Abstract
Objective
To investigate the predictive value of the prognostic nutritional index (PNI) for the effectiveness of infliximab (IFX) in patients with Crohn's disease (CD).
Methods
All data were retrospectively collected from Xiangya Hospital, Central South University between January 2016 and September 2021. Clinical remission at 52 weeks is the primary endpoint.
Results
Altogether, 193 CD patients were enrolled. PNI can identify clinical remission (p = 0.004), and the optimal cut-off value of the PNI was 39.2. 92/116 (79.3%) and 44/77 (57.1%) in the high- and low-PNI groups were in clinical remission at week 52 (p = 0.001). Patients with low PNI have poor general health at baseline. The body mass index, hemoglobin, platelet (PLT), serum creatinine, fibrinogen, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), Crohn's disease activity index (CDAI), and location of disease significantly differed between the two groups (p < 0.05). PNI was negatively correlated with CRP, ESR, PLT and CDAI (p < 0.05). The lower PNI, smoking history, and higher CDAI at baseline were the independent risk factors of disease activity at 52 weeks (p < 0.05). The high-PNI group is less likely to develop poor outcomes (p = 0.033).
Conclusion
The PNI may serve as a novel and promising biomarker in predicting the effectiveness of IFX and contribute to targeted management in CD.
KEY MESSAGES
The prognostic nutritional index could be a prognostic indicator in identifying the effectiveness of infliximab in CD patients. The lower PNI is an independent risk factor for the poor effectiveness of infliximab in CD patients. More attention should be given to assessing the immune and nutritional statuses in CD patients.
Introduction
Crohn's disease (CD), an inflammatory bowel disease (IBD) with an increasing incidence worldwide, is a nonspecific chronic inflammatory disease involving the gastrointestinal tract with symptoms evolving in a relapsing and remitting manner [Citation1]. Dysregulated innate and adaptive immune responses activated by a complex interplay between genetic susceptibility, environmental factors, and altered gut microbiota could be the culprit of CD [Citation1]. Over time, half of the CD patients will undergo surgical treatment due to serious complications [Citation2,Citation3], which may lead to poor quality of life. Malnutrition is seen in 80% of patients and may serve as a clinical marker of poor CD prognosis in hospitalized patients [Citation4–6]. The current treatment paradigm is shifting to an earlier, more aggressive treatment aiming for deep and long-lasting remission, preventing complications and poor outcomes, such as surgery, and inhibiting disease progression [Citation1,Citation7].
Infliximab (IFX), a human-murine chimeric monoclonal antibody directed against tumor necrosis factor (TNF), was the first biologic medication used to treat CD. IFX is highly effective in inducing mucosal healing and maintaining remission in CD patients [Citation8–10]. The current guidelines have recommended the use of IFX to induce remission in patients with moderate-to-severe CD not responding to conventional therapies [Citation8,Citation9,Citation11]. The use of TNF inhibitors in the first 2 years of the disease course is preferred in controlling disease progression based on the results of the post-hoc analyses of previous clinical trials [Citation12,Citation13]. However, as a monoclonal antibody administered by intravenous infusion, IFX may be prone to exposure systematically, which may induce subsequent autoantibody formation [Citation14,Citation15]. It was reported that 23%–46% of patients have a loss of response to IFX at 12 months after induction [Citation16]. The nutritional and immune statuses are closely associated with IFX efficacy [Citation17–19]. Thus, identifying patients at risk and conducting stringent regulations as early as possible are of great significance.
The prognostic nutritional index (PNI), which is calculated by using serum albumin (ALB) concentration and peripheral blood lymphocyte count, was first proposed by Onodera in 1984 as a nutritional index to comprehensively evaluate the surgical risks of patients with gastrointestinal cancer [Citation20]. Recently, as an easily accessible and non-invasive biomarker reflecting the nutritional and immunological statuses, PNI has attracted more attention and has been extensively used for the clinical evaluation of the prognosis in patients with cancer [Citation21], adverse cardiovascular events [Citation22], and diabetic nephropathy [Citation23]. Therefore, considering the strong association of CD and IFX with the nutritional and immunological statuses, it is reasonable to speculate that PNI could be used as a biomarker to predict CD severity and IFX's clinical efficacies.
Despite the extensive clinical study, until now, there have been a few clinical or experimental studies examining the association between PNI and the clinical efficacies of IFX. The present study aimed to investigate the correlation of PNI with the clinical efficacies of IFX.
