Abstract
Tirofiban is a small non-peptide ligand-mimetic Glycoprotein (GP) IIb/IIIa inhibitor which can reversibly bind to the arginine-glycine-aspartic acid (RGD) recognition site of GP IIb/IIIa to prevent platelet aggregation. It reduces the incidence of thrombotic cardiovascular events in patients with non-ST-segment elevation acute coronary syndrome (NSTE-ACS). Although generally considered safe, tirofiban has been reported to be associated with thrombocytopenia in several case reports and clinical trials. The pathogenesis for this adverse reaction is not entirely understood, is thought to be due to immune-mediated reaction. This side effect caused by tirofiban is especially concerning given how frequently it is used in the practice of contemporary cardiovascular care. The present review provides an overview of the pathophysiology, clinical presentation, management, and risk factors associated with tirofiban-induced thrombocytopenia.
KEY MESSAGES
Tirofiban-induced thrombocytopenia usually occurred within the first 24 h of treatment, frequently accompanied by bleeding symptoms. The majority of the time, supportive care is used to manage this adverse event, and the platelet count often returns to normal in a few days.
Although the exact cause of this adverse response is unknown, it is thought to be due to drug-dependent antibodies that bind to GP IIb/IIIa, presumably after tirofiban-induced conformational change.
Age ≥ 65 years, white blood cell ≥ 12 × 109/L, diabetes mellitus, congestive heart failure, and chronic kidney disease were identified as the risk factors for tirofiban-induced thrombocytopenia. Further investigations are needed for this.
1. Introduction
Glycoprotein (GP) IIb/IIIa is the dominant integrins on platelets, which can bind to the fibrinogen to crosslink platelets, and is essential for platelet activation and aggregation [Citation1]. By occupying the same pocket to block the binding of GP IIb/IIIa to fibrinogen, GP IIb/IIIa antagonists inhibit platelet aggregation (). Tirofiban, a highly selective small non-peptide platelet GP IIb/IIIa antagonist, was approved by the FDA in 1998 to reduce thrombotic cardiovascular events in patients with non-ST-elevation acute coronary syndrome (NSTE-ACS) () [Citation2]. It is used globally in patients with acute coronary syndrome or undergoing percutaneous coronary intervention (PCI) [Citation3]. According to previous clinical trials, tirofiban also appeared to have an acceptable tolerance, safety, and efficacy profile for the treatment of ischemic stroke, particularly for patients complicated with atherosclerotic stenosis [Citation4]. The SaTIS (Safety of Tirofiban in acute Ischemic Stroke) trial detected that tirofiban might be safe in acute ischemic stroke without increasing the incidence of cerebral hemorrhage and parenchymal hemorrhage [Citation5]. The safety of tirofiban for acute ischemic stroke patients with large artery atherosclerosis receiving endovascular therapy was verified in a multi-center prospective study, and it also showed a trend of reducing mortality [Citation6].
Figure 1. (A) The mechanism of tirofiban. (B) The Pathogenesis of tirofiban-induced thrombocytopenia. Created with BioRender.com.
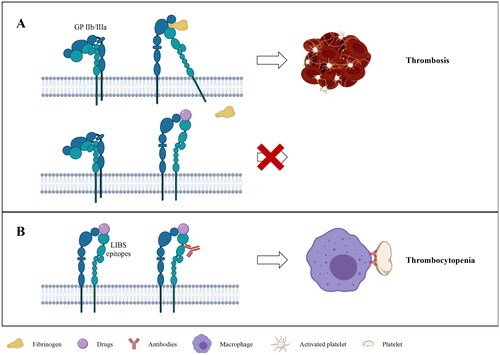
Table 1. Characteristics of tirofiban.
Tirofiban was generally well tolerated, with bleeding complications being the most commonly reported events, but there was no significant difference in the rate of major bleeding in tirofiban recipients compared with heparin in clinical trials [Citation7]. Although being regarded as widely safe, tirofiban has been reported to be associated with thrombocytopenia in rates ranging from 0.4% to 5.6% [Citation8]. This condition has been linked to a poor prognosis and higher mortality [Citation9]. The majority of patients who experienced thrombocytopenia following therapy with tirofiban recovered uneventfully, but severe bleeding and fatalities had been reported, which were associated with longer in-hospital times, increased healthcare costs, morbidity, and even mortality [Citation10]. The purpose of this article is to provide an overview of tirofiban-induced thrombocytopenia including its clinical presentations, pathogenetic mechanisms, and available treatments.
2. Thrombocytopenia caused by tirofiban
2.1. Clinical presentation
In most studies, mild thrombocytopenia was defined as the platelet count between 50 and 100 × 109/L, severe thrombocytopenia as the platelet count less than 50 × 109/L, and profound thrombocytopenia as the platelet count less than 20 × 109/L [Citation11,Citation12]. In the clinical trials of tirofiban, the incidence of severe thrombocytopenia ranged from 0.1% to 0.5%, approximately twice the incidence among individuals who were not administered tirofiban [Citation13,Citation14]. Tirofiban-induced thrombocytopenia usually developed within 24 h after treatment [Citation11], but in some cases might develop delayed thrombocytopenia up to 10 days later [Citation15,Citation16]. Strictly monitoring platelet count in patients receiving tirofiban was significant. Monitoring of platelet count at 6, 12, and 24 h after administrating tirofiban would detect most cases of acute thrombocytopenia [Citation17]. Bleeding symptoms were frequently present along the course of thrombocytopenia, and occasionally life-threatening consequences such as alveolar and gastrointestinal system hemorrhages could occur [Citation18,Citation19]. Angina, palpitations, dyspnea, fever, hypotension, and chills may be observed in some situations [Citation20,Citation21]. The reported cases and studies of tirofiban-induced thrombocytopenia were shown in and (table format and score/exclusion criteria refer to https://www.ouhsc.edu/platelets/ditp.html).
