Abstract
Objective
The primary objectives of this study were to 1) investigate the internal consistency 2) and construct validity of the Short Musculoskeletal Function Assessment Questionnaire (SMFA) in older adults commencing physical rehabilitation in an outpatient setting.
Methods
This cross-sectional study recruited older adults who had commenced physical rehabilitation in an outpatient setting. The SMFA consists of two indices: 1) dysfunction capturing the impact of musculoskeletal disorders on physical limitations, and 2) bothering capturing how the individual is emotionally affected by their disorder. SMFA holds four categories: ‘mobility’, ‘daily activities’, ‘emotional status’, and ‘function of the arm and hand’. Participants answered the SMFA alongside other patient-reported questionnaires (such as the 36-Item Short Form Survey, SF-36) and similar) and objectively measured muscle strength for the upper and lower body and functional capacity.
Results
We included 115 older adults with a median age of 74 years (IQR 9). Adequate internal consistency was seen with Cronbach’s alpha values of 0.90–0.94 for the SMFA indices and 0.77–0.91 for the SMFA categories. The strongest correlations between the SMFA indices were observed with the SF-36 physical component summary (SMFA-Dysfunction r = 0.74, p < 0.05, SMFA-Bother r = 0.72, p < 0.05). Only fair correlations were found between SMFA index scores and clinical outcome measures.
Discussion
This study demonstrated that the SMFA has adequate internal consistency and construct validity for self-reported health status in older adults, especially when considering components covering physical health status. However, we only observed fair correlations between SMFA and clinical outcome measures, indicating that SMFA does not adequately capture muscle strength and functional capacity.
Introduction
Older adults in outpatient settings are a heterogeneous group suffering from various health disorders such as musculoskeletal disorders (e.g. back and neck pain, osteoarthritis), chronic obstructive pulmonary disease, and depression. These conditions ultimately disperse the functional abilities. With increasing age, the risk of more than one chronic condition increases steadily, affecting physical functioning and health-related quality of life [Citation1,Citation2]. Furthermore, differences in function and recovery profiles exist among older adults with medical conditions [Citation3]. This indicates different rehabilitation needs within this heterogeneous population. Usually, physical rehabilitation is focused primarily on one condition, and many conditions in one individual challenge the choice of health outcomes to assess progress. Using patient-reported outcome measures (PRO’s) plays an important role in reflecting the context of patients’ everyday lives, as it allows the assessment of an individual’s perception of functional limitations and symptoms and how they think it affects their daily living [Citation4].
The Short Musculoskeletal Function Assessment Questionnaire (SMFA) captures the dysfunction of specific musculoskeletal disorders. The SMFA aims to provide a patient-reported perception of the impact of musculoskeletal disorders on physical limitations (SMFA dysfunction index, SMFA-D) and how the individual is emotionally affected by their disorder (SMFA bother index, SMFA-B) [Citation5]. Using a standardized questionnaire to compare different musculoskeletal diseases and injuries in patients undergoing surgical or conservative inpatient treatment [Citation6].
When choosing PROs that reflect the degree of dysfunction in physical rehabilitation contexts, it is essential to consider the measured construct, its value to the patient, and its psychometric properties. A commonly used holistic framework for classifying and revealing the impact of a condition on health in everyday life is the International Classification of Functioning, Disability, and Health (ICF), developed by the World Health Organization (WHO) [Citation7]. The ICF includes the constructs of body function and structure, activities, and participation while simultaneously considering the effects of the context as environmental and personal factors. Physical and occupational therapists often use the classification during diagnostics to assign information so that specific goals can be targeted for each construct [Citation8]. Using the ICF as a framework provides a common international language for collecting data on disability and functioning in a structured and consistent way.
Consequently, investigating the construct validity of the SMFA by examining its association with other health-related questionnaires representing different ICF domains and its association with typically used clinical outcome measures will provide valuable information to physical and occupational therapists regarding the clinical interpretation of SMFA scores. Hence, the primary objectives of this study were to 1) investigate the internal consistency 2) and the construct validity of the SMFA questionnaire in older adults commencing physical rehabilitation in an outpatient setting.
Methods
The reporting of this paper is based on the ‘COnsensus-based Standards for the selection of health Measurement Instruments’ (COSMIN) reporting guidelines for studies on measurement properties of PRO measures [Citation9]. The body funding this research played no role in this study’s design, conduct, or reporting.