Materials and methods
The research study was conducted according to the guidelines stipulated in the Declaration of Helsinki and was approved by the ethical committee of Xiangya Hospital of Central South University (No. 202108158).
Patients and design
This study retrospectively enrolled patients diagnosed with CD and who had undergone IFX therapy for the first time from January 2016 and September 2021 at the IBD Center of Xiangya Hospital. The inclusion criteria were as follows: (1) diagnosed with CD at the IBD Center; and (2) IFX was infused strictly according to the instructions (doses given were 5 mg/kg. The second and third doses should be administered following the first dose at 2 and 6 weeks; therefore, regular infusions were scheduled every 8 weeks). The following participants were excluded: (1) follow-up duration was <1 year; (2) unable or unwilling to continue the IFX treatment for objective reasons; (3) women who got pregnant during follow-up; and (4) patients with other malignant tumors, severe hepatic disease, severe active infections, congenital or acquired immunodeficiencies, cognitive disorders, and psychiatric disorders.
Clinical measurements
The data on the patient's baseline characteristics, including general conditions and disease characteristics, were collected. The general conditions included sex, age, body mass index (BMI), smoking history, and bowel surgery. The disease characteristics included disease activity, behavior, location, duration, corticosteroid use, history of treatment (immunomodulators or 5-aminosalicylates), and presence of perianal disease. Data on disease activity was collected, and the disease activity was calculated using the Crohn's disease activity index (CDAI). A CDAI ≥ 150 was defined as an active disease and a CDAI < 150 as remission [Citation24]. Furthermore, laboratory test results, including the hemoglobin (HGB) level; white blood cell (WBC), lymphocyte, and platelet (PLT) counts; ALB, serum creatinine (Scr), fibrinogen (FIB), C-reactive protein (CRP) levels; and erythrocyte sedimentation rate (ESR), before the first IFX infusion were collected from the electronic medical records of Xiangya Hospital. The PNI was calculated as follows: PNI = ALB (g/L) + 5 * lymphocyte count (109/L).
Follow-up
The follow-up period for this study lasted for 52 weeks. All of the patients were regularly followed up through medical record retrieval, telephone interview, and WeChat communication. The follow-up data obtained included general conditions, current medications, presence or absence of poor outcomes (either of the three: CD-related surgery, switching medication, and unplanned hospital readmissions), time to the first poor outcome, and CDAI score at 52 weeks.
Endpoints
The rate of clinical remission at 52 weeks is the primary endpoint. Moreover, the secondary endpoint was the occurrence of any poor outcome (CD-related surgery, switching medication, or unplanned hospital readmissions).
Statistical analysis
All statistical analyses were performed using SPSS for Mac OS X version 23 (SPSS Inc., Chicago, IL, USA) or GraphPad Prism 9 (GraphPad Software, Inc., La Jolla, CA) software. Continuous data were expressed as median [interquartile range: (IQR)] and categorical data as proportion. A receiver-operating characteristic (ROC) curve was built to evaluate the prediction performance of PNI, and the Youden index was calculated to determine the optimal cut-off points. Group comparisons were made by unpaired t-test, Wilcoxon rank-sum test, or chi-square test, as appropriate. The correlation between PNI and common clinical biochemical parameters was examined through linear regression analysis. Then, univariate and multivariate logistics regression analyses were performed to identify the risk factors associated with clinical remission. Finally, the Kaplan–Meier (KM) curves were plotted to compare the poor outcomes among different PNI groups. A two-tailed p-value < 0.05 was considered significant.
Results
Baseline characteristics
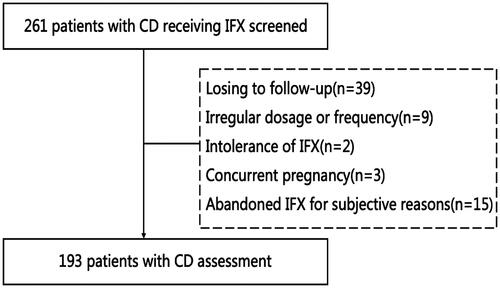
Altogether, 193 eligible patients were included in the study after the eligibility screening (). Of these, 142 patients (73.6%) were male, and 51 patients (26.4%) were female, with a median age of 26(IQR 18.5–33.0) years and a median BMI of 17.72(15.68–19.62) kg/m2. The median PNI was 41.40(35.40–46.70) in all patients. Altogether, 38(16.69%) patients were smokers, 96(49.7%) patients had a perianal lesion, 32(16.6%) patients had a history of bowel surgery, 18(9.3%) patients used oral corticosteroid, 33(17.1%) patients had a history of treatment with immunomodulators, and 38(19.7%) had prior 5-aminosalicylates therapy. The median CD duration was 12(4–36) months. Laboratory examination results and disease characteristics are shown in .
