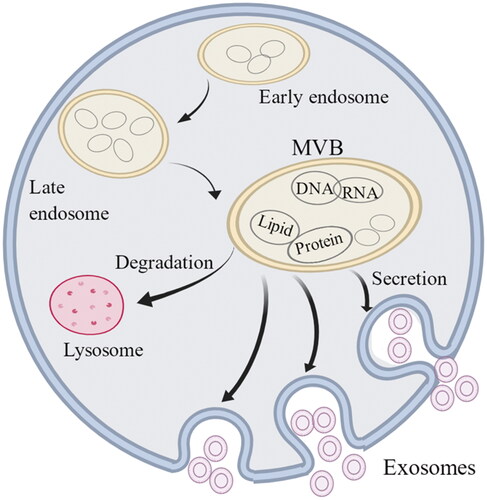
Exosomes contain various active molecules, including proteins, phospholipids, nucleic acids and non-coding RNA (ncRNA). Exosomes can be secreted and released, delivered as vectors, and transferred as signals between cells and tissues throughout the body [Citation9]. Additionally, blood, saliva and tissue fluids contain many exosomes that transport active molecules to target cells. Body fluid transport can effectively ensure rapid transmission, giving rise to the premise that exosomes can be used as a biomarker for early disease diagnosis [Citation10,Citation11]. miRNAs are the most studied mature ncRNAs. Their function is not to directly encode proteins but, rather, to combine with the 3′-UTR non-coding region of specific messenger RNA (mRNA) to promote or reduce protein expression via mRNA [Citation12–14]. Since miRNAs are small and have a relatively stable structure, they can be more effectively transmitted throughout the body than long non-coding RNA (lncRNA) and mRNA. Additionally, exosomes function mainly by influencing gene expression and signalling pathways, which are closely related to miRNA regulation of cellular communication [Citation15,Citation16].
These characteristics make it possible for exosomes to serve as both a biomarker and a new avenue for disease treatment. Studies have confirmed that exosomes can interfere with the mechanisms of disease formation through signal transduction, cellular immunity, angiogenesis and other pathways. Thus, playing a crucial role in the early formation and development of cardiovascular and nervous system diseases and tumours [Citation17,Citation18]. A growing number of studies have attempted to alleviate or treat diseases through exosomes, using methods like implanting exosomes derived from stem cells or synthetic exosomes [Citation19,Citation20]. Their results indicate that miRNAs in exosomes have great significance in preventing and controlling diseases in clinical research. We summarize these studies below to better understand their implications.
2. Origin and isolation of exosomes
EVs are nanoscale to micrometre-sized vesicles secreted by cells and play important roles in cell communication and transmission. According to the size, morphology and function of secretions, they are divided into three main categories: exosomes, microvesicles (MVs) and apoptotic bodies. Exosomes are vesicles formed by the fusion of MVBs with the plasma membrane and generally consist of two types. The first is natural exosomes, which have mainly been studied by scientists. The other is engineered exosomes, which are exosomes modified by molecular engineering techniques [Citation21]. Natural exosomes are secreted by various cells, primarily fibroblasts, endothelial cells, tumours and mesenchymal stem cells (MSCs) [Citation22,Citation23]. MSCs, adult stem cells that originate from the mesoderm and share all the commonalities of stem cells, are currently considered the most effective therapeutic cells. Their advantages include strong replication capacity, fast self-renewal and multi-directional differentiation potential. MSCs have been used in the clinical treatment stage of several studies [Citation24–27]. Additionally, macrophages can also produce exosomes to improve cell proliferation and inflammation by regulating endothelial growth factors and their related signalling pathways [Citation28].
Common methods for isolating exosomes include ultracentrifugation, ultrafiltration, immuno-affinity purification and microfluidic-based isolation techniques [Citation29]. The most classical extraction method, ultracentrifugation, is low-cost and suitable for the study and analysis of large sample numbers. However, the operation procedure is complicated and the extraction concentration is low. Thus, it is mainly used for analysing a small number of samples. Ultrafiltration is used for screening molecules based on their molecular weight, size, density and function. It has the advantages of high speed and purification concentration. However, some impure proteins remain in samples. Regarding new separation methods, immuno-affinity purification and microfluidics-based isolation both have high efficiency and good purification. However, because new technologies require specific reagents and instruments, they are not currently widely available and are primarily used for research on essential molecules and proteins [Citation30]. In addition to the above methods, size exclusion chromatography (SEC) and precipitation are also used for exosome isolation [Citation31]. Still, regardless of how they are isolated, exosomes must either be studied as soon as possible or stored in a refrigerator at −80 °C to ensure that the components are not degraded.
