Abstract
Background
This study aimed to identify the appropriate signature veins for the right adrenal gland using a 3D model fused with adrenal venography images and to verify their accuracy through the selectivity index (SI) >2.
Methods
We analyzed the right adrenal venography images of 41 patients who underwent adrenal venous sampling (AVS). These images were merged with a 3D structure of the adrenal gland to identify the signature veins of the right adrenal gland. We then used the signature veins observed during adrenal venography to determine the optimal position of the catheter tip during AVS for 53 other patients. Finally, we verified the accuracy of this method according to the SI.
Results
We successfully fused the 3D models of 41 cases with adrenal venography images. We identified the trunk branch type as the major venous morphology in the right anterior oblique at degrees of 30 (38 cases, 92.7%). In addition, the central vein, brush vein, uvula vein, and capsular vein were identified as signature veins for the right AVS. The accuracy of AVS was 100% in the other 53 patients, as verified by an SI >2.
Conclusions
Our study identified the right adrenal signature veins, including the previously overlooked uvula vein, which can be used to determine the position of the catheter tip and improve the success rate of AVS.
KEY MESSAGES
The majority of the venography types observed in patients in the right anterior oblique at 30 degrees during adrenal venography were trunk branch types, while irregular or hollow triangle types were infrequent.
The signature veins identified during right adrenal venous sampling were the central vein, brush vein, uvula vein, and capsular vein.
The right adrenal signature veins, particularly the uvula vein, which has not been given much attention in the past, can serve as a reference to verify the position of the catheter tip and enhance the success rate of adrenal venous sampling.
Introduction
Adrenal venous sampling (AVS) is considered the gold standard for subtype classification and lateralization of primary aldosteronism [Citation1,Citation2]. During the procedure, adrenal venography is required to confirm the proper placement of the catheter tip [Citation3]. However, identifying the right adrenal gland can be challenging due to the variability of right adrenal venography images [Citation4,Citation5]. Previous literature reports have shown that referral centres with experienced interventional radiologists can achieve an overall success rate of up to 90% in AVS procedures [Citation6–8]. However, this success rate drops significantly to 30.5% in centres lacking experienced interventional radiologists [Citation9].
We believe that the most crucial factor in successful AVS procedures is the ability of interventional radiologists to accurately interpret adrenal venography images. However, the success of AVS is also heavily dependent on the morphology of adrenal veins [Citation10,Citation11]. Unfortunately, the structural and distribution characteristics of adrenal veins and the reasons for the variability of adrenal venography are not well understood.
To address these issues, we investigated the concept of adrenal signature veins and assessed their potential utility in confirming proper catheter tip placement during AVS.
Methods
Research object
This retrospective clinical study consisted of two stages: the development model and the validation model. The development model included patients who underwent an adrenal CT examination at Xiangyang Central Hospital/Affiliated Xiangyang Hospital of Hubei College of Arts and Sciences between 1 June 2019 and 1 June 2021, and met the following inclusion criteria: (1) screening following the PA guidelines [Citation12] issued by the American Endocrine Society before surgery, (2) AVS conducted in a posterior–anterior position and the 30 degrees right anterior oblique, (3) patients over 18 years old, and (4) adrenal thin layer (thickness of 0.5–1 mm) enhanced CT. The exclusion criteria were as follows: (1) inability to distinguish the right adrenal gland from adjacent tissues in an enhanced CT scan, and (2) the right adrenal gland had a space-occupying lesion resulting in noticeable morphological changes. Based on these criteria, 18 patients were excluded, and 41 patients were included in the development model.
The validation model included all patients who underwent AVS in the hospital between 1 July 2021, and 1 July 2022, to verify the value of the signature vein.
Preoperative preparation: all patients have definitive diagnosis confirmed by 0.9% sodium chloride solution loading test or captopril test. Diuretics were discontinued for 4–6 weeks prior to AVS. Antihypertensives agents were limited to calcium antagonists in AVS. Adrenocorticotropic hormone (ACTH) provocation tests were not performed in all cases.
The study was approved by the Ethics Committee of Xiangyang Central Hospital/Affiliated Xiangyang Hospital to Hubei University of Arts and Sciences with ethical approval No.: 2022-023 and conducted in accordance with the Declaration of Helsinki. Informed consent was not required in view of the retrospective nature of the research and the anonymity of the study data.