Study design and participants
This was a multicenter cross-sectional study. Eligible participants were older adults aged ≥ 65 years who were referred for physical rehabilitation in municipality-based health centers from a medium-sized Danish municipality covering both rural and urban populations. Administrative personnel initially screened participants for eligibility in January-September 2021, a period partly characterized by lockdown due to COVID-19. If deemed eligible, older adults were invited to participate in a screening interview conducted at their own homes. The interview consisted of standardized questions, collecting information on the reasons for referral, comorbidities, use of medications, history of falls, and practical information regarding transportation. Risk evaluation for participating in clinical measurements was also performed. Participants were excluded from participation if any of the following criteria were met: inability to speak or read Danish, active cancer, upper or lower limb amputations, hypertension >180/110 mmHg for diastolic/systolic blood pressure, referral to rehabilitation primarily due to gynaecological or neurological conditions (apoplexies), surgeries where movement restrictions prohibit participating in most of the clinical outcome measures, or general practitioner advised against participation.
Ethics
The study protocol was reviewed and approved by the Ethical Committee in Region Zealand, Denmark (SJ-758), and the General Data Protection Regulation at the University of Southern Denmark, Odense (10.330). Further, the trial has been registered at clinicaltrials.gov (NCT04862481) as a sub-study of our ongoing randomized controlled trial (NCT0491308). Written consent was obtained from all the participants. Furthermore, all participants were informed about their right to withdraw from the study, as explicitly stated by the Ethics Committee in Region Zealand, Denmark.
Data collection
A standardized data collection protocol was approved by the regional ethical committee and followed across recruitment sites and personnel to ensure reproducibility and avoid selection bias. If eligible and willing to participate in the study, older adults received a paper-based 100-item survey. The survey was answered by participants in familiar surroundings using paper and pencil during home visits [Citation10–13]. If queries arose while answering the survey, participants were instructed to discuss this with the principal investigator. The questionnaire answers were double-entered in Excel (Microsoft Office 365) to blind the outcome assessors from the SMFA scores of each participant before the clinical testing session.
Patient-reported outcome measures
The 100-item survey consisted of the following questionnaires: the 36-Item Short Form 36 (SF-36), the Tilburg Frailty Indicator (TFI), Program of Research to Integrate Services for the Maintenance of Autonomy (PRISMA7), New Mobility Scale (NMS), and questions on sociodemographic variables). All the questionnaires are described in .
Table 1. Descriptive outcome table.
The SMFA is an abbreviated version of the more comprehensive 101-item Musculoskeletal Function Assessment questionnaire (MFA) and has been developed to render its usage in clinical practice [Citation23]. The SMFA is a 46-item self-reported generic questionnaire and takes approximately 10–20 min to complete. The SMFA consists of two indices:1) dysfunction capturing the impact of musculoskeletal disorders on physical limitations (SMFA-Dysfunction, 34-items) and 2) bother capturing how the individual is emotionally affected by their disorder (SMFA-Bother, 12-items). Items related to the SMFA-B index aim to clarify individuals’ approaches to dysfunction in recreation, housework, and leisure. The SMFA is scored using a 5-point Likert scale (from ‘Unable to do’ to ‘Not at all difficult’ and from ‘Extremely bothered’ to ‘Not at all bothered’), which can be transformed and summarized on a 0–100 score, where higher values indicate higher degrees of dysfunction or bother [Citation23]. The items of the SMFA can be categorized into four categories: ‘mobility’, ‘daily activities’, ‘emotional status’ and ‘function of the arm and hand’.
Clinical outcome measures
Before initiating the assessment of clinical outcome measures, the participants were asked to hand in signed written consent forms. The clinical test session was booked to 3–7 days after the screening interview. Clinical testing took 90–100 min to complete and was conducted by the principal investigator alone or with a physical therapy student. The equipment was calibrated each morning before initiating the testing procedure. On arrival, all participants were asked not to drink for one hour before the test and were encouraged to empty their bowels. The warm-up program preceded testing. The clinical outcome measures included maximal bilateral handgrip strength, elbow flexion strength, elbow extension strength, and shoulder abduction strength measured using a dynamometer fixed with a belt. Lower extremity strength was assessed as the maximal voluntary isometric contraction (MVIC) of knee extension in the left leg measured using a strain gauge device. Bilateral leg press, knee extension, and calf extension were assessed as five repetition maxima. Balance and functional capacity were assessed using the tandem balance test, two-minute walk test, and timed up and go test (TUG). All outcomes are presented in . The clinical outcome measures were conducted as described in our study protocol for a randomized controlled trial investigating whether individualized physical exercise programs combined with increased protein intake can improve the measure of all ICF levels when compared with usual care alone or care as usual in combination with increased protein intake [Citation24].