3. Relationship between exosomes and various diseases
3.1. Exosomes and cardiovascular diseases
The most common cardiovascular disease worldwide is coronary atherosclerotic heart disease (CHD), which is mainly caused by myocardial hypoxia and ischaemia due to the accumulation of lipid plaques and thrombosis [Citation32]. The characteristic plaque formation and aggregation of CHD can be controlled by inhibiting the early inflammatory response; therefore, reducing the inflammatory reaction and the resulting damage is the key to protecting the vascular intima [Citation33,Citation34]. Studies have found that MSC-derived exosome miR-133 regulates inflammation levels by controlling the snail 1 gene [Citation35]. Exosomal miR-34a, miR-124 and miR-135b can suppress inflammation through polarization of M2 macrophages [Citation36]. Serum-derived exosomal miR-126 can inhibit atherosclerosis and reduce the risk of stable coronary heart disease [Citation37]. Adipose-derived stem cell (ADSCs)-derived exosome miR-146a reduces cellular inflammation and fibrosis [Citation38]. One study of myocardial infarction found that the MSC-derived exosomes miR-19a and miR-144 protected the myocardium by reducing the apoptosis of cardiomyocytes mediated through the PTEN/AKT pathway [Citation39,Citation40]. MiR-125 also plays a cardioprotective role by regulating autophagic flux [Citation41,Citation42]. Clathrin is a highly conserved protein, which mainly plays a major role in transport in the human body. Exosome miR-214 from ADRCs has been proven to inhibit cell death through clathrin endocytosis and reduce the risk of heart rupture in acute myocardial infarction [Citation43]. Myocardial ischaemia–reperfusion injury (MIRI) increases the difficulty of follow-up treatment. Thus, the smaller the degree of reperfusion injury, the better the prognosis and recovery of the myocardium. Exosomes from MSCs – such as miR-132, miR-21 and miR-210 – can improve myocardial health by promoting vascular regeneration [Citation44–46]. Additionally, miR-25-3p and miR-486-5p can play a cardioprotective role by regulating myocardial apoptosis and necrosis, thereby reducing the area of myocardial infarction [Citation47,Citation48]. The exosomes miR-211/222 of ADSCs and miR-133a-3p of macrophage migration inhibitory factor-engineered umbilical cord MSCs (MIF-ucMSCs) regulate MIRI by reducing fibrosis and inhibiting apoptosis [Citation49,Citation50]. The abovementioned studies indicate a close relationship between exosomal miRNAs and the cardiovascular system ().
Table 1. Role of different kinds of exosome miRNAs in cardiovascular diseases.
Additionally, exosomal miRNAs play a critical role in protecting the heart under hypoxic conditions. Exosome expression is increased, promoting cell proliferation, migration and vascular regeneration. Zhu et al. found that exosomal miR-125b can inhibit proapoptotic factors p53 and BAK1 and reduce myocardial damage after myocardial infarction in a hypoxic environment [Citation41]. In addition to increased expression, the transport, binding and uptake efficiency of exosomes in a hypoxic environment are also improved. Preadaptation in ischaemia–reperfusion injury refers to the fact that several short periods of ischaemia and hypoxia can enhance organ resistance and reduce subsequent reperfusion injury before major and severe injury. This mechanism may be related to the enhancement of exosome function under hypoxia [Citation54]. Other studies have shown that miR-199a is involved in myocardial hypertrophy and fibrosis in patients with hypertrophic cardiomyopathy. In heart failure, miR-1, miR-499 and other circulating miRNAs are essential biomarkers in patients and require further study [Citation55,Citation56].
Among cardiovascular diseases, patients with angina pectoris or myocardial infarction are mainly treated with oral drugs such as antiplatelet agents, nitrates and low-density lipoprotein cholesterol (LDL-C) and patients with severe vascular stenosis require either percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG) to ensure smooth coronary flow. Exosomes can protect myocardial tissue by inhibiting the inflammatory response and apoptosis, promoting angiogenesis, reducing the extent of myocardial infarction, and rescuing the ischaemic myocardium by administering exosomes of different origins [Citation57]. Exosomes have a vital role in cardiovascular therapy. Intervention with exosomes can reduce injury in the early stages, providing a new target for the treatment of patients with myocardial infarction.
3.2. Exosomes and nervous system diseases
Nervous system diseases are diverse and complex, mainly including neurodegenerative, cerebrovascular and neuromuscular junction diseases. Studies have shown that exosomes are closely related to the nervous system function and are mainly involved in cellular transduction, transport and protein expression. Simultaneously, they can freely cross the blood–brain barrier and play an irreplaceable role in treating nervous system diseases () [Citation69–71].
Table 2. Role of different kinds of exosome miRNAs in nervous system diseases.
Neurodegenerative diseases are one of the most important diseases that affect the health and quality of life of the elderly. They include Alzheimer’s disease (AD) and Parkinson’s disease (PD). The mechanism of these diseases is currently believed to be mainly due to a damaged protein expression process, resulting in changes in the structure and properties of neurons or myelin sheaths, which subsequently cannot transmit signals normally [Citation60]. Although there is no cure for this type of disease, research on exosomal miRNAs has shown some exciting new possibilities. Sarkar et al. found that the expression of miR-34a activated synaptic linkage, revealing the polygenic AD formation mechanism [Citation58]. Gui et al. found that cerebrospinal fluid (CSF) secretions contain abundant miRNAs, which differ significantly between normal people and patients with neurodegenerative diseases, indicating that CSF miRNAs can be used as early biomarkers [Citation60].
Stroke is a highly disabling and lethal cerebrovascular disease that seriously threatens human health. Stroke is a sudden and rapidly progressing disease associated with cerebral ischaemia or intracerebral haemorrhage. Similar to critical cardiovascular illness, once it occurs, it needs to be treated within a few hours. Otherwise, it is life-threatening. Currently, thrombolysis and interventional thrombus retrieval are the gold standard early treatments for patients with cerebral infarction. The key to successful treatment is to reduce infarct size and ensure cerebral perfusion. Studies have found that, by establishing a mouse stroke model of exercise, miR-126 can effectively mitigate cerebral infarct size by regulating PI3K. Simultaneously, miR-126 promotes vascular regeneration, which has a significant neuroprotective effect [Citation62]. Supplementing exogenous exosome miR-17-92 has been found to restore the function of nerve cells and reduce the harm caused by ischaemia and hypoxia [Citation63]. By collecting blood samples from patients with acute cerebral infarction and extracting exosomes, Jing et al. found that miR-134 is closely related to inflammatory factors, infarct size and the risk of cerebrovascular events, which may be an essential indicator of ischaemic stroke [Citation64].