Table 2. Reported cases of thrombocytopenia associated with tirofiban.
Table 3. Summary of thrombocytopenia occurrence in studies of tirofiban.
The treatment strategies for tirofiban-induced thrombocytopenia included modification of the drug regimen, other interventions to minimize the risk of bleeding, and supportive care. The risk of bleeding increases when the patient’s platelet count is below 100 × 109/L, and the therapies which affect hemostasis may need to be discontinued [Citation57]. After discontinuation of treatment, the platelet count might normalize within 1–6 days (mean of 2.1 days) [Citation11]. However, platelet count recovery might be slower in individuals with decreased metabolism due to renal or hepatic insufficiency [Citation33]. Platelet transfusions were recommended in the presence of active bleeding associated with profound thrombocytopenia (platelet count < 20 × 109/L) by the European Society of Cardiology [Citation58], but maybe little effect while reversibly binding GP IIb/IIIa inhibitor tirofiban remains in circulation (half-life 2 h) [Citation59]. In patients with persistent major bleeding, fibrinogen supplementation with cryoprecipitate or fresh frozen plasma may be considered [Citation46]. Supportive treatments for profound thrombocytopenia might include corticosteroid and immunoglobulin therapy [Citation58]. Intravenous immunoglobulin (IVIG) was frequently used to successfully treat thrombocytopenia caused by tirofiban [Citation10,Citation15,Citation17], resulting in an immediate increase in the platelet count and rapid return to normal levels. However, due to thrombocytopenia evolving, inadequate antiplatelet therapy and the use of thrombopoietin may increase the risk of thrombosis, occasionally leading to the occurrence of thrombotic events [Citation38].
2.2. Pathogenesis
Currently, the pathophysiological mechanism of tirofiban-induced thrombocytopenia is still uncertain, it is thought to be associated with immune-mediated thrombocytopenia induced by drug-dependent antibodies (DDAbs) [Citation60]. Drug-induced immune thrombocytopenia (DITP) refers to thrombocytopenia due to drug-dependent antibody-mediated destruction of platelets. Evidence suggested that the combination of DDAbs, drug, and target antigen – the drug may be trapped at the antigen-antibody interface to form a three-molecule complex – was the mechanism of DITP associated with drugs like quinine and many others. Then, macrophages recognized the Fc ‘tail’ of DDAbs and cleared antibody-coated platelets from the circulation [Citation21,Citation61]. When the drug is present, it can bind to the complementarity-determining regions (CDR) of the antibody to remodel its structure and increase its binding affinity to the target antigenic, which in the absence of the drug is weak for the antibody response to the target antigen [Citation62]. The most common target antigens include platelet glycoprotein complexes such as GP IIb/IIIa and GPIb/IX [Citation63].
The conformation of GP IIb/IIIa was altered after binding to tirofiban, as demonstrated by numerous studies, enabling it to bind to specific DDAbs. Platelets were removed from circulation as a result of these DDAbs being subsequently recognized by immune-mediated reaction. Bougie et al. [Citation18] demonstrated DDAbs specific for GP IIb/IIIa in nine patients who developed acute and severe thrombocytopenia (platelet count < 25 × 109/L) after treatment with tirofiban (four patients) or eptifibatide (five patients). In the acute phase, each patient’s serum contained a high titer of IgG antibodies that reacted to the GP IIb/IIIa complex only in the presence of the therapeutic drugs. Neither tirofiban- nor eptifibatide-dependent antibodies were found in 100 randomly selected healthy blood donors. Of the 23 patients treated with tirofiban or eptifibatide without thrombocytopenia (platelets less than 100 × 109/L), 21 had no detectable antibodies and only 2 had extremely weak tirofiban-dependent antibodies. The results indicated that acute thrombocytopenia following tirofiban or eptifibatide treatment may be caused by DDAbs induced by prior exposure to the drug or produced naturally. This could provide an explanation for the instances of thrombocytopenia observed a few hours after the initial administration [Citation21]. In 2003 and 2005, Dunkley et al.confirmed that thrombocytopenia was caused by tirofiban by finding DDAbs in the blood of four individuals using flow cytometry [Citation16,Citation23]. In addition, Clofent-Sanchez et al. [Citation15] verified that the DDAbs were indeed exclusively generated in the presence of tirofiban by ELISA and flow cytometry, indicating the specificity of the immune response. It should be noted as well that no tirofiban-depended antibodies were detected by ELISA when the platelet count recovered.
Evidence was obtained that the antibodies might recognize multiple target epitopes on the GP IIb/IIIa-drug complex. The binding of GP IIb/IIIa antagonists to the RGD recognition site might induce conformational changes in GP IIb/IIIa, leading to the expression of novel epitopes or ligand-induced binding sites (LIBS) that can be recognized by the antibodies already present in the plasma () [Citation61]. Bougie et al. [Citation64] found that antibodies recognize subtle drug-induced structural changes that were mainly distributed around RGD recognition sites in the head region of GP IIb/IIIa heterodimer. After binding to antibodies, platelets are destructed by macrophages in the spleen or liver through Fcγ receptor signaling via spleen tyrosine kinase (Syk) [Citation11,Citation65,Citation66].