Table 1. Baseline characteristics of 193 patients with Crohn's disease.
PNI can be a better IFX effectiveness predictor than CRP and CDAI
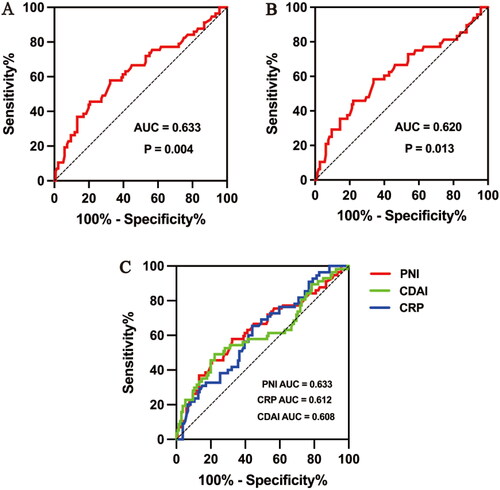
Of the 193 patients receiving IFX, 136 (70.5%) were in clinical remission at week 52 (the primary endpoint), 48(24.9%) developed poor outcomes during the 52-week follow-up, and 9(4.6%) patients without poor outcomes did not achieve clinical remission at week 52. The ROC curves were applied to evaluate the performances of PNI at baseline for prognosticating the clinical remission and poor outcomes at 52 weeks after IFX infusion. The ROC curve in predicting clinical remission showed an area under the curve (AUC) of 0.633 (95% CI: 0.543–0.722, p = 0.004) (). The optimal cut-off value for PNI was 39.2, which was used to divide all patients into a high (PNI > 39.2) and a low (PNI ≤ 39.2) PNI group. The AUC for poor outcomes at 52 weeks after IFX infusion was 0.620(95% CI: 0.523–0.718, p = 0.013) (). Similarly, the predictive abilities of CRP, ESR, PLT, and CDAI on clinical remission were also calculated. The AUC for PNI (AUC 0.633) was greater than that for CRP [AUC 0.612(95% CI: 0.526–0.699, p = 0.015)] and CDAI [AUC 0.608(95% CI: 0.515–0.702, p = 0.018)] (). The ROC curves of ESR (p = 0.173) and PLT (p = 0.290) were not statistically significant.
Figure 2. Receiver-operating curve (ROC) analysis for prognostic nutritional index (PNI) to predict (A) clinical remission with optimal PNI level cut-off of 39.2, (B) poor outcomes for 52 weeks. (C) ROC analysis of PNI, C-reactive protein (CRP) and Crohn's disease activity index (CDAI) to predict clinical remission.
Patients with low PNI seem to have poor general health at baseline
The high-PNI group has a higher number of patients than the low-PNI group (n = 116 vs. n = 77). Age (p = 0.066) and sex (p = 0.121) distributions did not differ between the two groups. The high-PNI group had a significantly higher BMI (17.93[16.31–19.59] vs 16.63[15.22–19.83], p = 0.038) than the low-PNI group. Regarding the laboratory findings, the high-PNI group had higher HGB (126.50 [114.00–137.00] vs 98.00 [84.00–115.00], p < 0.001) and Scr (73.00 [65.38–80.00] vs 66.80 [56.85–76.50], p = 0.007) levels than the low-PNI group. The PLT count (321.50 [228.00–384.00] vs 364.00 [288.00–453.00], p = 0.001), FIB level (4.10 [3.13–4.85] vs 4.69 [4.05–5.51], p < 0.001), CRP level (12.85 [4.10–36.63] vs 35.00 [17.80–77.35], p < 0.001), ESR (41.50 [18.00–70.50] vs 70.50 [33.50–97.75], p = 0.001), and CDAI scores (173.04 [143.61–259.60] vs 248.12 [198.53–297.06], p < 0.001) were significantly lower in the high-PNI group than in the low-PNI group. The disease locations (27[23.3%] vs 7[9.1%] patients involved in the ileum; 22[19.0%] vs 15[19.5%] patients involved in the colon; 67[57.8%] vs 55[71.4%] patients involving in ileum and colon simultaneously, p = 0.036) significantly differed between the two groups. No significant differences in the history of smoking, disease duration, WBC count, disease behavior, perianal lesion rate, previous bowel surgery, corticosteroid use and History of treatment with immunomodulators or 5-aminosalicylates were observed between the two groups (all p > 0.05, ). Further, the linear regression analysis indicated that PNI was negatively correlated with CRP (r = −0.476, p < 0.001), ESR (r = −0.238, p = 0.002), PLT (r = −0.313, p < 0.001) and CDAI scores (r = −0.461, p < 0.001) ().