The mechanisms of some nervous system diseases are complex and regulated by many factors, such as genes and the environment. Epilepsy is caused by the abnormal excitation of neurons in the brain, often occurring without any apparent reason, and its mechanism is complex. A recent study found that miR-346 and miR-331-3p were significantly downregulated after miRNA sequencing verification in a rat epilepsy model. Signal pathways and targets were identified through enrichment analysis, which provided a new research direction for determining the underlying mechanism of epilepsy [Citation72].
Amyotrophic lateral sclerosis (ALS) is a severe disease that begins with mild weakness, progresses gradually to severe general weakness, and ends with general muscle atrophy and difficulty in breathing. Currently, only supportive symptomatic treatment is available to such patients. Daniel et al. found that the expression of miR-494-3p in exosomes from astrocytes can affect the function of motor neurons and play a role in ALS [Citation65]. In addition to the diseases discussed above, Huntington’s disease (HD), multiple sclerosis (MS) and other neurological diseases have also been confirmed to involve exosome miRNAs, indicating their potential as diagnostic and prognostic indicators [Citation66,Citation67].
3.3. Exosomes and tumours
Tumours are malignant diseases with abnormal gene expression caused by multiple factors. Owing to changes in the surrounding environment, exposure conditions, metabolism and heredity, some gene mutations cause abnormal expression of tissue cells. The biomolecular basis of these diseases is mainly an imbalance between oncogenes and tumour suppressors, apoptosis, and DNA repair genes [Citation73]. Unlike normal cells, tumour cells are characterized by rapid growth and reproduction rates, strong metastatic invasiveness, and the ability to evade normal surveillance by the organism which, once achieved, is difficult to contain and causes great harm to human health and socioeconomics. The focus of current research is mainly to target the characteristics of tumour cells, aiming to intervene in advance and eliminate them in the early stages. Exosomes are essential components of cell communication because of their high transmissibility and free mobility. They carry most of the essential molecules in life, such as proteins, lipids and nucleic acids, all of which participate in tumour formation and progression [Citation74,Citation75]. We have identified possible future treatment strategies to retard and treat tumours by summarizing current exosome and tumour research and analysing the links between the two ().
Table 3. Role of different kinds of exosome miRNAs in tumours.
3.3.1. Promoting proliferation and migration
The rapid rate of cell proliferation and migration is the most prominent characteristic of tumour cells and identifying the regulated genes is key. In a study on non-small cell lung cancer, the engineered exosome miR-499a was constructed, which could be specifically recognized and absorbed by lung cancer basal epithelial cells. Exosomal miR-499a plays an antitumour role by inhibiting the proliferation, growth and spread of lung cancer cells by regulating the apoptotic gene Bcl-2 [Citation76]. Additionally, miR-17-92 was found to be significantly upregulated in lung cancer, playing a role in promoting tumour cell proliferation and gene amplification [Citation84]. Ascites is inevitable in patients with mid- to late-stage gastric cancer, when cancer cells undergo ascites and cause malignant ascites, resulting in accelerated disease progression. Hu et al. studied exosomes derived from malignant ascites in patients with gastric cancer and found that it could increase the invasion and metastasis of gastric cancer cells. Through high-throughput sequencing of ascites samples from patients with gastric cancer and normal patients, miR-196, miR-92 and other genes were differentially expressed, which may serve as novel therapeutic targets for peritoneal metastasis [Citation77].
3.3.2. Promoting tumour angiogenesis
Tumour angiogenesis is the formation of new blood vessels from existing capillaries. It plays an essential role in the nutrient supply of tumour cells and distant metastasis. Angiogenesis is mainly through the process of endothelial cell proliferation, differentiation and fusion into new vascular lumen and basement membrane. In studies on metastatic breast cancer (BRCa), exosomal miR-105 is overexpressed, which enhances vascular regeneration and permeability and induces the proliferation of tumour cells. MiR-105 acts by targeting the regulatory ZO-1 protein, and the inhibition of miR-105 and ZO-1 can reduce tumour angiogenesis. These results suggest that miR-105 may serve as an early biomarker for BRCa [Citation78]. There is a close relationship between exosomes and nervous system tumours, and studies have found that miRNAs play an essential role in various brain tumours by participating in cellular processes such as angiogenesis and apoptosis [Citation85]. In glioblastoma (GBM)-derived exosomes, miR-9 can promote tumour cell growth by hindering the expression of angiostatic proteins and plays a crucial role in inducing apoptosis and promoting tumour angiogenesis [Citation79].
3.3.3. Tumour immune escape
Some tumour cells evade the regulation of the immune system by changing the properties of their self-antigens, causing body cells to proliferate extensively. In liver cancer, exosome miR-23a-3p secreted by hepatocellular carcinoma (HCC) cells suppresses the signal of the PTEN/AKT pathway and reduces T cell function. Transfection of tunicamycin-treated HCC cells into macrophages results in increased apoptosis of T cells and affects normal immune function, which may be a novel mechanism for tumour cells to escape the immune system [Citation80].
The acquisition of drug resistance by tumour cells is also responsible for accelerated tumour progression. Exosome miR-21, derived from adipocytes and fibroblasts in ovarian cancer (OVCa), targets APAF1, a factor that regulates apoptosis, resulting in decreased APAF1 protein expression and suppression of tumour cell apoptosis. When exogenous miR-21 is transfected into cells, drug resistance and invasiveness of tumour cells increase significantly, which could be a potential direction for treating chemotherapeutic drug resistance in OVCa [Citation81]. The tumour microenvironment, which can alter tumour growth and invasion and is considered a new target for antitumour therapy, has been a topic of much research in recent years. Studies have found that GBM-derived exosomes are more prone to disrupting vascular endothelial stability under hypoxic environments, inducing tumour cell proliferation and migration [Citation86]. The formation of other tumours has also been found to be directly related to the exosomes, such as multiple myeloma (MM) and prostate cancer (PCa) [Citation82,Citation83].