Parameters of thin-slice CT
The thin-slice CT scan was performed using a Philips 64-slice spiral CT with the following parameters: tube voltage of 120 kV, tube current of 200 mA, layer thickness of 1.0 mm, and screw pitch of 1. During the contrast-enhanced CT scan, the non-ionic contrast agent iohexol (320 mg/mL) was injected through the vein of the upper extremity at a flow rate of 3.0 mL/s, with a total volume of 80–100 mL. Hepatic portal venous phase dynamic imaging was performed during the contrast-enhanced CT scan, with the second phase imaging starting at 30 and 40 s after the initiation of the iohexol injection [Citation13,Citation14]. The scan ranged from the diaphragm to the upper edge of the iliac wings, and the patients were asked to hold their breath before the scan was started. The scan data were reconstructed transversely using images with a thickness of 0.5–1 mm.
AVS procedure
For AVS, we inserted the 4F MPA 1 (Cordis Co., USA) catheter into the right adrenal vein (RAV) through the right basilic vein, median cubital vein, or cephalic vein [Citation15]. Additionally, the 5F cobra (Cordis Co., USA) catheter was used for AVS through the right femoral vein. During the procedure, adrenal venography was performed in a posterior–anterior position and at a right anterior oblique angle of 30 degrees to confirm that the catheter tip was in the correct position. We collected peripheral venous blood from the external iliac vein.
Order of AVS: right adrenal vein, external iliac vein, and finally left adrenal vein.
Protocol of 3D reconstruction of adrenal glands
The following were the brief steps taken: (1) The adrenal CT data were imported into the Mimics 21.0 software (Material Co., Belgium) in DICOM format. (2) By adjusting the window width and position, the margin of the adrenal gland was clearly distinguished from surrounding tissues. (3) The boundary of the adrenal gland in the image was outlined from top to bottom. (4) Isolated impurities were removed, and edges were trimmed on the transverse, sagittal, and coronal planes. (5) 3D reconstruction was carried out after removing isolated impurities and trimming the edges.
The same method was used to reconstruct the RAV (fusion reference) and the lumbar vertebral body (scaling reference) in the same plane as the right adrenal gland. Two senior radiologists (Zhenglin Shen and Qingan Li) outlined the boundary of the adrenal gland in the image.
Fusion of adrenal venography images and 3D models
To match the angle of the adrenal vein image in AVS, we created a 3D model with the RAV and vertebral body, rotated it accordingly, and saved the resulting image. We then set the venography image as the bottom layer and the 3D model image as the top layer and fused the two layer-translucent images. During the fusion process, we used the opening of the RAV as the reference point. To match the image size, we overlapped the upper and lower edges of the same vertebral body.
Experience guidance and inspection verification
Observations of the development model images are summarized as follows: (1) the shape and structure of the 3D adrenal model; (2) the distribution of adrenal veins in the 3D model, and their distribution regulation in a posterior–anterior position and at a right anterior oblique angle of 30 degrees; (3) exploration of signature veins in the right adrenal gland during AVS.
The signature veins in the development model were used to locate the catheter tip optically during AVS. The right adrenal venous blood was collected from 53 patients in the verification model, and the postoperative selectivity index (SI) values were used to confirm the accuracy of the catheter position.
Statistical analysis
The data analysis was conducted using SPSS 22.0 statistical software. Quantitative data with normal distribution, including age, height, weight, body mass index (BMI), systolic blood pressure, diastolic blood pressure, and blood potassium, were presented as mean ± standard deviation, and the t-test was used to compare the differences between groups. Categorical data were presented as numbers and compared using the chi-square test. Statistical significance was defined as p < .05.
Results
Baseline data of patients
In the development model, 41 patients (22 males and 19 females) underwent AVS, with an average age of 48.02 ± 11.26 years (range 28–67). The validation model included 53 patients (25 males and 28 females) who underwent AVS, with an average age of 51.30 ± 9.1 years (range 29–68). The general characteristics and clinical examinations of the two groups were compared, and no significant differences in variables were observed between the two models (p > .05; ).
Table 1. General information of the patients (mean ± SD).