Figure 3. Correlations of prognostic nutritional index (PNI) with (A) C-reactive protein (CRP) (B) erythrocyte sedimentation rate (ESR), (C) platelet (PLT), (D) Crohn's disease activity index (CDAI) at baseline.
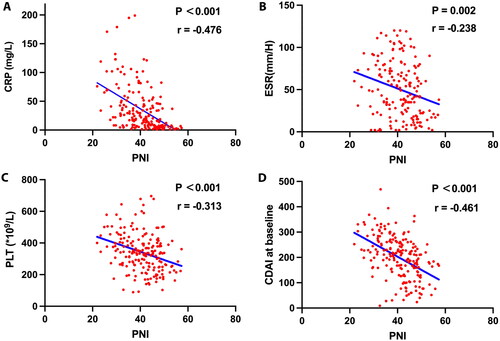
Table 2. Associations of prognostic nutritional index (PNI) with clinical characteristics of patients with Crohn's disease.
The lower PNI at baseline was a risk factor for clinical activity at 52 weeks
At week 52, we observed a total of 92 of the 116 patients (79.3%) in high-PNI groups, 44 of the 77 patients (57.1%) in low-PNI groups were in clinical remission (p = 0.001). The population was divided into two groups based on the clinical activity at 52 weeks. shows the baseline characteristics of both groups. Subsequently, univariate and multivariate regression analyses were used to explore the risk factors for clinical activity (). Multivariate regression analysis revealed that the lower PNI (OR: 0.832, 95% CI: 0.712–0.973, p = 0.021), history of smoking (3.684[1.607–8.444], p = 0.002), high CDAI score (1.005[1.000–1.010], p = 0.036) before IFX infusion were the independent risk factors of clinical activity at 52 weeks.
Table 3. Baseline characteristics grouped by clinical activity and logistic regression analyses to explore the risk factors for clinical activity at 52 weeks.
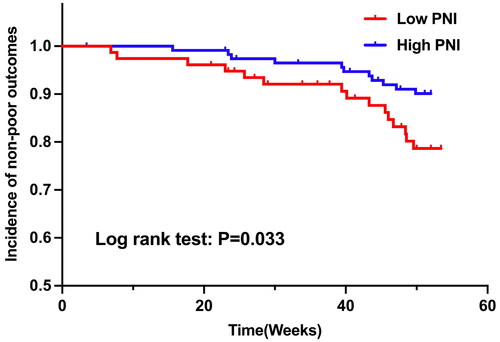
High PNIs have a lower probability of developing poor outcomes
To further explore whether PNI at baseline could be a prognostic factor for poor outcomes, KM survival curves were drawn (). The result implies that patients with high PNI have a lower probability of developing poor outcomes (p = 0.033).
Discussion
In the present study involving 193 individuals, we investigated the predictive value of PNI at baseline for the effectiveness of IFX in patients with CD. The outcome failure rate to maintain clinical remission at 52 weeks was 29.5%, which is similar to the results of previous studies16. The current study suggests that PNI is strongly associated with CD severity, and PNI has a predictive value for clinical remission and poor outcomes of IFX. Therefore, the PNI at baseline could serve as a predictor for determining the effectiveness of IFX.