3.4. Exosomes and other diseases
Cardiovascular diseases, nervous system diseases and tumours are currently the most studied diseases that involve exosomes. Additionally, it is associated with skeletal muscle, rheumatic and endocrine diseases. For instance, in a tendon injury model, MSC-Exo-miR-29a-3p promoted tendon recovery and reduced muscle atrophy and is now considered a new approach for the treatment of tendon injury [Citation87]. In an osteoarthritis model, delivering exosome miR-140 into chondrocytes inhibited osteocyte destruction and protected tissues [Citation88]. Rheumatology-generated joint synovial fluid contains many exosomes, and the T cell-derived exosome miR-204-5p can inhibit inflammatory responses [Citation89], which are free to pass through biological membranes and delay rheumatoid arthritis (RA) progression.
In blood, exosomes act as important signalling molecules and can transfect leukaemia cells with miR-365, which can lead to the development of stronger drug resistance and affect the efficacy of chemotherapy drugs [Citation90]. The mechanisms of type 1 diabetes in endocrine diseases are complex, and their development is related to immunity and genetics. Mouse model studies have found that ADSCs derived exosomes exert immunomodulatory effects by altering the function of T cells, since mice treated with exosomes had significantly better control of blood glucose [Citation91]. Stem cell-derived exosomes have also been used to treat oral diseases such as periodontitis. Implanting exosomes can contribute to periodontal tissue regeneration and reduce inflammatory responses [Citation92]. These studies all show a close link between exosomes and a variety of diseases, which requires further in-depth analysis.
4. New advances and limitations of exosomes
The rapid development of stem cell technology and genetic engineering in recent years has made exosome research more convenient, and the effects of exosomes derived from stem cells originating from different sources can be investigated in various disease models, helping probe this connection better. MiRNAs are involved in the expression, transport and modification of biological genes, and play essential roles in living organisms ().
Figure 2. Role of exosomal miRNAs in human body. AD: Alzheimer’s disease; PD: Parkinson’s disease; ALS: amyotrophic lateral sclerosis; MS: multiple sclerosis; HD: Huntington’s disease; BRCa: breast cancer; GBM: glioblastoma; HCC: hepatocellular carcinoma; OVCa: ovarian cancer; MM: multiple myeloma; PCa: prostate cancer; CHD: coronary heart disease; MI: myocardial infarction; MIRI: myocardial ischaemia–reperfusion injury.
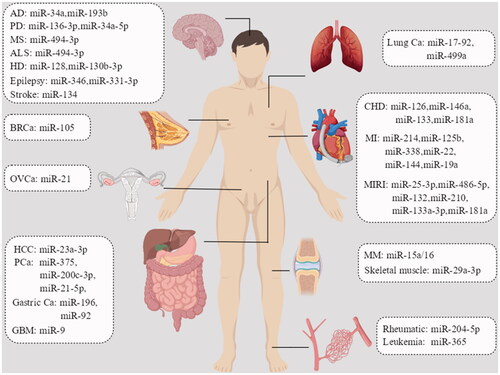
Exosome miRNAs have great research outlook in clinical therapy due to their ease of synthesis and modification. The advantage of exosome miRNAs is that they can function freely through the cell membrane and blood–brain barrier with high delivery efficiency and accuracy. They can not only act directly on nearby cells but also transmit information to distant cells through signals. Another advantage of exosomes is that they can either transport substances themselves or act as carriers. Compared to traditional carriers, they have better stability, lower immunogenicity and easier availability [Citation93]. MSC-derived exosomes used for drug delivery have been found to have good effects with few side effects, indicating that they could serve as a new direction in the treatment of patients with graft-versus-host-disease [Citation94].
Exosomes are also of great value as biomarkers and are the subject of several clinical trials. For example, by collecting urine from patients with PCa and healthy people, the expression of exosomal miRNAs can be analysed to evaluate the accuracy of disease diagnosis [Citation95]. The other trial has examined the specific expression of neurogenic exosomal miRNAs in the blood of suicidal individuals, exploring how miRNA/mRNA-regulatory pathways contribute to suicide pathogenesis [Citation96]. In tumour studies, exosomal miRNAs have been found to be more suitable for the diagnosis of early gastrointestinal cancers than plasma free miRNAs, and some exosomes have been used for the early identification of tumours [Citation97,Citation98]. These characteristics and advantages make us believe that exosomes can have better research prospects in the future.
Previous exosome studies have mainly focused on certain diseases. In this review, we summarized the relationship between exosomal miRNAs and various diseases, comprehensively presenting the current progress and future direction of research. However, exosomes also have limitations. Owing to technical and economic reasons, the large-scale extraction of exosomes cannot be widely performed. The purity of isolated exosomes also needs improvement, which requires better-quality technologies and equipment. Additionally, although exosome miRNAs do have the characteristics necessary to be used as biomarkers for disease diagnosis, they still lack high accuracy for disease diagnosis. Thus, more research needs to be conducted [Citation99].
This article introduces the role of exosome miRNAs in living organisms, demonstrating its value and potential use in terms of exosome source and mechanism of action. Due to the limitations of current technology, studies on exosomal miRNAs cannot be fully applied to clinical programmes, and need more research. Fortunately, an increasing number of researchers have begun to explore the link between exosomal miRNAs and diseases, especially in cardiovascular diseases and tumours, and have already made considerable progress. More exosome therapeutics will be used in future clinical trials, which may bring new hope for the diagnosis and treatment of several diseases.