Additionally, various studies have demonstrated that tirofiban-induced antibodies can result in platelet activation and/or microparticle formation [Citation67]. This may explain why the adverse ischemic outcomes observed in the EPIC trail that patients occurred thrombocytopenia had an approximately 12-fold higher mortality rate at 30 days and a roughly four-fold increase rate of myocardial infarction [Citation68]. It could be assumed that platelets may be activated in GP IIb/IIIa inhibitor-induced thrombocytopenia, increasing the risk of thrombosis events and accelerated platelet clearance [Citation69]. The evidence of platelet activation caused by tirofiban-dependent antibodies found by Dunkley et al. [Citation23] may further support the hypothesis. They identified the tirofiban-dependent antibodies in two patients who had occurred profound thrombocytopenia by flow cytometry. The results of the serotonin release test (SRA) represented that tirofiban-dependent antibodies detected in the serum of two patients caused platelet activation. They followed cases receiving tirofiban in order to confirm that this was caused by tirofiban-dependent antibodies [Citation51]. 11 of 871 patients treated with tirofiban developed profound thrombocytopenia (platelet count < 20 × 109/L). Tirofiban-dependent antibodies were confirmed in all cases by flow cytometric assay. Thrombotic events occurred in seven of the eleven patients, including six patients with persistent or recurrent cardiac ischemia and one patient with deep vein thrombosis. Platelet activation was analyzed by various methods, including SRA, P-selectin, and annexin V, showed that thrombotic events were significantly associated with platelet activation. Six patients had evidence of platelet activation, significantly related to further coronary ischemic events occurring during acute thrombocytopenia. Nevertheless, the amount of data is still very limited currently, and more data will be needed to verify such a hypothesis. Further research should be undertaken to investigate how platelets activate and what factors influence them.
2.3. Risk factors
Thrombocytopenia may have negative effects on clinical outcomes, yet clinicians have difficulty identifying high-risk patients with thrombocytopenia. Some patients with thrombocytopenia may not be noticed until severe bleeding complications develop. Therefore, it may be crucial to identify patients at high risk of thrombocytopenia for early and timely intervention.
Yi et al. [Citation8] conducted a study to investigate risk factors of tirofiban-induced thrombocytopenia and developed a simple clinical assessment tool that can be used to predict the occurrence of thrombocytopenia before using tirofiban in patients undergoing PCI. Five independent risk factors for tirofiban-induced thrombocytopenia were ultimately identified by univariable and multivariable analysis, involving age ≥ 65 years, white blood cell ≥ 12 × 109/L, diabetes mellitus, congestive heart failure, and chronic kidney disease (CKD). Patients with thrombocytopenia were found to be older than those without it (62.78 ± 10.93 vs. 60.66 ± 10.87) in this study. To date, the majority of patients reported in cases of tirofiban-induced thrombocytopenia were older than 65 years. The results of this study also showed a higher incidence of thrombocytopenia in diabetes mellitus patients (5.51% vs. 2.66%). Diabetes mellitus was a comorbidity in 664 patients (47.6%) in the study. Additionally, Chen et al. [Citation70] found that patients with diabetes mellitus tended to increase the severity of thrombocytopenia. Another significant risk factor was congestive heart failure, which is defined as New York Heart Association Functional Class III to IV. In this research, the rate of thrombocytopenia in patients with congestive heart failure was 6.37%. Thrombocytopenia has also been reported as a clinical complication in heart failure patients. Mondal et al. [Citation71,Citation72] reported that the modulation of GP IIb/IIIa expression and shedding induced by oxidative stress may play a potential role in platelet apoptosis, leading to thrombocytopenia and non-surgical bleeding in patients with heart failure. It is well known that patients with CKD were at high risk of both bleeding and thrombosis [Citation73]. According to the study, 11.1% of patients with thrombocytopenia had CKD, and patients with CKD were more likely to develop thrombocytopenia than those with normal renal function (7.74% vs. 3.28%). Furthermore, the univariate analysis indicated that white blood cell count was an independent risk factor for thrombocytopenia in patients receiving tirofiban. The cut point of 12 × 109/L is further determined by the LOWESS program. Fountain et al. [Citation74] found that 15% of 533 patients diagnosed with clostridium difficile infection (CDI) patients and elevated levels of white blood cell had moderate thrombocytopenia (platelet count < 100 × 109/L at CDI diagnosis). Numerous studies have shown that infection was often accompanied by the activation of platelets, resulting in increased platelet consumption and clearance, leading to the development of thrombocytopenia [Citation75].
For scoring purposes, age ≥ 65 years, DM, and congestive heart failure were counted as two points, CKD, and white blood cell ≥ 12 × 109/L were scored one point (). Patients with higher risk scores were more likely to develop thrombocytopenia. This model can not only help clinicians identify patients at high risk for thrombocytopenia, but also reduce unnecessary platelet count monitoring in low-risk patients using tirofiban. If a patient is determined to be at high risk of tirofiban-induced thrombocytopenia, prompt treatments such as improving indicator monitoring and altering anticoagulation methods may be necessary. However, further large-scale studies are needed before the scoring system can be used in clinical practice.
Table 4. Scores points for the independent variables [Citation8].
In addition, Adamo et al. [Citation76] found that the incidence of thrombocytopenia was affected by differences in buffers used in the formulation of tirofiban to maintain a slightly acidic pH in undiluted drugs. In the PRISM trial, two different formulations of tirofiban were used sequentially. The study drug used in the early stage of the research was a phosphate-buffered product, which was later replaced by citrate-buffered formulation due to a report of drug instability. The results indicated that the incidence of thrombocytopenia was significantly higher in the early stage. Thrombocytopenia occurred more frequently in patients treated with phosphate buffer-tirofiban than in patients treated with unfractionated heparin (UFH) during the same period (1.7% vs. 0.5% at platelet nadir < 90 × 109/L, 2.0% vs. 0.7% at platelet nadir < 100 × 109/L), with approximately three-fold increased risk of thrombocytopenia. However, during the later stage, no significant difference was observed between citrate-buffered tirofiban and UFH (0.3% vs. 0.1% at platelet nadir < 90 × 109/L, and 0.7% vs. 0.7% at platelet nadir < 100 × 109/L). When compared to UFH, citrate-buffered tirofiban had a lower 30-day risk of mortality (HR: 0.49; 95% CI: 0.27–0.89; p = 0.019), whereas phosphate-buffered tirofiban did not vary from UFH (HR: 0.87; 95% CI: 0.49–1.54; p = 0.629). Thrombocytopenia is associated with a 5- to 10-fold increased risk of TIMI (Thrombolysis In Myocardial Infarction) major or minor bleeding. Patients with platelet nadir < 100 × 109/L also had a two-fold increased risk of developing net adverse cardiovascular events. It is yet unknown why different buffer formulations of the same pharmacological molecule cause thrombocytopenia in variable degrees. It was speculated that the conformational changes in GP IIb/IIIa induced by phosphate-buffered tirofiban were more frequently recognized by naturally occurring antibodies than citrate-buffered tirofiban, and therefore more prone to thrombocytopenia. Phosphate-buffered tirofiban was associated with a higher incidence of thrombocytopenia compared with citrate-buffered tirofiban and may therefore increase the risk of adverse clinical outcomes. Therefore, comprehensive post-marketing surveillance of the formulations of drugs is necessary.