Hypotheses testing for construct validity
One method for investigating the construct validity of a measurement instrument is hypothesis testing [Citation25,Citation26].
We hypothesized a strong correlation between the SMFA-Dysfunction index and the SMFA-Bother index, as has previously been shown in other heterogeneous populations [Citation5]. Moreover, we hypothesized that higher scores on the SMFA-Dysfunction and SMFA-Bother would reflect higher scores on the PRISMA7 and TFI and lower scores on the SF-36 and NMS questionnaires, thereby reflecting the ability of the SMFA to measure health status. We expect SMFA-Dysfunction to show stronger correlations with patient-reported outcomes than SMFA-Bother [Citation4].
Additionally, we hypothesized that SMFA-Dysfunction and SMFA-Bother scores are only fairly to moderately correlated with clinical outcome measures. Hence, higher SMFA scores are expected to correlate with lower strength measures, and poor balance and mobility. However, we expected that higher scores on the SMFA would reflect a longer time to complete the TUG test. As the SMFA-Dysfunction and SMFA-Bother are sum scores comprising items covering several body areas, the assessment of a specific function (e.g. strength, balance) is not necessarily correlated. Therefore, we hypothesized that specific clinical outcome measures will not be as strongly correlated to SMFA-Dysfunction and SMFA-Bother as they will be in a specific SMFA category [Citation4,Citation5]. Finally, we expect the SMFA-Dysfunction to correlate more strongly with the clinical outcome measures than the SMFA-Bother.
Sample size calculation
The sample size calculation was performed using Stata (v. 17) and is based on correlations (Spearman’s rank order correlation coefficient) between SMFA-Dysfunction and subscales from the SF-36 [Citation27]. To detect a correlation coefficient of minimum r = ±0.3 with a significance level of 0.05, and a power of 0.8, between the SMFA-Dysfunction and the generic health-related quality of life questionnaire SF-36, a minimum of 85 participants were needed based on the Fisher’s z test. Some studies have shown a response rate for the SMFA to be approximately 65% for the studies [Citation28]. Consequently, 35% extra participants were added to the calculated sample size, resulting in a target of 115 participants initially included in the study.
Statistical analyses
Scoring instructions for the SMFA were followed, and missing responses were handled as suggested in the original scoring manual [Citation23]. We anticipated that some participants would not complete all clinical tests due to discomfort, pain, or fear of injury. In these circumstances, participants were excluded from the analysis, as no data were available. All statistical analyses were performed using Stata BE (ver. 17), and the do files were stored in the dataset. Initially, the raw data were inspected for Gaussian distribution and were considered not to be normally distributed. Summary statistics were presented as medians with interquartile ranges (IQR). Categorical data are presented as counts and percentages.
Cronbach’s α was calculated to investigate the internal consistency of the specific categories of the SMFA (daily activities, emotional status, arm and hand function, mobility). Internal consistency was considered acceptable, with Cronbach’s α above 0.70, as previously stated in our statistical analyses plan published at clinicaltrials.gov (NCT04862481). Construct validity was investigated using Spearman’s rank-order correlation coefficients. The rho values were interpreted as r = ±0.1 to 0.25 weak, r> ±0.25 to 0.50 a fair, r> ±0.50 to 0.75 moderate, and above r> ±0.75 strong [Citation29].
Results
Participants
The study included 115 participants with a median age of 74 years (IQR: 9), and 61% were females, . In comparison, the excluded older adults had a median age of 76 years (IQR: 10), and 58% were females. Approximately 20% of the participants had a low BMI, 59% had a normal BMI, and 21% had a BMI > 30.9. Regarding physical activity, 29.5% of the participants reported being sedentary, and 50% reported that their physical activity level could be categorized as ‘standing or walking without physical exertion’. The prevalence of musculoskeletal pain or discomfort was highest in the lower extremities (35.7%), followed by the upper extremities (31.3%), spinal region (25.2%), and other body areas (7.8%). Approximately half of the participants (50.9%) reported living alone. More than half (62%) of the participants had a history of falls in the previous year. The median scores (IQR) for the patient-reported outcomes and clinical measurements are shown in .