Author contributions
Senjie Li: conceptualization, data curation, formal analysis, investigation, writing-original draft, writing-review and editing. Dongqing Lv: conceptualization, investigation, methodology, writing-original draft, writing-review and editing. Hong Yang: conceptualization, investigation, methodology, project administration and software. Yan Lu: writing-original draft, writing-review and editing. Yongping Jia: conceptualization, methodology, project administration, supervision, writing-review and editing.
Consent form
All participants provided informed consent.
Acknowledgements
We thank Professor Jia for guidance on the content of the article and Doctor Yang for his support in data analysis.
Disclosure statement
The authors declare no conflict of interest.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.
Additional information
Funding
References
- Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):1–12. doi: 10.1083/jcb.201211138.
- Murillo D, Thistlethwaite W, Rozowsky J, et al. A exRNA atlas analysis reveals distinct extracellular RNA cargo types and their carriers present across human biofluids. Cell. 2019;177(2):463–477.e15. doi: 10.1016/j.cell.2019.02.018.
- Stahl D, Raposo G. Extracellular vesicles: exosomes and microvesicles, integrators of homeostasis. Physiology. 2019;34(3):169–177. doi: 10.1152/physiol.00045.2018.
- Lasser C. Mapping extracellular RNA sheds lights on distinct carriers. Cell. 2019;177(2):228–230. doi: 10.1016/j.cell.2019.03.027.
- Fritz J, Heintz-Buschart A, Ghosal A, et al. Sources and functions of extracellular small RNAs in human circulation. Annu Rev Nutr. 2016;36:301–336. doi: 10.1146/annurev-nutr-071715-050711.
- Hu W, Liu C, Bi Z, et al. Comprehensive landscape of extracellular vesicle-derived RNAs in cancer initiation, progression, metastasis and cancer immunology. Mol Cancer. 2020;19(1):102. doi: 10.1186/s12943-020-01199-1.
- Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci. 2018;75(2):193–208. doi: 10.1007/s00018-017-2595-9.
- van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213–228. doi: 10.1038/nrm.2017.125.
- Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478):eaau6977. doi: 10.1126/science.aau6977.
- Valadi H, Ekström K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596.
- Vlassov AV, Magdaleno S, Setterquist R, et al. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 2012;1820(7):940–948. doi: 10.1016/j.bbagen.2012.03.017.
- Jennifer B, Michelyn J, Mugdha J, et al. MicroRNAs as prognostic markers in acute coronary syndrome patients—a systematic review. Cells. 2019;8(12):1572.
- Berezikov E. Evolution of microRNA diversity and regulation in animals. Nat Rev Genet. 2011;12(12):846–860. doi: 10.1038/nrg3079.
- Anastasiadou E, Jacob S, Slack J. Non-coding RNA networks in cancer. Nat Rev Cancer. 2018;18(1):5–18. doi: 10.1038/nrc.2017.99.
- Angela M, Adriana L, William J, et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood. 2012;119(3):756–766.
- Wang W, Han Y, Jo HA, et al. Non-coding RNAs shuttled via exosomes reshape the hypoxic tumor microenvironment. J Hematol Oncol. 2020;13(1):67. doi: 10.1186/s13045-020-00893-3.
- Xian W, Yi T, Zhao L, et al. The application potential and advance of mesenchymal stem cell-derived exosomes in myocardial infarction. Stem Cells Int. 2021;2021:5579904. doi: 10.1155/2021/5579904.
- Zhong W, Zeng W, Hai X, et al. Exosomal noncoding RNAs in central nervous system diseases: biological functions and potential clinical applications. Front Mol Neurosci. 2022;15:1004221.
- Xu C, Qi L. Potential clinical applications of exosomes in the diagnosis, treatment, and prognosis of cardiovascular diseases: a narrative review. Ann Transl Med. 2022;10(6):372.
- Selvaraj J, Dhanavathy G, Johnson R, et al. Stem cell-derived exosomes potential therapeutic roles in cardiovascular diseases. Front Cardiovasc Med. 2021;8:723236.
- Gege L, Jun W, Guo C, et al. The potential therapeutic value and application prospect of engineered exosomes in human diseases. Front Cell Dev Biol. 2022;10:1051380.
- Stephanie P, Amy B, Mark H, et al. Endothelial cell apoptosis and the role of endothelial cell-derived extracellular vesicles in the progression of atherosclerosis. Cell Mol Life Sci. 2019;76(6):1093–1106.
- Wang C, Zhang C, Liu L, et al. Macrophage-derived mir-155-containing exosomes suppress fibroblast proliferation and promote fibroblast inflammation during cardiac injury. Mol Ther. 2017;25(1):192–204. doi: 10.1016/j.ymthe.2016.09.001.
- Zhang H, Hai Z, Daniela F, et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat Cell Biol. 2018;20(3):332–343. doi: 10.1038/s41556-018-0040-4.
- Pathan M, Pamali F, Sai C, et al. Vesiclepedia 2019: a compendium of RNA, proteins, lipids and metabolites in extracellular vesicles. Nucleic Acids Res. 2019;47(D1):D516–D519. doi: 10.1093/nar/gky1029.
- Van B, Eisele A, Pegtel D, et al. Quantitative and qualitative analysis of small RNAs in human endothelial cells and exosomes provides insights into localized RNA processing, degradation and sorting. J Extracell Vesicles. 2015;4(4):26760. doi: 10.3402/jev.v4.26760.
- Kamerkar S, Valerie S, Hi S, et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546(7659):498–503. doi: 10.1038/nature22341.
- Niu C, Wang X, Zhao M, et al. Macrophage foam cell-derived extracellular vesicles promote vascular smooth muscle cell migration and adhesion. J Am Heart Assoc. 2016;5(10):e004099. doi: 10.1161/JAHA.116.004099.