3. GP IIb/IIIa inhibitors-induced thrombocytopenia
In most countries, currently clinically available GP IIb/IIIa inhibitors include abciximab, eptifibatide and tirofiban, the former being monoclonal antibodies and the latter two being small molecule compounds [Citation1]. Abciximab is the Fab fragment of the mouse-human monoclonal antibody 7E3 that binds to the epitope near the ligand binding site of GP IIb/IIIa, resulting in steric hindrance, thus inhibiting platelet aggregation. Eptifibatide is an 832 Da cyclic heptapeptide ligand mimetic containing a lysine-glycine-aspartic acid (KGD) sequence that reversibly blocks platelet aggregation by binding and inhibiting GP IIb/IIIa receptors.
As with tirofiban, abciximab or eptifibatide can also cause acute, severe thrombocytopenia. The various GP IIb/IIIa inhibitors have different propensities to induce thrombocytopenia [Citation60]. A pooled, patient-level analysis of the CHAMPION trials [Citation77] showed a significantly higher incidence of thrombocytopenia in patients treated with GP IIb/IIIa inhibitors compared with patients who did not (2.0% vs. 0.68%). Thrombocytopenia developed in 18 of 558 (3.2%) patients treated with abciximab, 31 of 2105 patients (1.5%) with eptifibatide, and 9 of 327 patients (2.8%) with tirofiban. The final multivariate model analysis revealed that GP IIb/IIIa inhibitors use, age, hyperlipidemia, diabetes mellitus, and prior coronary artery bypass grafting were independently associated with thrombocytopenia, and GP IIb/IIIa inhibitors use was the strongest predictor. A meta-analysis of 29 randomized trials involving 123,419 patients showed that the use of GP IIb/IIIa inhibitors increased the risk of thrombocytopenia (platelet count < 100 × 109/L) by 63% compared with placebo [Citation9]. The results of the subgroup analysis showed that abciximab [risk ratio (RR) = 2.93, 95% confidence interval (CI) 2.43–3.52] and tirofiban (RR = 2.79, 95% CI 1.17–6.63) significantly increased the risk of thrombocytopenia compared with placebo, while the risk of thrombocytopenia with eptifibatide (RR = 1.05, 95% CI 0.86–1.29) did not increase significantly. The different rates of thrombocytopenia observed with various GP IIb/IIIa inhibitors indicated that the new epitopes produced by drugs were different. Thrombocytopenia occurred more frequently in patients with abciximab. It may be that abciximab is an antibody derived and has a higher antigenicity than small molecule compounds such as tirofiban, and thus is more likely to induce an immune response [Citation11]. In the EPIC trial, lower baseline platelet count (< 200 × 109/L), older age (> 65 years), and lighter weight (< 80 kg) were considered to be important risk predictors of thrombocytopenia [Citation26,Citation68].
Five patterns of GP IIb/IIIa inhibitors-induced thrombocytopenia had been described: (1) acute severe thrombocytopenia (platelet count < 10 × 109/L) within 12 h of first exposure, (2) acute thrombocytopenia within 12 h of the second exposure, (3) delayed thrombocytopenia (in 5–7 days after treatment), (4) anaphylaxis after first or second exposure, and (5) pseudo-thrombocytopenia due to platelet clumping [Citation78].
Given that some patients who developed thrombotic events after drug-induced thrombocytopenia may need further treatment, switching to another GP IIb/IIIa inhibitor with a lower or absent risk of thrombocytopenia is critical. So far, at least four patients with thrombocytopenia during abciximab administration were subsequently treated with tirofiban [Citation79,Citation80] or eptifibatide [Citation81,Citation82]. Clofent-Sanchez et al. [Citation15] reported that a patient with profound and prolonged thrombocytopenia induced by tirofiban was treated with abciximab without thrombocytopenia. Lev et al. [Citation83] preliminarily evaluated the clinical safety of sequential treatment with abciximab after tirofiban or eptifibatide in patients undergoing PCI. Of them, 25 patients were treated with post-tirofiban abciximab (tirofiban-abciximab group), 10 with post-eptifibatide abciximab (eptifibatide-abciximab group), and 15 with abciximab alone (abciximab control group). First, tirofiban and eptifibatide were administered over 20–24 h in the tirofiban-abciximab group and eptifibatide-abciximab group, respectively. Prior to PCI, abciximab was initiated and administered continuously over 12h in all patients after obtaining arterial access, and tirofiban or eptifibatide was discontinued after 5 min of overlap with the abciximab infusion. None of the 50 patients developed severe thrombocytopenia. Only 1 in the 25 patients in the tirofiban-abciximab group, and 2 in the abciximab control group had mild thrombocytopenia. This finding indicated that the administration of abciximab after tirofiban or eptifibatide may be effective and safe. It was speculated that the epitopes recognized by the presumably pathogenic antibodies could be different for the three drugs. In vitro, abciximab has inhibited the antibody reaction to thrombocytopenia induced by the ligand-mimetic agents (eptifibatide and tirofiban). The proposed mechanism for this lack of cross-reactivity was the steric hindrance provided by the molecule after binding to the GP IIb/IIIa receptor [Citation18]. However, abciximab-associated thrombocytopenia had been reported years after previous tirofiban-induced thrombocytopenia [Citation29]. Based on the aforementioned evidence, patients with a history of thrombocytopenia induced by these agents should be cautious when readministered GP IIb/IIIa inhibitors.