3D model of the right adrenal gland
3D reconstruction was successfully performed in all 41 cases of the right adrenal gland. The gland’s 3D structure was displayed on three surfaces: the front outer surface, the rear inner surface, and the rear lower surfaces. The gland was made up of three ridges in the anterior–posterior direction: the top limb, medial limb, and lateral limb, with the outer edge of the three limbs referred to as the margo liber. These three adjacent limbs combined into a part and formed a cord from back to front called the combination cord, which was divided into three equal parts. The ventral side of the three limbs and combination cord was the head, the middle part was the body, and the dorsal side was the tail. The tail of the combination cord was the starting point of the combination part, which extended forward to form the head of the combination cord. The top limb and lateral limb constituted the uvula, while the surfaces of every two adjacent limbs together formed a surface, with three surfaces in total. The surfaces of the top limb and medial limb formed the inner surface, the surfaces of the top and lateral limb formed the outer surface, and the surfaces of the medial limb and lateral limb formed the inferior surface. A sulcus was often formed at the combination surface of every two limbs on the corresponding surface, namely the medial sulcus, lateral sulcus, and inferior sulcus. These grooves were deep, usually covering the front top of the kidney (). In general, the right adrenal gland in 90% of patients (37 cases) exhibited a ‘Lama hat’ shape (), while four cases (10%) showed the shape of a ‘triangular leaf of pitaya’ ().
Figure 1. The 3D model of the right adrenal gland of a 40-year-old male patient. (A) Right anterior view. (B) Left posterior view. (C) Inferior posterior view.
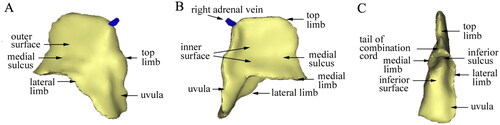
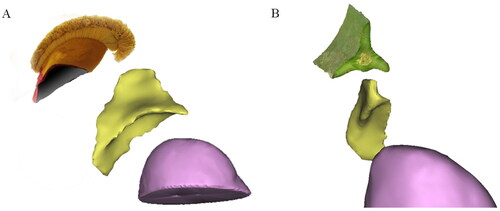
Figure 2. The 3D structure of adrenal glands. (A) The overall shape of the right adrenal gland with a deeper inferior sulcus looked like the ‘llama hat’ in a 32-year-old male patient. (B) The overall shape of the right adrenal gland was similar to the triangular leaf of a pitaya in a 62-year-old female patient.
Types of right adrenal venography
Adrenal venography images and their corresponding adrenal 3D images were fused at two different angles, namely the anteroposterior fusion and the right anterior oblique at a 30-degree angle, for a total of 82 fusion maps. The fused images provided information on the course and distribution of blood vessels in the gland. The morphology of the RAV in the angiographic image was classified and described according to the venographic morphology of the RAV as summarized by the University of Queensland [Citation10]. In the anteroposterior position, the starburst type was observed in five cases (12%; ), the solid triangle type in 13 cases (32%; ), the trunk branch type in 12 cases (29%), the hollow triangle type in four cases (9%; ), the irregular type in four cases (9%; ), and the spider type in three cases (7%). When the venography image was taken at a 30-degree right anterior oblique angle in the same patients, the trunk branch type was observed in most cases (38 cases, 93%), while only a few cases were of the irregular or hollow triangle type (three cases, 7%; ).
Figure 3. Effect of fusion of the adrenal venographic image and the 3D model. (A) An anteroposterior fusion image of a 50-year-old male patient. The right central vein was in the axial direction, and its image was a point at the catheter tip. The venographic image conformed to the ‘starburst type‘. Veins in the ‘starburst type’ were distributed in the upper part of the 3D model, while the uvula veins in the lower part of the 3D model were not shown. (B) An anteroposterior fusion image of a 34-year-old male patient. The right central vein was in the oblique axial direction and gradually shown, forming a ‘solid triangle type’ with its branches. The three limb veins were distributed in three limbs, referring to the central vein as the centre. No visible veins were shown in the uvula. (C) An anteroposterior fusion image of a 52-year-old female patient. The right central vein was further shown and communicated with renal capsular veins, forming a ‘hollow triangle type‘. The brush vein shown could be a signature vein of adrenal veins. No visible veins were shown in the uvula.