- Ashley J, Cordy B, Lucia D, et al. Retrovirus-like gag protein Arc1 binds RNA and traffics across synaptic boutons. Cell. 2018;172(1–2):262–274.e211. doi: 10.1016/j.cell.2017.12.022.
- Gurunathan S, Kang MH, Jeyaraj M, et al. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells. 2019;8(4):307. doi: 10.3390/cells8040307.
- Li P, Kaslan M, Lee SH, et al. Progress in exosome isolation techniques. Theranostics. 2017;7(3):789–804. doi: 10.7150/thno.18133.
- Gregory R, George M, Catherine J, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010.
- Guo Y, Yu Y, Hu S, et al. The therapeutic potential of mesenchymal stem cells for cardiovascular diseases. Cell Death Dis. 2020;11(5):349. doi: 10.1038/s41419-020-2542-9.
- Majka M, Sułkowski M, Badyra B, et al. Concise review: mesenchymal stem cells in cardiovascular regeneration: emerging research directions and clinical applications. Stem Cells Transl Med. 2017;6(10):1859–1867. doi: 10.1002/sctm.16-0484.
- Chen Y, Zhao Y, Chen W, et al. MicroRNA-133 overexpression promotes the therapeutic efficacy of mesenchymal stem cells on acute myocardial infarction. Stem Cell Res Ther. 2017;8(1):268. doi: 10.1186/s13287-017-0722-z.
- Heo S, Lim Y, Yoon W, et al. Exosome and melatonin additively attenuates inflammation by transferring miR-34a, miR-124, and miR-135b. Biomed Res Int. 2020;2020:1621394. doi: 10.1155/2020/1621394.
- Jansen F, Yang X, Proebsting S, et al. MicroRNA expression in circulating microvesicles predicts cardiovascular events in patients with coronary artery disease. J Am Heart Assoc. 2014;3(6):e001249. doi: 10.1161/JAHA.114.001249.
- Pan J, Alimujiang M, Chen Q, et al. Exosomes derived from miR-146a-modified adipose-derived stem cells attenuate acute myocardial infarction-induced myocardial damage via downregulation of early growth response factor 1. J Cell Biochem. 2019;120(3):4433–4443. doi: 10.1002/jcb.27731.
- Bolandi Z, Mokhberian N, Eftekhary M, et al. Adipose derived mesenchymal stem cell exosomes loaded with miR-10a promote the differentiation of Th17 and Treg from naive CD4(+) T cell. Life Sci. 2020;259:118218. doi: 10.1016/j.lfs.2020.118218.
- Wen Z, Mai Z, Zhu X, et al. Mesenchymal stem cell-derived exosomes ameliorate cardiomyocyte apoptosis in hypoxic conditions through microRNA144 by targeting the PTEN/AKT pathway. Stem Cell Res Ther. 2020;11(1):36. doi: 10.1186/s13287-020-1563-8.
- Zhu P, Tian T, Wang Y, et al. Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics. 2018;8(22):6163–6177. doi: 10.7150/thno.28021.
- Xiao C, Wang K, Xu Y, et al. Transplanted mesenchymal stem cells reduce autophagic flux in infarcted hearts via the exosomal transfer of miR-125b. Circ Res. 2018;123(5):564–578. doi: 10.1161/CIRCRESAHA.118.312758.
- Eguchi S, Takefuji M, Sakaguchi T, et al. Cardiomyocytes capture stem cell-derived, anti-apoptotic microRNA-214 via clathrin-mediated endocytosis in acute myocardial infarction. J Biol Chem. 2019;294(31):11665–11674. doi: 10.1074/jbc.RA119.007537.
- Ma T, Chen Y, Chen Y, et al. MicroRNA-132, delivered by mesenchymal stem cell-derived exosomes, promote angiogenesis in myocardial infarction. Stem Cells Int. 2018;2018:1–11. doi: 10.1155/2018/3290372.
- Wang K, Jiang Z, Webster A, et al. Enhanced cardioprotection by human endometrium mesenchymal stem cells driven by exosomal microRNA-21. Stem Cells Transl Med. 2017;6(1):209–222. doi: 10.5966/sctm.2015-0386.
- Wang N, Chen C, Yang D, et al. Mesenchymal stem cells-derived extracellular vesicles, via miR-210, improve infarcted cardiac function by promotion of angiogenesis. Biochim Biophys Acta Mol Basis Dis. 2017;1863(8):2085–2092. doi: 10.1016/j.bbadis.2017.02.023.
- Peng Y, Zhao L, Peng Y, et al. Exosomal miR-25-3p from mesenchymal stem cells alleviates myocardial infarction by targeting pro-apoptotic proteins and EZH2. Cell Death Dis. 2020;11(5):317. doi: 10.1038/s41419-020-2545-6.
- Sun XH, Wang X, Zhang Y, et al. Exosomes of bone-marrow stromal cells inhibit cardiomyocyte apoptosis under ischemic and hypoxic conditions via miR-486-5p targeting the PTEN/PI3K/AKT signaling pathway. Thromb Res. 2019;177:23–32. doi: 10.1016/j.thromres.2019.02.002.
- Lee L, Lai C, Lin R, et al. Conditioned medium from adipose-derived stem cells attenuates ischemia/reperfusion-induced cardiac injury through the microRNA-221/222/PUMA/ETS-1 pathway. Theranostics. 2021;11(7):3131–3149. doi: 10.7150/thno.52677.
- Zhu W, Sun L, Zhao P, et al. Macrophage migration inhibitory factor facilitates the therapeutic efficacy of mesenchymal stem cells derived exosomes in acute myocardial infarction through upregulating miR-133a-3p. J Nanobiotechnology. 2021;19(1):61. doi: 10.1186/s12951-021-00808-5.