In this study, we first confirmed the prognostic value of PNI at baseline for clinical remission at 52 weeks and poor outcomes. Further analysis revealed that the predictive abilities of PNI were even better than CRP and CDAI scores. CRP, an acute-phase reactant, is secreted by the liver in response to inflammatory stimuli [Citation25]. It is often closely correlated with mucosal damage, disease activity, disease recurrence, and disease progression of CD [Citation26–28]. CDAI score is among the most common methods used to assess clinical activity [Citation24]. CRP and CDAI were proven to be significant predictors for IFX effectiveness in other studies [Citation29–32], consistent with our findings. The predictive superiority of PNI indicates that, besides the inflammatory activity, the baseline nutritional status is also closely related to the effectiveness of IFX. It is suggested that the predictive ability of the combination of inflammation and nutrition was more superior than that of the inflammatory activity alone.
The results of further analysis of the clinical features of both the low- and high-PNI groups indicate that patients with low PNI have poor general health conditions at baseline. This observation is in line with the findings of previous studies on other disorders, such as melanoma [Citation33] and hypertrophic cardiomyopathy [Citation34]. In the current study, PNI was related not only to the known indicators of nutritional status, including BMI and HGB, and to the indicators of immune and inflammatory conditions, including ESR and CRP. PLT, Scr [Citation35], FIB, and CDAI. Notably, PNI was also found to be strongly associated with disease location. A greater percentage of patients with high and low PNI had isolated ileum and ileocolonic involvements, respectively. A reduction in the area of intestinal mucosal injury could lead to a reduction in the inflammatory response, which may account for the disease location differences. As also reported by Gecse et al. the CRP level of CD patients with isolated ileum involvement was lower than that of patients with ileocolonic involvement [Citation36]. The subsequent linear regression showed that a strong negative correlation was found between PNI and inflammatory biomarkers of CD severity (CRP, ESR, PLT, and CDAI score), indicating that the lower the PNI, the more severe the CD, which is similar to our other study findings.
In the present study, the lower PNI is a risk factor for clinical activity at 52 weeks, and it can predict a trend toward higher incidences of poor outcomes. The lower PNI may affect the effectiveness of IFX based on the following aspects. On the one hand, serum ALB is involved in the transport of IFX to prevent the drug from being eliminated from the body. Compared to those with lower serum ALB levels, patients with a higher serum ALB level had a lower IFX clearance, a longer IFX half-life and, therefore, a higher IFX exposure [Citation37,Citation38]. The pharmacokinetic evaluation showed that the early-stage IFX trough level in patients with a decreased ALB level was too low to be detected, leading to an increased risk of developing anti-IFX-antibodies [Citation39,Citation40]. On the other hand, the recruitment of the circulating leukocytes to the gut is a pivotal mechanism in CD pathogenesis [Citation41,Citation42]. Lymphocyte homing to the intestinal mucosa and lymphatic infiltration in gut-associated lymphoid tissues may decrease peripheral lymphocyte counts in CD patients [Citation43]. The ability of circulating lymphocytes to migrate into lymphoid tissues has also been documented in other disease models [Citation44–46]. The decreasing number of lymphocytes predicts severe intestinal inflammation, upregulating protein catabolism in the reticuloendothelial system and accelerating the IFX clearance [Citation38]. Therefore, the PNI can reflect the immune and nutritional statuses simultaneously, which may be the main reason for the poor prognosis of CD patients.
Smoking was also identified as one of the most potent risk factors for clinical activity in the present study. Indeed, smoking has been associated not only with CD occurrence [Citation47] but also with CD progression [Citation48]. A higher recurrence rate reportedly occurs after drug or surgical treatment in smokers [Citation49]. Consequently, quitting smoking is recommended for CD patients [Citation50].
With the deepening understanding of CD and its high disability rate, it is generally accepted that only finding a reliable and sensitive way to predict progression and drug effectiveness might contribute to guiding clinical decision-making. Endoscopy, the most useful tool to assist in CD diagnosis and management, is invasive, and its clinical application is limited [Citation51]. Recently, many studies have focused on developing new biomarkers to predict IFX effectiveness. Serological markers, such as the type IV collagen [Citation52,Citation53] and B-cell activating factor [Citation54], histological markers, such as ileocolonic expression of IL13RA2 [Citation55], metabolite markers, such as fecal calprotectin [Citation56] and fecal bacterial and fungal microbiota composition [Citation57], were found to predict IFX effectiveness by comparing their microbiota diversity, integrity of the intestinal mucosal barrier, and so on. However, they are not as non-invasive and readily accessible as the PNI. Moreover, as routine test items, detecting lymphocyte count and ALB concentration does not increase the economic burden on patients; in fact, it is well accepted by patients.