Figure 1. Flow chart.*Other indicates severe and complex health issues that excluded the older adults from participating in most of the selected clinical outcome measures (<50% of the clinical outcome measures).
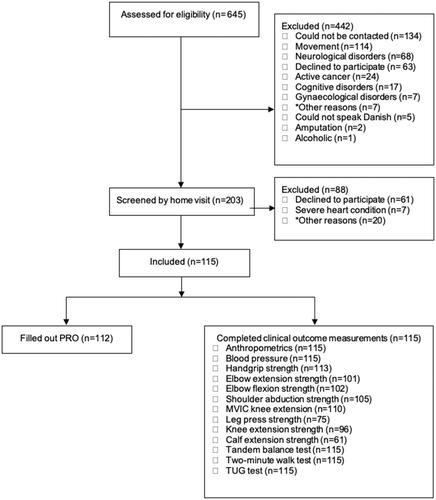
Table 2. Scores from patient-reported outcome and clinical outcome measures of the participants.
Internal consistency
No flooring or ceiling effects were observed in the scores for SMFA-D and SMFA-B [Citation30]. Adequate internal consistency for the SMFA was found with Cronbach’s alpha values of 0.94 for the SMFA-Dysfunction and 0.90 for the SMFA-Bother. Furthermore, Cronbach’s alpha values were calculated for the SMFA categories. The values are 0.91 for the Daily Activities Category, 0.89 for the Mobility Category, 0.77, and 0.90, respectively. This indicates a close relationship between the set of items for specific indices and categories of SMFA in older adults.
Construct validity
Correlation with PRO measures
Our a priori hypothesis that SMFA-Dysfunction and SMFA-Bother are strongly correlated was confirmed (r = 0.87, p ≤ 0.001). As expected, the SMFA indices and SMFA categories were negatively correlated with SF36 and NMS, and positively correlated with PRISMA-7 and TFI (TFI total, physical, and psychological scores) (). Consequently, if participants reported high levels of dysfunction, they similarly reported lower levels of health and mobility, and higher levels of lost autonomy and frailty. As hypothesized, the correlations between SMFA indices and PRO were generally indicative of moderate construct validity (r > 0.50–0.75, p ≤ 0.001), (). We hypothesized that SMFA-Dysfunction would show stronger correlations with the listed PROs than SMFA-Bother; however, this was not the case. Both SMFA indices were correlated to a similar extent with the listed PROs (). The SMFA-Bother was poorly correlated with the SF36 General Health dimension (r = −0.49, p ≤ 0.001), which was contrary to the hypothesis. The TFI social score was not correlated with any SMFA index or category ().
Table 3. Construct validity: Correlation table for Short musculoskeletal function Assessment questionnaire, indices/categories, and other patient-reported outcome measures.
Correlation with clinical outcome measures
As expected, the SMFA indices and categories correlated negatively with all clinical outcome measures except for the TUG test. Higher SMFA-Dysfunction scores indicated a higher degree of dysfunction and were correlated with lower levels of muscle strength and functional capacity. Overall, the correlations were fair (r > 0.25–0.50, p ≤ 0.001), as hypothesized (). In addition, stronger correlations were found between SMFA-Dysfunction and clinical outcome measures than between SMFA-Bother and clinical measures. In addition, as hypothesized, clinical outcome measures assessing upper extremity strength showed higher correlations with the SMFA category arm and hand functions than the SMFA indices. Further, the SMFA category Mobility correlated more strongly with the two-minute walk test and TUG compared to the SMFA indices, as hypothesized ().
Table 4. Correlation table for Short musculoskeletal function Assessment questionnaire, indices/categories, and clinical outcome measures.