- Wei Z, Qiao S, Zhao J, et al. miRNA-181a over-expression in mesenchymal stem cell-derived exosomes influenced inflammatory response after myocardial ischemia–reperfusion injury. Life Sci. 2019;232:116632. doi: 10.1016/j.lfs.2019.116632.
- Fu L, Jiang H, Li Y, et al. MicroRNA-338 in MSCs-derived exosomes inhibits cardiomyocyte apoptosis in myocardial infarction. Eur Rev Med Pharmacol Sci. 2020;24(19):10107–10117. doi: 10.26355/eurrev_202010_23230.
- Feng Y, Huang W, Wani M, et al. Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via miR-22. PLOS One. 2014;9(2):e88685. doi: 10.1371/journal.pone.0088685.
- Lei Z, Qing W, Xiao L, et al. Exosomal microRNA-98-5p from hypoxic bone marrow mesenchymal stem cells inhibits myocardial ischemia–reperfusion injury by reducing TLR4 and activating the PI3K/Akt signaling pathway. Int Immunopharmacol. 2021;101:107592.
- Roncarati R, Viviani C, Losi MA, et al. Circulating miR-29a, among other up-regulated microRNAs, is the only biomarker for both hypertrophy and fibrosis in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2014;63(9):920–927. doi: 10.1016/j.jacc.2013.09.041.
- Akat M, Moore V, Morozov P, et al. Comparative RNA-sequencing analysis of myocardial and circulating small RNAs in human heart failure and their utility as biomarkers. Proc Natl Acad Sci USA. 2014;111(30):11151–11156. doi: 10.1073/pnas.1401724111.
- Xin H, Yin X, Zhi X, et al. A large-scale investigation of hypoxia-preconditioned allogeneic mesenchymal stem cells for myocardial repair in nonhuman primates: paracrine activity without remuscularization. Circ Res. 2016;118(6):970–983. doi: 10.1161/CIRCRESAHA.115.307516.
- Sarkar S, Jun S, Rellick S, et al. Expression of MicroRNA-34a in Alzheimer’s disease brain targets genes linked to synaptic plasticity, energy metabolism, and resting state network activity. Brain Res. 2016;1646:139–151. doi: 10.1016/j.brainres.2016.05.026.
- Liu G, Song J, Zhang Q, et al. MicroRNA-193b is a regulator of amyloid precursor protein in the blood and cerebrospinal fluid derived exosomal microRNA-193b is a biomarker of Alzheimer’s disease. Mol Med Rep. 2014;10(5):2395–2400. doi: 10.3892/mmr.2014.2484.
- Gui Y, Liu H, Zhang L, et al. Altered microRNA profiles in cerebrospinal fluid exosome in Parkinson disease and Alzheimer disease. Oncotarget. 2015;6(35):37043–37053. doi: 10.18632/oncotarget.6158.
- Grossi I, Radeghieri A, Paolini L, et al. MicroRNA-34a-5p expression in the plasma and in its extracellular vesicle fractions in subjects with Parkinson’s disease: an exploratory study. Int J Mol Med. 2021;47(2):533–546. doi: 10.3892/ijmm.2020.4806.
- Jin W, Hua L, Shu C, et al. Moderate exercise has beneficial effects on mouse ischemic stroke by enhancing the functions of circulating endothelial progenitor cell-derived exosomes. Exp Neurol. 2020;330:113325.
- Hong X, Mark K, Feng W, et al. MicroRNA cluster miR-17-92 cluster in exosomes enhance neuroplasticity and functional recovery after stroke in rats. Stroke. 2017;48(3):747–753.
- Jing Z, Lin C, Bocan C, et al. Increased serum exosomal miR-134 expression in the acute ischemic stroke patients. BMC Neurol. 2018;18(1):198.
- Daniel G, Ygor S, Raghavendra U, et al. Extracellular vesicles in the forebrain display reduced miR-346 and miR-331-3p in a rat model of chronic temporal lobe epilepsy. Mol Neurobiol. 2020;57(3):1674–1687.
- André V, Monika M, Lydia C, et al. Micro-RNAs secreted through astrocyte-derived extracellular vesicles cause neuronal network degeneration in C9orf72 ALS. EBioMedicine. 2019;40:626–635.
- Maryam A, Mojdeh G, Abdolreza M, et al. Altered expression of miR-326 in T cell-derived exosomes of patients with relapsing-remitting multiple sclerosis. Iran J Allergy Asthma Immunol. 2019;18(1):108–113.
- Kumar S, Vijayan M, Bhatti JS, et al. MicroRNAs as peripheral biomarkers in aging and age-related diseases. Prog Mol Biol Transl Sci. 2017;146:47–94. doi: 10.1016/bs.pmbts.2016.12.013.
- Ratajczak J, Miekus K, Kucia M, et al. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20(5):847–856. doi: 10.1038/sj.leu.2404132.
- Stahl D, Raposo G. Exosomes and extracellular vesicles: the path forward. Essays Biochem. 2018;62(2):119–124. doi: 10.1042/EBC20170088.
- Kawahara H, Hanayama R. The role of exosomes/extracellular vesicles in neural signal transduction. Biol Pharm Bull. 2018;41(8):1119–1125. doi: 10.1248/bpb.b18-00167.
- Fisher S, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55(4):475–482. doi: 10.1111/epi.12550.
- Kalluri R. The biology and function of exosomes in cancer. J Clin Invest. 2016;126(4):1208–1215. doi: 10.1172/JCI81135.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013.
- Rajagopal C, Harikumar KB. The origin and functions of exosomes in cancer. Front Oncol. 2018;8:66. doi: 10.3389/fonc.2018.00066.