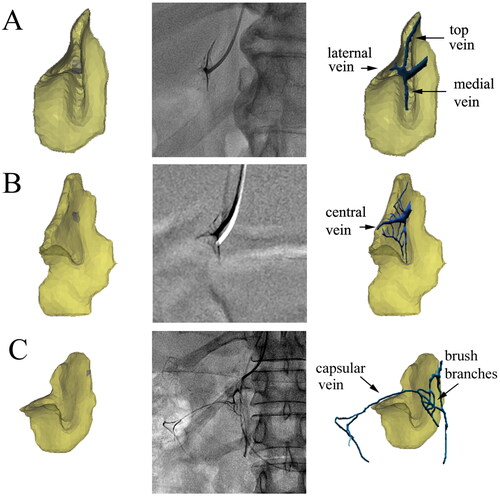
Figure 4. Effect of fusion of the irregular type and 3D model. (A) Adrenal fusion image of a 52-year-old female patient. The distribution of the adrenal veins was disordered, forming an ‘irregular type‘. Brush veins and central veins were shown at the catheter tip. (B) In the same patient, because of the catheter tip bending to the medial gland, more contrast agents entered the uvula vein.
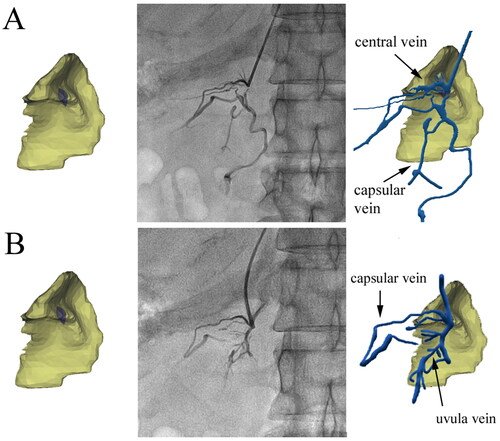
Table 2. Morphology classification of right adrenal venography (n = 41).
The primary and secondary veins were found to cover the entire model, as depicted in . The course and distribution of adrenal veins can be summarized as follows: (1) The central vein was the first primary branch of RAV and was concomitant with the combination cord. (2) The top vein, medial vein, and lateral vein, which drained almost vertically into the central vein, were primarily arranged in a brush shape, forming ‘brush veins’ (). In some cases, these veins were not arranged in a brush shape and instead had an irregular shape (). (3) The ‘uvula vein’ was another primary branch of RAV. The small veins distributed in the uvula gradually converged in the form of uvula veins, which also drained almost vertically into RAV. (4) There were also signature veins outside the adrenal gland, such as renal capsular veins, which formed apparent communication with the adrenal internal signature veins. If the renal capsular vein was thickened and tortuous, the contrast agent would be temporarily detained in it. If the renal capsular vein was communicated with the renal vein, hepatic vein, hepatic capsular vein, or even inferior vena cava, the contrast agent would rapidly return from these veins.
Figure 5. Effect of fusion of the trunk branch type and 3D model. (A) An anteroposterior fusion image of a 37-year-old female patient. The course of the central vein was close to the horizontal direction, and the uvula veins extended downward, with multiple branches distributed in the uvula. (B) Adrenal fusion image at 30 degrees right anterior oblique of a 47-year-old male patient. The angle between the uvula and central veins was close to 90 degrees. The distribution of branches of the uvula veins was similar to leaf texture.