4. Conclusion
Although tirofiban-induced thrombocytopenia is self-limiting, it has independent prognostic effects because it is associated with a higher risk of death, ischemic events, and bleeding than patients without thrombocytopenia [Citation11]. This tirofiban-related adverse effect resulting from tirofiban usually develops within 24 h after initial treatment, and the platelet count may return to normal in 1–6 days. Close monitoring is essential during the first few hours of treatment, and if thrombocytopenia is detected and confirmed, the drug should be promptly discontinued. If thrombocytopenia persists or active clinical bleeding complications are observed, the patient should be treated with platelet transfusions. It is reasonable to attempt corticosteroids or immunoglobulins in case of profound thrombocytopenia if there are no contraindications. Advanced age (≥ 65), white blood cell ≥ 12 × 109/L, DM, congestive heart failure, and CKD are considered to be independent risk factors for predicting patients at high risk of thrombocytopenia, and clinicians should pay particular attention to those groups. Tirofiban-induced thrombocytopenia is thought to be an immune-mediated reaction. Since the drug-dependent antibodies may persist for years, patients should avoid using the drug in the future and should be cautious when using other GP IIb-IIIa inhibitors [Citation84].
Ethics approval
Ethics committee approval is not required for narrative reviews.
Authors contributions
JW conceptualized the manuscript and performed the literature search and manuscript drafting. DZ supervised and revised the manuscript. All authors contributed to the article and approved the submitted version.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Additional information
Funding
References
- Huang J, Li X, Shi X, et al. Platelet integrin αIIbβ3: signal transduction, regulation, and its therapeutic targeting. J Hematol Oncol. 2019;12(1):1. doi: 10.1186/s13045-019-0709-6.
- King S, Short M, Harmon C. Glycoprotein IIb/IIIa inhibitors: the resurgence of tirofiban. Vascul Pharmacol. 2016;78:10–11. doi: 10.1016/j.vph.2015.07.008.
- Karvouni E, Katritsis DG, Ioannidis JP. Intravenous glycoprotein IIb/IIIa receptor antagonists reduce mortality after percutaneous coronary interventions. J Am Coll Cardiol. 2003;41(1):26–32. doi: 10.1016/s0735-1097(02)02666-9.
- Yang M, Huo X, Miao Z, et al. Platelet glycoprotein IIb/IIIa receptor inhibitor tirofiban in acute ischemic stroke. Drugs. 2019;79(5):515–529. doi: 10.1007/s40265-019-01078-0.
- Siebler M, Hennerici MG, Schneider D, et al. Safety of tirofiban in acute ischemic stroke: the SaTIS trial. Stroke. 2011;42(9):2388–2392. doi: 10.1161/STROKEAHA.110.599662.
- Huo, Xiaochuan, Wang, Anxin, Mo, Dapeng, et al., Safety and efficacy of tirofiban for acute ischemic stroke patients with large artery atherosclerosis stroke etiology undergoing endovascular therapy. Front Neurol. 2021;12:630301. doi: 10.3389/fneur.2021.630301.
- McClellan KJ, Goa KL. Tirofiban. A review of its use in acute coronary syndromes. Drugs. 1998;56(6):1067–1080. doi: 10.2165/00003495-199856060-00017.
- Yi Y-H, Yin W-J, Gu Z-C, et al. A simple clinical pre-procedure risk model for predicting thrombocytopenia associated with periprocedural use of tirofiban in patients undergoing percutaneous coronary intervention. Front Pharmacol. 2018;9:1456. doi: 10.3389/fphar.2018.01456.
- Wessler JD, Giugliano RP. Risk of thrombocytopenia with glycoprotein IIb/IIIa inhibitors across drugs and patient populations: a meta-analysis of 29 large placebo-controlled randomized trials. Eur Heart J Cardiovasc Pharmacother. 2015;1(2):97–106. doi: 10.1093/ehjcvp/pvu008.
- Teke HÜ, Teke D. Profound thrombocytopenia related with tirofiban: will it be enough to only stop medicine? Platelets. 2013;24(4):335–337. doi: 10.3109/09537104.2012.696749.
- Merlini PA, Rossi M, Menozzi A, et al. Thrombocytopenia caused by abciximab or tirofiban and its association with clinical outcome in patients undergoing coronary stenting. Circulation. 2004;109(18):2203–2206. doi: 10.1161/01.CIR.0000127867.41621.85.
- Topol EJ, Moliterno DJ, Herrmann HC, et al. Comparison of two platelet glycoprotein IIb/IIIa inhibitors, tirofiban and abciximab, for the prevention of ischemic events with percutaneous coronary revascularization. N Engl J Med. 2001;344(25):1888–1894. doi: 10.1056/NEJM200106213442502.
- The PRISM study investigators. A comparison of aspirin plus tirofiban with aspirin plus heparin for unstable angina. N Engl J Med. 1998;338(21):1498–1505.
- The RESTORE Investigators. Effects of platelet glycoprotein IIb/IIIa blockade with tirofiban on adverse cardiac events in patients with unstable angina or acute myocardial infarction undergoing coronary angioplasty. Circulation. 1997;96(5):1445–1453. doi: 10.1161/01.CIR.96.5.1445.
- Clofent-Sanchez G, Harizi H, Nurden A, et al. A case of profound and prolonged tirofiban-induced thrombocytopenia and its correction by intravenous immunoglobulin G. J Thromb Haemost. 2007;5(5):1068–1070. doi: 10.1111/j.1538-7836.2007.02440.x.
- Bosco A, Kidson-Gerber G, Dunkley S. Delayed tirofiban-induced thrombocytopenia: two case reports. J Thromb Haemost. 2005;3(5):1109–1110. doi: 10.1111/j.1538-7836.2005.01296.x.