Discussion
This study investigated the construct validity of the SMFA using a cross-sectional design, including a population of 115 older adults referred for outpatient-based rehabilitation. As hypothesized, SMFA indices showed moderate correlations with PROs assessing health status in older adults. The results demonstrate that the SMFA indices cover many of the same constructs (health status ‘SF-36’, frailty ‘PRISMA-7/TFI’, mobility ‘NMS’) as the presented PROs in older adults commencing physical rehabilitation in an outpatient setting. Consequently, the SMFA indices have adequate construct validity for assessing self-reported health status in older adults, especially when considering components covering physical health status. These results underline the value of using clinical outcome measures in rehabilitation, as SMFA does not capture this and consequently is not closely related to the degree of dysfunction. SMFA indices cover mental and social features to a lesser degree than the components linked to physical ability ().
Our findings support the previous results of SMFA conducted in other populations. This shows that the SMFA can function as a measure of older adults’ health status across different musculoskeletal disorders. In the first validity study of the SMFA questionnaire, Swiontkowski et al. demonstrated sufficient construct validity by comparing scores of the SMFA indices to SF-36 dimensions [Citation5]. Almost equivalent measurement properties were obtained in the present study even though Swiontkowski et al. included younger participants who had sustained musculoskeletal disease or injury. Other studies have demonstrated good reliability, validity, and responsiveness in different populations suffering from musculoskeletal disorders and injuries [Citation4, Citation6, Citation31–40]. Concerning the Danish translation, an investigation of the content validity for the SMFA showed that the SMFA covers all the ICF constructs [Citation37]. Similarly, it was recently reported that the SMFA could cover all constructs and contextual factors of the ICF and capture the injuries’ consequences for everyday life in 160 middle-aged fracture patients [Citation41]. Thus, despite the different target groups, the SMFA has the same ability to cover similar constructs to those of the SF-36. However, interestingly, the severity of an injury can perhaps impact the construct validity of the SMFA when compared to SF-36, as some studies have demonstrated significantly lower correlations in participants with severe or acute injuries, although the one-week recall version of SF-36 was used [Citation37]. The lower correlations might partly be explained by the fact that SF-36 Physical functioning mainly includes items related to the function of the lower extremities. In contrast, the SMFA has more detailed upper and lower body items, which is a noteworthy advantage compared to the SF-36 [Citation32]. Nevertheless, this partly explains why no correlation between the TFI and the SMFA indices and categories can be found. However, in a study by Gobbens et al. the authors correlated TFI subscales with adverse outcomes of frailty. They found significant but low correlations between the TFI social frailty subscale and disability measured by the Groningen Activity Restriction Scale (r = 0.257, p < 0.001) in comparison to the TFI physical frailty (r = 0.488, p < 0.001) and TFI psychological frailty (r = 0.361, p < 0.001) [Citation42]. In the present study, the social component of the SF-36 showed moderate correlations with the SMFA indices, which is also supported by other studies, even though the participants in the present study also demonstrated very good scores on the SF-36 social functioning dimension () [Citation5, Citation35, Citation38]. The explanation might be that the SF-36 is more sensitive for measuring social well-being, making it easier to spot minor differences between individuals than the scale used in TFI.
The correlations between the SMFA indices and clinical outcome measures were mostly fair, which was expected because the instruments measured different constructs. We expected stronger correlations between some SMFA categories and clinical outcome measures in the same body area. The SMFA arm and hand function category had the highest correlation with clinical measurements assessing upper extremity strength (handgrip, elbow, and shoulder strength). Swiontkowski et al. compared handgrip strength and range of motion in the upper and lower extremity joints to the SMFA-D, but not the subcategories, and found similar results to those of the present study when comparing SMFA-D and handgrip strength (r = 0.45, p < 0.05). Conversely, correlations between the SMFA mobility category and measures assessing lower extremity function (leg press strength, knee extension strength, calf extension strength, tandem balance test, two-minute walk test, and TUG) were mainly fair. Remarkably, shoulder abduction strength correlated more strongly with the SMFA mobility category than with the MVIC of knee extension. One explanation could be that more than half the participants had a history of falls within the last year, hurting their shoulders, which could have influenced their ability to use them during activities. This could have impaired shoulder function and strength over time. Suppose that impaired function or strength is accompanied by fear of falling. In that case, it can lead to further declines in physical functioning, influencing their ability to perform the activities listed in the mobility category of the SMFA [Citation43]. Kirschner et al. found a stronger correlation between SMFA-D and walking speed than that between the two-minute walk distance and SMFA-D [Citation35]. The difference could be due to different methods of assessing walking. Kirschner et al. used a course of 25 meters to determine gait speed, as in the study by Swiontkowski et al. [Citation35]
Strengths and limitations
One limitation of this study is the significant number of participants who scored well on the PRISMA-7, NMS, and TFI questionnaires, indicating a lower degree of frailty or immobility. This study was conducted during the COVID-19 pandemic when most older adults did not attend habitual activities. This could have influenced their understanding of the context, looking at the items addressed in the questionnaires, thereby scoring themselves better than they otherwise would have. Another explanation might be that the frailest older adults stayed at home during the pandemic, so we included more functionally fit older adults. Nevertheless, these instruments were considered reasonable for use based on observations of older adults and discussions with health professionals from a specific clinical setting before the COVID-19 pandemic. Another significant limitation of this study is that we only measured reliability as internal consistency, and not as repeated measures.