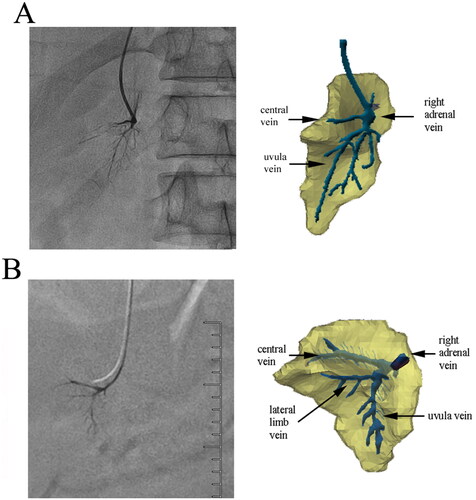
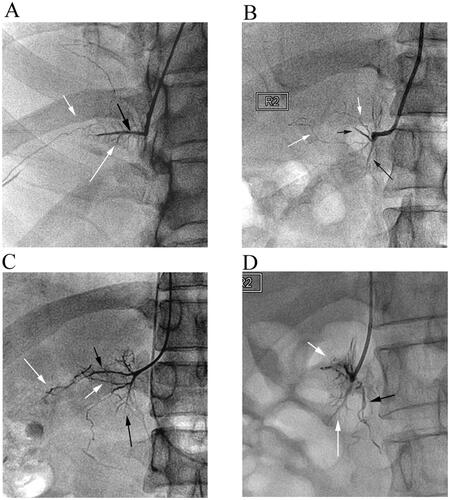
Figure 6. The signature veins of the right adrenal gland. (A) A 27-year-old male patient’s brush vein (long white arrow) almost vertically drained into the central vein (black arrow). The renal capsular vein (short white arrow) communicated with the brush vein expanded laterally. (B) In the ‘spider type’ of a 46-year-old male patient, the central vein (short black arrow) and the uvula vein (long black arrow) can be distinguished, but the brush vein was not shown. It could be seen that the renal capsular veins (white arrows), like soft vines, drained into nearly the outlet of the central vein. The overall shape of these renal capsular veins was similar to the spider’s foot. (C) A 42-year-old male patient’s venographic image showed relatively complete adrenal veins: the central vein (short black arrow), the uvula vein and its branch veins (long black arrow), the lateral limb vein (short white arrow), and the renal capsular vein (long white arrow) communicated with the distal end of the central vein. (D) The ‘solid triangle type’ of a 57-year-old female patient included the solid upper triangle venous enhancement area (short white arrow) and the lower uvula vein (long white arrow). It was common that the renal capsular vein (long black arrow) connected with the renal vein.
Validation of adrenal signature veins
The signature veins in the development model used to be the criterion for determining the position of the catheter tip during AVS. In the subsequent 12 months, a total of 53 cases were validated through AVS, and in all 53 cases, AVS was successfully performed based on SI values (). Adrenal venography revealed that 22 cases (42%) were of the complete type (with the central vein, uvula vein, brush vein, and renal capsular vein displayed simultaneously; ), while 31 cases (58%) were of the incomplete type (with only the central vein and uvula vein displayed). In each of the 53 cases, not all of the signature veins were displayed. The number of signature veins displayed during adrenal venography was as follows: the central vein was displayed in 48 cases (90%), the uvula vein was displayed in 33 cases (62%), the brush vein was displayed in 39 cases (73%), and the renal capsular vein was displayed in 44 cases (83%; ).
Table 3. Ratio of cortisol concentration of RAV and its primary branches to peripheral blood samples.
Table 4. Number of signature veins displayed during right adrenal venography with 4F MPA1 catheter (n = 53).
Discussion
Previous studies have not given much attention to the course and distribution of adrenal veins, resulting in confusion among clinicians regarding adrenal venography [Citation16,Citation17]. To better understand the characteristics of adrenal vein distribution, it is necessary to reconstruct the 3D anatomical structure of the adrenal gland [Citation18]. Through fusion images of adrenal venography and 3D structure, we made some important discoveries, as follows: (1) When adrenal venography was performed at a right anterior oblique angle of 30 degrees, 93% of venous morphology was of the trunk branch type. (2) The ‘uvula vein’ that we discovered, along with the central vein, was the primary branch of the RAV. The central vein was accompanied by a combination cord, while the ‘uvula vein’ was distributed in the uvula. (3) Adrenal internal signature veins included the central vein, brush vein, and uvula vein. (4) Adrenal external signature veins were the renal capsular veins [Citation19]. A previous study reported that the inferior emissary vein, which belongs to the renal capsular veins, could serve as a reliable landmark in AVS [Citation20].
With the development of segmental AVS [Citation18], other primary branches of the RAV have received significant attention [Citation21,Citation22]. Based on our 3D reconstruction, we have introduced the concept of the ‘adrenal uvula’. We have named the internal adrenal veins according to the names of each part of the adrenal gland in our 3D model, which is convenient for understanding, memory, and accurate surgical positioning. Anatomically, the uvula, resembling the shape of a tongue, is located in the anterior lower part of the adrenal gland and is formed by the downward extension of the lateral limb and the top limb of the margo liber. We have three pieces of evidence to support its existence. Firstly, in the solid triangle type, the central vein and brush vein are only distributed in the three limbs, suggesting the presence of unknown veins in the uvula. Secondly, in the trunk branch type, a prominent vein branch can be seen in the uvula, which drains into the central vein and forms the RAV. Lastly, when the catheter is super-selectively advanced to the uvula for AVS, the ratio of cortisol in the uvula vein to peripheral venous cortisol is more than 2 [Citation23].