- Velibey Y, Golcuk Y, Ekmekci A, et al. Tirofiban-induced acute profound thrombocytopenia: what is the optimal approach to treatment? Platelets. 2015;26(2):197–198. doi: 10.3109/09537104.2013.787406.
- Bougie DW, Wilker PR, Wuitschick ED, et al. Acute thrombocytopenia after treatment with tirofiban or eptifibatide is associated with antibodies specific for ligand-occupied GPIIb/IIIa. Blood. 2002;100(6):2071–2076. doi: 10.1182/blood.V100.6.2071.h81802002071_2071_2076.
- Zhou X, Peng H, Yin Y, et al. Diffused alveolar hemorrhage: a rare and severe complication of tirofiban-induced thrombocytopenia. Int J Cardiol. 2016;206:93–94. doi: 10.1016/j.ijcard.2016.01.020.
- Ibrahim T, El Karak F, Araji A, et al. Tirofiban-induced thrombocytopenia occurring with crohn’s disease. Case Rep Cardiol. 2016;2016:4605139. doi: 10.1155/2016/4605139.
- Vayne C, Guéry E-A, Rollin J, et al. Pathophysiology and diagnosis of Drug-Induced immune thrombocytopenia. JCM. 2020;9(7):2212. doi: 10.3390/jcm9072212.
- Hofer CK, Straumann E, Genoni M, et al. Profound drug-induced thrombocytopenia before urgent cardiopulmonary bypass✩. Interact Cardiovasc Thorac Surg. 2002;1(2):63–65. doi: 10.1016/s1569-9293(02)00016-6.
- Dunkley S, Lindeman R, Evans S, et al. Evidence of platelet activation due to tirofiban-dependent platelet antibodies: double trouble. J Thromb Haemost. 2003;1(10):2248–2250. doi: 10.1046/j.1538-7836.2003.00429.x.
- Eryonucu B, Tuncer M, Erkoc R. Repetitive profound thrombocytopenia after treatment with tirofiban: a case report. Cardiovasc Drugs Ther. 2004;18(6):503–505. doi: 10.1007/s10557-004-6228-9.
- Mulot A, Moulin F, Fohlen-Walter A, et al. Practical approach to the diagnosis and management of thrombocytopenia associated with tirofiban treatment. Am J Hematol. 2004;77(1):67–71. doi: 10.1002/ajh.20149.
- Patel S, Patel M, Din I, et al. Profound thrombocytopenia associated with tirofiban: case report and review of literature. Angiology. 2005;56(3):351–355. doi: 10.1177/000331970505600319.
- Tuhta AG, Yeşildağ O, Köprülü D. Tirofiban-associated acute thrombocytopenia. Acta Cardiol. 2006;61(5):577–579. doi: 10.2143/AC.61.5.2017776.
- Demirkan B, Guray Y, Guray U, et al. Differential diagnosis and management of acute profound thrombocytopenia by tirofiban: a case report. J Thromb Thrombolysis. 2006;22(1):77–78. doi: 10.1007/s11239-006-7789-1.
- Dorsch MP, Montague D, Rodgers JE, et al. Abciximab-associated thrombocytopenia after previous tirofiban-related thrombocytopenia. Pharmacotherapy. 2006;26(3):423–427. doi: 10.1592/phco.26.3.423.
- Höchtl T, Pachinger L, Unger G, et al. Antiplatelet drug induced isolated profound thrombocytopenia in interventional cardiology: a review based on individual case reports. J Thromb Thrombolysis. 2007;24(1):59–64. doi: 10.1007/s11239-006-9052-1.
- Beiras-Fernandez A, Kowert A, Jiru P, et al. Acute profound thrombocytopenia after treatment with tirofiban and off-pump coronary artery bypass grafting. Ann Thorac Surg. 2009;87(2):629–631. doi: 10.1016/j.athoracsur.2008.06.040.
- Sakellariou D, et al. First report of tirofiban-induced anemia (found in combination with severe thrombocytopenia). Tex Heart Inst J. 2009;36(1):55–57.
- Rahman N, Jafary FH. Vanishing platelets: rapid and extreme tirofiban-induced thrombocytopenia after percutaneous coronary intervention for acute myocardial infarction. Tex Heart Inst J. 2010;37(1):109–112.
- Panduranga P, Sulaiman K. Severe thrombocytopenia following tirofiban infusion. Indian J Pharmacol. 2011;43(6):726–728.
- Elcioglu OC, Ozkok A, Akpınar TS, et al. Severe thrombocytopenia and alveolar hemorrhage represent two types of bleeding tendency during tirofiban treatment: case report and literature review. Int J Hematol. 2012;96(3):370–375. doi: 10.1007/s12185-012-1133-7.
- Dursunoglu D, Taskoylu O, Gür S, et al. Tirofiban-induced acute profound thrombocytopenia after primary angioplasty. Asian Cardiovasc Thorac Ann. 2013;21(1):74–76. doi: 10.1177/0218492312445142.
- Yurtdaş M, Yaylali YT, Aladağ N, et al. Acute serious thrombocytopenia associated with intracoronary tirofiban use for primary angioplasty. Case Rep Med. 2014;2014:190149.
- Li Y, Xu Q, Guo X. Thromboembolic events secondary to tirofiban-induced thrombocytopenia being treated with thrombopoietin: a case report. Exp Ther Med. 2016;12(2):1177–1180. doi: 10.3892/etm.2016.3439.
- Meghrajani V, Sabharwal N, Namana V, et al. A case of hyperacute severe thrombocytopenia occurring less than 24 hours after intravenous tirofiban infusion. Case Rep Hematol. 2018;2018:4357981.
- Patti R, Sinha A, Rodriguez D, et al. Severe thrombocytopenia due to intravenous tirofiban therapy. Am J Ther. 2019;26(4):e534–e537. doi: 10.1097/MJT.0000000000000756.