Additionally, in this cross-sectional study, we could not investigate the responsiveness of SMFA, which would have been helpful. We utilized the COSMIN taxonomy and assessed construct validity through hypothesis testing. We chose to present correlations between the SMFA and PROs and clinical outcome measures to cover aspects of construct validity. We acknowledge differences in the research literature regarding validity terminology, as acknowledged by the COSMIN study [Citation44]. However, the strength of this study was the investigation of the association between the SMFA indices, categories, and clinical outcome measures often used in clinical practice. These results provide insight into the relationship between clinical measures, often targeted as primary goals, and patients’ reflection of their level of dysfunction and bother. Overall, the SMFA is a valuable instrument for health professionals to use with older adults in an outpatient rehabilitation setting alongside clinical measurements to measure health status. Still, future research should examine the responsiveness of SMFA in a population of older adults.
Author contributors
All authors have formed a project description (November 2020). ST wrote the first draft and is the guarantor of the manuscript, and ML, CMM, GS, KS, and LFS elaborated on the final paper. All authors contributed to and approved the final version. None of the sponsors was involved in the trial design, data collection, management, analysis, or interpretation of the data.
Acknowledgements
The authors thank all funding parties for financially supporting this trial. The authors also thank all the older adults who commenced this study and the Slagelse Municipality for allowing us to conduct the study in a real clinical setting.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Research data supporting this research will be uploaded to the Danish State Archives after the end of the project (https://www.sa.dk/en/research-researchers-research-service-the-danish-national-archives/).
Additional information
Funding
References
- Dillin A, Gottschling DE, Nystrom T. The good and the bad of being connected: the integrons of aging. Curr Opin Cell Biol. 2014;26:1–11. doi:10.1016/j.ceb.2013.12.003.
- Brown K, Boot D, Groom L, et al. Problems found in the over-75s by the annual health check. Br J Gen Pract. 1997;47(414):31–35.
- Juul-Larsen HG, Andersen O, Bandholm T, et al. Differences in function and recovery profiles between patterns of multimorbidity among older medical patients the first year after an acute admission-An exploratory latent class analysis. Arch Gerontol Geriatr. 2020;86:103956. doi:10.1016/j.archger.2019.103956.
- Bouffard J, Bertrand-Charette M, Roy JS. Psychometric properties of the musculoskeletal function assessment and the short musculoskeletal function assessment: a systematic review. Clin Rehabil. 2016;30(4):393–409. doi:10.1177/0269215515579286.
- Swiontkowski MF, Engelberg R, Martin DP, et al. Short musculoskeletal function assessment questionnaire: validity, reliability, and responsiveness. J Bone Joint Surg Am. 1999;81(9):1245–1260. doi:10.2106/00004623-199909000-00006.
- Wollmerstedt N, Kirschner S, Faller H, et al. Reliability, validity and responsiveness of the german short musculoskeletal function assessment questionnaire in patients undergoing surgical or conservative inpatient treatment. Qual Life Res. 2006;15(7):1233–1241. doi:10.1007/s11136-006-0066-0.
- WHO. Towards a common language for functioning, disability and health. Inter Classific. 2002;1149:1–22.
- Maribo T, Petersen KS, Handberg C, et al. Systematic literature review on ICF From 2001 to 2013 in the nordic countries focusing on clinical and rehabilitation context. J Clin Med Res. 2016;8(1):1–9. doi:10.14740/jocmr2400w.