The reasons for obtaining adrenal venography images are varied. Alongside anatomical variants and imaging angles of the adrenal gland, operational factors can also lead to a range of venous morphologies. Incorrect placement of the catheter tip or insufficient injection pressure, inadequate contrast agent doses, and short image acquisition times can result in venous insufficiency. Additionally, veins that are squeezed and deformed by tumours, as well as thicker and numerous capsular veins, contribute to the diversity of venography [Citation20, Citation24].
From the fusion of venography images and a 3D model, we were able to identify the adrenal internal signature veins: the central vein, brush vein, and uvula vein. Furthermore, the renal capsular vein served as the adrenal external signature vein, which usually communicates with the adrenal internal signature veins. These signature veins are critical for successful catheterization in AVS, regardless of the type of adrenal venography.
In AVS, catheters are susceptible to dislocation from their original position due to shorter RAV or large respiratory excursions. To prevent catheter dislocation, deeper implanted catheters are often used to reinforce the stability of the catheter tip. However, deeper implanted catheters may result in super-selective AVS. In this study, 58% of patients underwent super-selective AVS in the central vein and uvula vein. If the central vein or vertical vein is omitted in AVS, the cortisol and aldosterone concentrations may not accurately reflect the actual secretion of the entire right adrenal gland. By identifying the signature veins during AVS, we can determine whether any regions were missed during the procedure.
AVS is a technically challenging procedure, primarily due to difficulties in identifying and catheterizing the RAV. Georgiades et al. have suggested that C-arm CT during AVS is an excellent method for verifying catheter placement [Citation25,Citation26]. However, our experience has shown that the small scanning aperture and poor imaging quality of C-arm CT scans performed in roll mode, make it unsuitable for AVS via upper limb veins, obese patients, or patients with significant respiratory excursions. The use of signature veins and venography in the right anterior oblique at 30-degree angles has been shown to achieve a 100% success rate for AVS. Super-selective AVS performed by inserting the catheter into the central vein and uvula vein is similar to the segmental AVS (s-AVS) proposed by Satani [Citation18]. Additionally, many studies have demonstrated that rapid cortisol assays can significantly improve the success rate of AVS [Citation27–29]. Nonetheless, it is still essential to understand the structure and morphology of the adrenal veins. A short review by Zelinka et al. suggests that normalization of blood pressure after adrenalectomy or mineralocorticoid receptor antagonists is rarely seen in primary aldosteronism patients [Citation30]. The reason why these findings are hard-to-interpret is due to unclear anatomical structure of the adrenal gland.
The 3D reconstruction technology associated with the adrenal gland finds wide application in clinical practice [Citation31,Citation32]. However, due to the similarity in CT value between the adrenal gland and adjacent tissues, it can be challenging to eliminate interference from adjacent adrenal tissues using CT threshold values. The adrenal gland is typically surrounded by fat, and the CT value of fat in a plain CT scan is approximately −80 Hu, whereas the CT value of the adrenal gland is around 30 Hu [Citation33,Citation34]. Since there is a substantial difference in CT values between the two tissues [Citation12,Citation13], the Mimics software can accurately distinguish this difference.
In the current study, anatomical features were utilized as positioning and scaling references, ensuring the fusion’s high reliability. However, our study has several limitations. Firstly, it was a retrospective study conducted in a single centre. Secondly, the outline of the adrenal veins and adrenal CT images were selected using threshold values and manually corrected, which may have affected the accuracy due to the researcher’s experience. Thirdly, there was no autopsy verification.
Conclusions
In summary, this study provides a thorough investigation of the course and distribution of the RAVs and introduces the concept of signature veins, including the central vein, brush vein, and uvula vein for right AVS. These signature veins, particularly the uvula veins that have not received significant attention in the past, not only improve the success rate of AVS but also provide a reliable location to interpret data accurately.