- Liu Y-Y, Dong Q-T, Guo Y-L, et al. Tirofiban-Induced severe thrombocytopenia. Am J Ther. 2019;26(5):e659–e661. doi: 10.1097/MJT.0000000000000861.
- Kabadi RA, Danelich IM, Entwistle JW, et al. Use of cangrelor as a bridge to left ventricular assist device implantation in a patient with a recent drug-eluting stent who developed acute Tirofiban-Related thrombocytopenia. Pharmacotherapy. 2019;39(4):521–525. doi: 10.1002/phar.2219.
- Wang S, Sawalha K, Khan A. An unusual case of drug-induced thrombocytopenia. J Investig Med High Impact Case Rep. 2020;8:2324709620947891. doi: 10.1177/2324709620947891.
- Rawala MS, Ahmed AS, Posina K, et al. Tirofiban induced thrombocytopenia: a rare but severe adverse effect. J Community Hosp Intern Med Perspect. 2020;10(2):171–173. doi: 10.1080/20009666.2020.1747783.
- Gulati A, Tiwari A, Shetty V, et al. Tirofiban: a rare cause of thrombocytopenia in a patient undergoing percutaneous coronary intervention. Cureus. 2021;13(9):e18217. doi: 10.7759/cureus.18217.
- Abdeladim S, Elharras M, Elouarradi A, et al. Thrombocytopenia induced by glycoprotein (GP) IIb-IIIa antagonists: about two cases. Pan Afr Med J. 2021;38:9. doi: 10.11604/pamj.2021.38.9.27215.
- Ran Y, Xu H, Huo Y, et al. Acute profound thrombocytopenia within 1 hour after small doses of tirofiban. Am J Ther. 2022. doi: 10.1097/MJT.0000000000001553.
- Piao B, Li T, Zhong H, et al. Acute profound thrombocytopenia induced by tirofiban in stent assisted embolization of intracranial ruptured aneurysm-a rare case report. Heliyon. 2023;9(4):e14504. doi: 10.1016/j.heliyon.2023.e14504.
- The PRISM-PLUS Study Investigators. Inhibition of the platelet glycoprotein IIb/IIIa receptor with tirofiban in unstable angina and non-Q-wave myocardial infarction. N Engl J Med. 1998;338(21):1488–1497.
- Valgimigli M, Percoco G, Barbieri D, et al. The additive value of tirofiban administered with the high-dose bolus in the prevention of ischemic complications during high-risk coronary angioplasty: the ADVANCE trial. J Am Coll Cardiol. 2004;44(1):14–19. doi: 10.1016/j.jacc.2004.03.042.
- Dunkley S, Evans S, Gaudry L, et al. Two distinct subgroups of tirofiban-induced thrombocytopenia exist due to drug dependent antibodies that cause platelet activation and increased ischaemic events. Platelets. 2005;16(8):462–468. doi: 10.1080/09537100500140141.
- Valgimigli M, Percoco G, Malagutti P, et al. Tirofiban and sirolimus-eluting stent vs abciximab and bare-metal stent for acute myocardial infarction: a randomized trial. JAMA. 2005;293(17):2109–2117. doi: 10.1001/jama.293.17.2109.
- Valgimigli M, Campo G, Percoco G, et al. Comparison of angioplasty with infusion of tirofiban or abciximab and with implantation of sirolimus-eluting or uncoated stents for acute myocardial infarction: the MULTISTRATEGY randomized trial. JAMA. 2008;299(15):1788–1799. doi: 10.1001/jama.299.15.joc80026.
- Van’t Hof AW, ten Berg J, Heestermans T, et al. Prehospital initiation of tirofiban in patients with ST-elevation myocardial infarction undergoing primary angioplasty (on-TIME 2): a multicentre, double-blind, randomised controlled trial. Lancet. 2008;372(9638):537–546. doi: 10.1016/S0140-6736(08)61235-0.
- Garbe E, Andersohn F, Bronder E, et al. Drug-induced immune thrombocytopaenia: results from the Berlin case-control surveillance study. Eur J Clin Pharmacol. 2012;68(5):821–832. doi: 10.1007/s00228-011-1184-3.
- Zanaty M, Allan L, Samaniego EA, et al. Phase 1/2a trial of ISPASM. Stroke. 2021;52(12):3750–3758. doi: 10.1161/STROKEAHA.121.034578.
- Huxtable LM, Tafreshi MJ, Rakkar AN. Frequency and management of thrombocytopenia with the glycoprotein IIb/IIIa receptor antagonists. Am J Cardiol. 2006;97(3):426–429. doi: 10.1016/j.amjcard.2005.08.066.
- Collet J-P, Thiele H. ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42(20):2020–2021. doi: 10.1093/eurheartj/ehaa909.
- Wang HL, Aguilera C, Knopf KB, et al. Thrombocytopenia in the intensive care unit. J Intensive Care Med. 2013;28(5):268–280. doi: 10.1177/0885066611431551.
- Aster RH. Immune thrombocytopenia caused by glycoprotein IIb/IIIa inhibitors. Chest. 2005;127(2 Suppl):53s–59s. doi: 10.1378/chest.127.2_suppl.53S.
- Warkentin TE. Drug-induced immune-mediated thrombocytopenia–from purpura to thrombosis. N Engl J Med. 2007;356(9):891–893. doi: 10.1056/NEJMp068309.
- Aster RH, Bougie DW. Drug-induced immune thrombocytopenia. N Engl J Med. 2007;357(6):580–587. doi: 10.1056/NEJMra066469.
- Marini I, Uzun G, Jamal K, et al. Treatment of drug-induced immune thrombocytopenias. Haematologica. 2022;107(6):1264–1277. doi: 10.3324/haematol.2021.279484.