- Gagnier JJ, Lai J, Mokkink LB, et al. COSMIN reporting guideline for studies on measurement properties of patient-reported outcome measures. Qual Life Res. 2021;30(8):2197–2218. doi:10.1007/s11136-021-02822-4.
- Parker MJ, Palmer CR. A new mobility score for predicting mortality after hip fracture. J Bone Joint Surg Br. 1993;75(5):797–798. doi:10.1302/0301-620X.75B5.8376443.
- Hoogendijk EO, van der Horst HE, Deeg DJ, et al. The identification of frail older adults in primary care: comparing the accuracy of five simple instruments. Age Ageing. 2013;42(2):262–265. doi:10.1093/ageing/afs163.
- Gobbens RJ, Schols JM, van Assen MA. Exploring the efficiency of the tilburg frailty indicator: a review. Clin Interv Aging. 2017;12:1739–1752. doi:10.2147/CIA.S130686.
- McHorney CA, Ware JE, Jr., Lu JF, et al. The MOS 36-item Short-Form health survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32(1):40–66. doi:10.1097/00005650-199401000-00004.
- Ware JE, Kosinski M, Sd K. SF-36 Physical and Mental Health Summary Scales: A User’s Manual. 1994.
- Gobbens RJ, van Assen MA, Luijkx KG, et al. The tilburg frailty indicator: psychometric properties. J Am Med Dir Assoc. 2010;11(5):344–355. doi:10.1016/j.jamda.2009.11.003.
- Verney J, Schwartz C, Amiche S, et al. Comparisons of a Multi-Frequency bioelectrical impedance analysis to the Dual-Energy X-Ray absorptiometry scan in healthy young adults depending on their physical activity level. J Hum Kinet. 2015;47:73–80. doi:10.1515/hukin-2015-0063.
- Pescatello LS, Arena R, Riebe D, et al. General principles of exercise prescription. 9th ed. ACSM’s Guidelines for Exercise Testing and Prescription. Lippincott Williams And Wilkins; 2014. p. 162–193.
- Roberts HC, Denison HJ, Martin HJ, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. 2011;40(4):423–429. doi:10.1093/ageing/afr051.
- Burich R, Teljigovic S, Boyle E, et al. Aerobic training alone or combined with strength training affects fitness in elderly: randomized trial. Eur J Sport Sci. 2015;15(8):773–783. doi:10.1080/17461391.2015.1060262.
- Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–94. doi:10.1093/geronj/49.2.m85.
- Bohannon RW. Normative reference values for the two-minute walk test derived by meta-analysis. J Phys Ther Sci. 2017;29(12):2224–2227. doi:10.1589/jpts.29.2224.
- Podsiadlo D, Richardson S. The timed “Up & go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–148. doi:10.1111/j.1532-5415.1991.tb01616.x.
- Scoring instructions for Short Musculoskeletal Function Assessment (SMFA). University of Minnesota. 2021. https://med.umn.edu/ortho/research/project-resources
- Teljigovic S, Sogaard K, Sandal LF, et al. Individualised physical exercise training and enhanced protein intake in older citizens during municipality-based rehabilitation: protocol for a randomised controlled trial. BMJ Open. 2020;10(11):e041605. doi:10.1136/bmjopen-2020-041605.
- Frost MH, Reeve BB, Liepa AM, et al. What is sufficient evidence for the reliability and validity of patient-reported outcome measures? Value Health. 2007;10(Suppl 2):S94–S105. doi:10.1111/j.1524-4733.2007.00272.x.
- Mokkink LB, Terwee CB, Patrick DL, et al. The COSMIN study reached international consensus on taxonomy, terminology, and definitions of measurement properties for health-related patient-reported outcomes. J Clin Epidemiol. 2010;63(7):737–745. doi:10.1016/j.jclinepi.2010.02.006.
- Cieza A, Stucki G. Content comparison of health-related quality of life (HRQOL) instruments based on the international classification of functioning, disability and health (ICF). Qual Life Res. 2005;14(5):1225–1237. doi:10.1007/s11136-004-4773-0.
- Hunsaker FG, Cioffi AD. The American academy of orthopaedix surgeons outcomes instruments normative values From the general population. J Bone Joint Surg. 2002;84(2):208–215.
- Portney LG, Watkins MP. Foundations of clinical research: applications to practice. 3 ed. Prentice Hall: Pearson; 2015. p. 892.