- Wen Z, Ming X, Zhi W, et al. Engineered exosomes loaded with miR-449a selectively inhibit the growth of homologous non-small cell lung cancer. Cancer Cell Int. 2021;21(1):485.
- Hu Y, Qi C, Liu X, et al. Malignant ascites-derived exosomes promote peritoneal tumor cell dissemination and reveal a distinct miRNA signature in advanced gastric cancer. Cancer Lett. 2019;457:142–150. doi: 10.1016/j.canlet.2019.04.034.
- Wei Z, Miranda F, Yong M, et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell. 2014;25(4):501–515. doi: 10.1016/j.ccr.2014.03.007.
- Xu C, Fan Y, Tianze Z, et al. MiR-9 promotes tumorigenesis and angiogenesis and is activated by MYC and OCT4 in human glioma. J Exp Clin Cancer Res. 2019;38(1):99. doi: 10.1186/s13046-019-1078-2.
- Jia L, Lu F, Han Y, et al. Endoplasmic reticulum stress promotes liver cancer cells to release exosomal miR-23a-3p and up-regulate PD-L1 expression in macrophages. Hepatology. 2019;70(1):241–258. doi: 10.1002/hep.30607.
- Chi Y, Ngai-Na C, Tetsushi T, et al. Exosomal transfer of stroma-derived miR21 confers paclitaxel resistance in ovarian cancer cells through targeting APAF1. Nat Commun. 2016;7:11150. doi: 10.1038/ncomms11150.
- Ming D, Huan Y, Su L, et al. Exosome-transmitted LINC00461 promotes multiple myeloma cell proliferation and suppresses apoptosis by modulating microRNA/BCL-2 expression. Cytotherapy. 2019;21(1):96–106. doi: 10.1016/j.jcyt.2018.10.006.
- Danny W, Tao T, Emily A, et al. Extracellular RNA as a kind of communication molecule and emerging cancer biomarker. Front Oncol. 2022;12:960072. doi: 10.3389/fonc.2022.960072.
- Yoji H, Hirotaka O, Yoshio T, et al. A polycistronic microRNA cluster, miR-17-92, is overexpressed in human lung cancers and enhances cell proliferation. Cancer Res. 2005;65(21):9628–9632.
- Heather A, Marc H, Fausto R, et al. miRNA regulation in gliomas: usual suspects in glial tumorigenesis and evolving clinical applications. J Neuropathol Exp Neurol. 2017;76(4):246–254.
- Paulina K, Helena C, Johanna W, et al. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc Natl Acad Sci U S A. 2013;110(18):7312–7317.
- Ya Z, Jue L, Hao X, et al. MicroRNA engineered umbilical cord stem cell-derived exosomes direct tendon regeneration by mTOR signaling. J Nanobiotechnol. 2021;19(1):169. doi: 10.1186/s12951-021-00906-4.
- Yu L, Xiao X, Xing L, et al. Chondrocyte-targeted MicroRNA delivery by engineered exosomes toward a cell-free osteoarthritis therapy. ACS Appl Mater Interfaces. 2020;12(33):36938–36947. doi: 10.1021/acsami.0c10458.
- Long W, Qin Z, Xing M, et al. Identification of novel rheumatoid arthritis-associated MiRNA-204-5p from plasma exosomes. Exp Mol Med. 2022;54(3):334–345. doi: 10.1038/s12276-022-00751-x.
- Qing M, Xiao W, Jing Z, et al. Exosomes derived from imatinib-resistant chronic myeloid leukemia cells mediate a horizontal transfer of drug-resistant trait by delivering miR-365. Exp Cell Res. 2018;362(2):386–393. doi: 10.1016/j.yexcr.2017.12.001.
- Shahrzad N, Sara S, Ardeshir H, et al. Immunomodulatory effects of mesenchymal stem cell-derived exosomes on experimental type-1 autoimmune diabetes. J Cell Biochem. 2018;119(11):9433–9443.
- Xiu W, Jin C, Wei T, et al. Strategies of cell and cell-free therapies for periodontal regeneration: the state of the art. Stem Cell Res Ther. 2022;13(1):536.
- Yasunari M, Ryu Y. Therapeutic strategy of mesenchymal-stem-cell-derived extracellular vesicles as regenerative medicine. Int J Mol Sci. 2022;23(12):6480.
- Xiao Z, Mohamed B, Steven P, et al. Comprehensive toxicity and immunogenicity studies reveal minimal effects in mice following sustained dosing of extracellular vesicles derived from HEK293T cells. J Extracell Vesicles. 2017;6(1):1324730. doi: 10.1080/20013078.2017.1324730.
- National Institutes of Health. ClinicalTrials.gov. identifier: NCT03911999. Available from: https://clinicaltrials.gov/ct2/show/NCT03911999?term=NCT03911999&draw=2&rank=1
- National Institutes of Health. ClinicalTrials.gov. identifier: NCT02418195. Available from: https://clinicaltrials.gov/ct2/show/NCT02418195?term=NCT02418195&draw=2&rank=1
- Min L, Zhu S, Chen L, et al. Evaluation of circulating small extracellular vesicles derived miRNAs as biomarkers of early colon cancer: a comparison with plasma total miRNAs. J Extracell Vesicles. 2019;8(1):1643670. doi: 10.1080/20013078.2019.1643670.
- Suyash P, Shruti S, Nitesh K, et al. Exosomes as drug delivery systems: a brief overview and progress update. Eur J Pharm Biopharm. 2020;154:259–269.
- Yong S, Youngeun K, Sun H, et al. The emerging role of exosomes as novel therapeutics: biology, technologies, clinical applications, and the next. Am J Reprod Immunol. 2021;85(2):e13329.