Author contributions
Conceptualization: Zhenglin Shen, Shaoyong Xu, Siyu Guan, and Zhao Gao. Data curation: Zhenglin Shen and Bo Chen. Formal analysis: Zhenglin Shen, Shaoyong Xu, and Bo Chen. Funding acquisition: Shaoyong Xu. Investigation: Zhenglin Shen, Qingan Li, and Ming Yu. Methodology: Zhenglin Shen. Project administration: Zhenglin Shen, Qingan Li, and Ming Yu. Resources: Zhenglin Shen, Qingan Li, and Ming Yu. Software: Zhenglin Shen. Supervision: Zhenglin Shen, Shaoyong Xu, and Zhao Gao. Validation: Zhenglin Shen, Shaoyong Xu, and Zhao Gao. Visualization: Zhenglin Shen. Writing – original draft: Zhenglin Shen and Shaoyong Xu. Writing – review and editing: Zhao Gao.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets generated or analyzed during the study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Galati SJ, Hopkins SM, Cheesman KC, et al. Primary aldosteronism: emerging trends. Trends Endocrinol Metab. 2013;24(9):1–11. doi: 10.1016/j.tem.2013.05.003.
- Ohno Y, Naruse M, Beuschlein F, et al. Adrenal venous sampling-guided adrenalectomy rates in primary aldosteronism: results of an international cohort (AVSTAT). J Clin Endocrinol Metab. 2021;106(3):e1400–e1407. doi: 10.1210/clinem/dgaa706.
- Quencer KB. Adrenal vein sampling: technique and protocol, a systematic review. CVIR Endovasc. 2021;4(1):38. doi: 10.1186/s42155-021-00220-y.
- Scholten A, Cisco RM, Vriens MR, et al. Variant adrenal venous anatomy in 546 laparoscopic adrenalectomies. JAMA Surg. 2013;148(4):378–383. doi: 10.1001/jamasurg.2013.610.
- Cesmebasi A, Du Plessis M, Iannatuono M, et al. A review of the anatomy and clinical significance of adrenal veins. Clin Anat. 2014;27(8):1253–1263. doi: 10.1002/ca.22374.
- Young WF, Stanson AW. What are the keys to successful adrenal venous sampling (AVS) in patients with primary aldosteronism? Clin Endocrinol. 2009;70(1):14–17. doi: 10.1111/j.1365-2265.2008.03450.x.
- Rossi GP, Auchus RJ, Brown M, et al. An expert consensus statement on use of adrenal vein sampling for the subtyping of primary aldosteronism. Hypertension. 2014;63(1):151–160. doi: 10.1161/HYPERTENSIONAHA.113.02097.
- So CB, Leung AA, Chin A, et al. Adrenal venous sampling in primary aldosteronism: lessons from over 600 single-operator procedures. Clin Radiol. 2022;77(2):e170–e179. doi: 10.1016/j.crad.2021.11.005.
- Vonend O, Ockenfels N, Gao X, et al. Adrenal venous sampling: evaluation of the German Conn’s registry. Hypertension. 2011;57(5):990–995. doi: 10.1161/HYPERTENSIONAHA.110.168484.
- Daunt N. Adrenal vein sampling: how to make it quick, easy, and successful. Radiographics. 2005;25(Suppl 1):S143–S158. doi: 10.1148/rg.25si055514.
- Omura K, Ota H, Takahashi Y, et al. Anatomical variations of the right adrenal vein: concordance between multidetector computed tomography and catheter venography. Hypertension. 2017;69(3):428–434. doi: 10.1161/HYPERTENSIONAHA.116.08375.
- Ota H, Seiji K, Kawabata M, et al. Dynamic multidetector CT and non-contrast-enhanced MR for right adrenal vein imaging: comparison with catheter venography in adrenal venous sampling. Eur Radiol. 2016;26(3):622–630. doi: 10.1007/s00330-015-3872-3.
- Morita S, Nishina Y, Yamazaki H, et al. Dual adrenal venous phase contrast-enhanced MDCT for visualization of right adrenal veins in patients with primary aldosteronism. Eur Radiol. 2016;26(7):2073–2077. doi: 10.1007/s00330-015-4073-9.
- Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(5):1889–1916. doi: 10.1210/jc.2015-4061.
- Shen ZL, Li QA, Li LH, et al. Bilateral adrenal vein sampling by single 4F multipurpose angiography catheter through right upper limb vein. J Interv Radiol. 2022;31(3):282–285.
- Trerotola SO, Asmar M, Yan Y, et al. Failure mode analysis in adrenal vein sampling: a single-center experience. J Vasc Interv Radiol. 2014;25(10):1611–1619. doi: 10.1016/j.jvir.2014.06.029.
- Ma G, Liu SW, Zhao ZM, et al. Sectional anatomy of the adrenal gland in the coronal plane. Surg Radiol Anat. 2008;30(3):271–280. doi: 10.1007/s00276-008-0308-7.