- Terwee CB, Bot SD, de Boer MR, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. 2007;60(1):34–42. doi:10.1016/j.jclinepi.2006.03.012.
- Bohm TD, Kirschner S, Kohler M, et al. The german short musculoskeletal function assessment questionnaire: reliability, validity, responsiveness, and comparison with the short form 36 and constant score–a prospective evaluation of patients undergoing repair for rotator cuff tear. Rheumatol Int. 2005;25(2):86–93. doi:10.1007/s00296-003-0423-z.
- Busse JW, Bhandari M, Guyatt GH, et al. Use of both short musculoskeletal function assessment questionnaire and short form-36 among tibial-fracture patients was redundant. J Clin Epidemiol. 2009;62(11):1210–1217. doi:10.1016/j.jclinepi.2009.01.014.
- Dattani R, Slobogean GP, O’Brien PJ, et al. Psychometric analysis of measuring functional outcomes in tibial Plateau fractures using the short form 36 (SF-36), short musculoskeletal function assessment (SMFA) and the Western Ontario McMaster osteoarthritis (WOMAC) questionnaires. Injury. 2013;44(6):825–829. doi:10.1016/j.injury.2012.10.020.
- Jung KS, Jung JH, In TS, et al. Reliability and validity of the korean version of the short musculoskeletal function assessment questionnaire for patients with musculoskeletal disorder. J Phys Ther Sci. 2016;28(9):2568–2571. doi:10.1589/jpts.28.2568.
- Kirschner S, Walther M, Bohm D, et al. German short musculoskeletal function assessment questionnaire (SMFA-D): comparison with the SF-36 and WOMAC in a prospective evaluation in patients with primary osteoarthritis undergoing total knee arthroplasty. Rheumatol Int. 2003;23(1):15–20. doi:10.1007/s00296-002-0253-4.
- Kirschner S, Walther M, Mehling E, et al. [Reliability, validity and responsiveness of the german short musculoskeletal function assessment questionnaire (SMFA-D) in patients with osteoarthritis of the hip undergoing total hip arthroplasty]. Z Rheumatol. 2003;62(6):548–554. doi:10.1007/s00393-003-0514-y.
- Lindahl M, Andersen S, Joergensen A, et al. Cross-cultural adaptation and validation of the danish version of the short musculoskeletal function assessment questionnaire (SMFA). Qual Life Res. 2018;27(1):267–271. Jan doi: 10.1007/s11136-017-1643-0.
- Reininga IH, el Moumni M, Bulstra SK, et al. Cross-cultural adaptation of the dutch short musculoskeletal function assessment questionnaire (SMFA-NL): internal consistency, validity, repeatability and responsiveness. Injury. 2012;43(6):726–733. doi:10.1016/j.injury.2011.07.013.
- Sepehri A, Lefaivre KA, O’Brien PJ, et al. Comparison of generic, Musculoskeletal-Specific, and foot and Ankle-Specific outcome measures Over time in tibial plafond fractures. Foot Ankle Orthop. 2019;4(4):2473011419884008. doi:10.1177/2473011419884008.
- Wang Y, He Z, Lei L, et al. Reliability and validity of the chinese version of the short musculoskeletal function assessment questionnaire in patients with skeletal muscle injury of the upper or lower extremities. BMC Musculoskelet Disord. 2015;16:161. doi:10.1186/s12891-015-0617-z.
- Lindahl M, Teljigović S, Nielsen NO. Six-months outcome after fracture for working-age persons analyzed using the international classification of functioning, disability, and health – a prospective cohort observational study. Physiother Theor Pract. 2023;39(8):1636–1649. doi:10.1080/09593985.2022.2048932.
- Gobbens RJ, Boersma P, Uchmanowicz I, et al. The tilburg frailty indicator (TFI): new evidence for its validity. Clin Interv Aging. 2020;15:265–274. doi:10.2147/CIA.S243233.
- WHO. WHO Global Report on Falls Prevention in Older Age. 2007. https://extranet.who.int/agefriendlyworld/wp-content/uploads/2014/06/WHo-Global-report-on-falls-prevention-in-older-age.pdf.
- Mokkink LB, Terwee CB, Patrick DL, et al. The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: an international delphi study. Qual Life Res. 2010;19(4):539–549. doi:10.1007/s11136-010-9606-8.