- Bougie DW, Rasmussen M, Zhu J, et al. Antibodies causing thrombocytopenia in patients treated with RGD-mimetic platelet inhibitors recognize ligand-specific conformers of αIIb/β3 integrin. Blood. 2012;119(26):6317–6325. doi: 10.1182/blood-2012-01-406322.
- McMillan R. The pathogenesis of chronic immune thrombocytopenic purpura. Semin Hematol. 2007;44(4 Suppl 5):S3–s11. doi: 10.1053/j.seminhematol.2007.11.002.
- Sun S, Urbanus RT, ten Cate H, et al. Platelet activation mechanisms and consequences of immune thrombocytopenia. Cells. 2021;10(12):3386. doi: 10.3390/cells10123386.
- Aster RH, Curtis BR, Bougie DW. Thrombocytopenia resulting from sensitivity to GPIIb-IIIa inhibitors. Semin Thromb Hemost. 2004;30(5):569–577. doi: 10.1055/s-2004-835677.
- Berkowitz SD, Sane DC, Sigmon KN, et al. Occurrence and clinical significance of thrombocytopenia in a population undergoing high-risk percutaneous coronary revascularization. Evaluation of c7E3 for the Prevention of Ischemic Complications (EPIC) study group. J Am Coll Cardiol. 1998;32(2):311–319. doi: 10.1016/s0735-1097(98)00252-6.
- Abrams CS, Cines DB. Platelet glycoprotein IIb/IIIa inhibitors and thrombocytopenia: possible link between platelet activation, autoimmunity and thrombosis. Thromb Haemost. 2002;88(12):888–889. doi: 10.1055/s-0037-1613328.
- Chen C-Y, Lee M-Y, Lin K-D, et al. Diabetes mellitus increases severity of thrombocytopenia in dengue-infected patients. Int J Mol Sci. 2015;16(2):3820–3830. doi: 10.3390/ijms16023820.
- Mondal NK, Chen Z, Trivedi JR, et al. Oxidative stress induced modulation of platelet integrin α2bβ3 expression and shedding may predict the risk of major bleeding in heart failure patients supported by continuous flow left ventricular assist devices. Thromb Res. 2017;158:140–148. doi: 10.1016/j.thromres.2017.09.006.
- Mondal NK, Li T, Chen Z, et al. Mechanistic insight of platelet apoptosis leading to non-surgical bleeding among heart failure patients supported by continuous-flow left ventricular assist devices. Mol Cell Biochem. 2017;433(1–2):125–137. doi: 10.1007/s11010-017-3021-1.
- Fox CS, Muntner P, Chen AY, et al. Use of evidence-based therapies in short-term outcomes of ST-segment elevation myocardial infarction and non-ST-segment elevation myocardial infarction in patients with chronic kidney disease: a report from the National Cardiovascular Data Acute Coronary Treatment and Intervention Outcomes Network registry. Circulation. 2010;121(3):357–365. doi: 10.1161/CIRCULATIONAHA.109.865352.
- Fountain EM, Moses MC, Park LP, et al. Thrombocytopenia in hospitalized patients with severe clostridium difficile infection. J Thromb Thrombolysis. 2017;43(1):38–42. doi: 10.1007/s11239-016-1423-7.
- Assinger A. Platelets and infection - an emerging role of platelets in viral infection. Front Immunol. 2014;5:649. doi: 10.3389/fimmu.2014.00649.
- Adamo M, Ariotti S, Costa F, et al. Phosphate- or citrate-buffered tirofiban versus unfractionated heparin and its impact on thrombocytopenia and clinical outcomes in patients with acute coronary syndrome: a post hoc analysis from the PRISM trial. JACC Cardiovasc Interv. 2016;9(16):1667–1676. doi: 10.1016/j.jcin.2016.05.031.
- Groves EM, et al. Incidence, predictors, and outcomes of acquired thrombocytopenia after percutaneous coronary intervention: a pooled, patient-level analysis of the CHAMPION trials (cangrelor versus standard therapy to achieve optimal management of platelet inhibition). Circ Cardiovasc Interv. 2018;11(4):e005635.
- Aster RH, Curtis BR, Bougie DW, et al. Thrombocytopenia associated with the use of GPIIb/IIIa inhibitors: position paper of the ISTH working group on thrombocytopenia and GPIIb/IIIa inhibitors. J Thromb Haemost. 2006;4(3):678–679. doi: 10.1111/j.1538-7836.2006.01829.x.
- Desai M, Lucore CL. Uneventful use of tirofiban as an adjunct to coronary stenting in a patient with a history of abciximab-associated thrombocytopenia 10 months earlier. J Invasive Cardiol. 2000;12(2):109–112.
- Simon BC, Herzum M, Klisch A, et al. Acute severe thrombocytopenia after c7E3 Fab (abciximab) therapy in a patient with unstable angina and stenting of the right coronary artery. Occurrence of subacute stent thrombosis and safe readministration of the GPIIb/IIIa inhibitor tirofiban. Int J Cardiovasc Intervent. 2000;3(3):185–188. doi: 10.1080/14628840050516118.
- Coto H. Platelet receptor glycoprotein IIb/IIIa inhibition with eptifibatide in a patient with thrombocytopenia after treatment with abciximab. J Invasive Cardiol. 2000;12(10):528–531.
- Rao J, Mascarenhas DA. Successful use of eptifibatide as an adjunct to coronary stenting in a patient with abciximab-associated acute profound thrombocytopenia. J Invasive Cardiol. 2001;13(6):471–473.
- Lev EI, Osende JI, Richard MF, et al. Administration of abciximab to patients receiving tirofiban or eptifibatide: effect on platelet function. J Am Coll Cardiol. 2001;37(3):847–855. doi: 10.1016/s0735-1097(00)01181-5.
- George JN, Aster RH. Drug-induced thrombocytopenia: pathogenesis, evaluation, and management. Hematology Am Soc Hematol Educ Program. 2009;2009(1):153–158. doi: 10.1182/asheducation-2009.1.153.