- Satani N, Ota H, Seiji K, et al. Intra-adrenal aldosterone secretion: segmental adrenal venous sampling for localization. Radiology. 2016;278(1):265–274. doi: 10.1148/radiol.2015142159.
- Lee BC, Chang CC, Liu KL, et al. Evaluation of right adrenal vein anatomy by Dyna computed tomography in patients with primary aldosteronism. Sci Rep. 2016;6:28305. doi: 10.1038/srep28305.
- Kohi MP, Agarwal VK, Naeger DM, et al. The inferior emissary vein: a reliable landmark for right adrenal vein sampling. Acta Radiol. 2015;56(4):454–457. doi: 10.1177/0284185114529107.
- Tannai H, Makita K, Koike Y, et al. Usefulness and accuracy of segmental adrenal venous sampling on localisation and functional diagnosis of various adrenal lesions in primary aldosteronism. Clin Radiol. 2022;77(8):e652–e659. doi: 10.1016/j.crad.2022.05.010.
- Tannai H, Makita K, Matsui S, et al. Radiological characteristics and diagnostic impact of duplicated right adrenal veins on adrenal venous sampling in primary aldosteronism. Diagn Interv Radiol. 2021;27(6):754–761. doi: 10.5152/dir.2021.21388.
- Noda Y, Goshima S, Nagata S, et al. Utility of microcatheter in adrenal venous sampling for primary aldosteronism. Br J Radiol. 2020;93(1109):20190636. doi: 10.1259/bjr.20190636.
- Harsha A, Trerotola SO. Technical aspects of adrenal vein sampling. J Vasc Interv Radiol. 2015;26(2):239. doi: 10.1016/j.jvir.2014.11.006.
- Georgiades CS, Hong K, Geschwind JF, et al. Adjunctive use of C-arm CT may eliminate technical failure in adrenal vein sampling. J Vasc Interv Radiol. 2007;18(9):1102–1105. doi: 10.1016/j.jvir.2007.06.018.
- Park CH, Hong N, Han K, et al. C-arm computed tomography-assisted adrenal venous sampling improved right adrenal vein cannulation and sampling quality in primary aldosteronism. Endocrinol Metab. 2018;33(2):236–244. doi: 10.3803/EnM.2018.33.2.236.
- Auchus RJ, Michaelis C, Wians FHJr, et al. Rapid cortisol assays improve the success rate of adrenal vein sampling for primary aldosteronism. Ann Surg. 2009;249(2):318–321. doi: 10.1097/SLA.0b013e3181961d77.
- Rossi E, Regolisti G, Perazzoli F, et al. Intraprocedural cortisol measurement increases adrenal vein sampling success rate in primary aldosteronism. Am J Hypertens. 2011;24(12):1280–1285. doi: 10.1038/ajh.2011.148.
- Wolley M, Thuzar M, Stowasser M. Controversies and advances in adrenal venous sampling in the diagnostic workup of primary aldosteronism. Best Pract Res Clin Endocrinol Metab. 2020;34(3):101400. doi: 10.1016/j.beem.2020.101400.
- Widimsky JJr, Strauch B, Petrák O, et al. Vascular disturbances in primary aldosteronism: clinical evidence. Kidney Blood Press Res. 2012;35(6):529–533. doi: 10.1159/000340031.
- Hurley ME, Herts BR, Remer EM, et al. Three-dimensional volume-rendered helical CT before laparoscopic adrenalectomy. Radiology. 2003;229(2):581–586. doi: 10.1148/radiol.2292021390.
- Mitterberger M, Pinggera GM, Peschel R, et al. The use of three-dimensional computed tomography for assessing patients before laparoscopic adrenal-sparing surgery. BJU Int. 2006;98(5):1068–1073. doi: 10.1111/j.1464-410X.2006.06489.x.
- Elsayes KM, Emad-Eldin S, Morani AC, et al. Practical approach to adrenal imaging. Urol Clin North Am. 2018;45(3):365–387. doi: 10.1016/j.ucl.2018.03.005.
- Boland GW, Lee MJ, Gazelle GS, et al. Characterization of adrenal masses using unenhanced CT: an analysis of the CT literature. AJR Am J Roentgenol. 1998;171(1):201–204. doi: 10.2214/ajr.171.1.9648789.