There are, of course, some limitations to our study. First, this is a single-center retrospective study. The sample size is small, and the follow-up period is short. There may be selection bias in patient selection and data collection. The second limitation is the heterogeneity of our population, especially regarding age and BMI, which could limit the generalizability and extrapolation of our results. Third, this study does not include the objective markers of evaluating the IFX effectiveness, such as fecal calprotectin, endoscopy, histology, etc. We did not include these markers because the missing data of these parameters is too much to get an accurate evaluation. A multicenter randomized prospective study with a larger sample size, longer follow-up duration and more comprehensive evaluation is needed to confirm our preliminary results in the future.
Conclusions
Our study data show for the first time that PNI has a predictive value for the effectiveness of IFX in CD patients. Attention should be given to assessing the immune and nutritional statuses before the IFX treatment. Moreover, patients with lower PNI should be followed closely.
Author contributions
ZP, YP, FL, XL designed research. ZP, DX, YL conducted research, provided essential reagents, or provided essential materials and analyzed data or performed statistical analysis. ZP wrote paper. FL, YP had primary responsibility for final content. All authors approved the final version of the manuscript, including the authorship list.
Acknowledgements
Not applicable.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Torres J, Mehandru S, Colombel J-F, et al. Crohn's disease. Lancet. 2017;389(10080):1–10. doi: 10.1016/S0140-6736(16)31711-1.
- Thia KT, Sandborn WJ, Harmsen WS, et al. Risk factors associated with progression to intestinal complications of Crohn's disease in a population-based cohort. Gastroenterology. 2010;139(4):1147–1155. doi: 10.1053/j.gastro.2010.06.070.
- Peyrin-Biroulet L, Loftus EV, Colombel J-F, et al. The natural history of adult Crohn's disease in population-based cohorts. Am J Gastroenterol. 2010;105(2):289–297. doi: 10.1038/ajg.2009.579.
- Donnellan CF, Yann LH, Lal S. Nutritional management of Crohn's disease. Therap Adv Gastroenterol. 2013;6(3):231–242. doi: 10.1177/1756283X13477715.
- Balestrieri P, Ribolsi M, Guarino MPL, et al. Nutritional aspects in inflammatory bowel diseases. Nutrients. 2020;12:372. doi: 10.3390/nu12020372.
- Nguyen GC, Munsell M, Harris ML. Nationwide prevalence and prognostic significance of clinically diagnosable protein-calorie malnutrition in hospitalized inflammatory bowel disease patients. Inflamm Bowel Dis. 2008;14(8):1105–1111. doi: 10.1002/ibd.20429.
- Sandborn WJ. Current directions in IBD therapy: what goals are feasible with biological modifiers? Gastroenterology. 2008;135(5):1442–1447. doi: 10.1053/j.gastro.2008.09.053.
- Zhou Z, Zhang H-S, Liu Y, et al. Loss of TET1 facilitates DLD1 colon cancer cell migration via H3K27me3-mediated down-regulation of E-cadherin. J Cell Physiol. 2018;233(2):1359–1369. doi: 10.1002/jcp.26012.
- Gomollón F, Dignass A, Annese V, et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn's disease 2016: part 1: diagnosis and medical management. J Crohns Colitis. 2017;11(1):3–25. doi: 10.1093/ecco-jcc/jjw168.
- Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002;359(9317):1541–1549. doi: 10.1016/S0140-6736(02)08512-4.
- Torres J, Bonovas S, Doherty G, et al. ECCO guidelines on therapeutics in Crohn's disease: medical treatment. J Crohns Colitis. 2020;14(1):4–22. doi: 10.1093/ecco-jcc/jjz180.
- Schreiber S, Reinisch W, Colombel JF, et al. Subgroup analysis of the placebo-controlled CHARM trial: increased remission rates through 3 years for adalimumab-treated patients with early Crohn's disease. J Crohns Colitis. 2013;7(3):213–221. doi: 10.1016/j.crohns.2012.05.015.
- Colombel JF, Reinisch W, Mantzaris GJ, et al. Randomised clinical trial: deep remission in biologic and immunomodulator naïve patients with Crohn's disease – a SONIC post hoc analysis. Aliment Pharmacol Ther. 2015;41(8):734–746. doi: 10.1111/apt.13139.
- Maser EA, Villela R, Silverberg MS, et al. Association of trough serum infliximab to clinical outcome after scheduled maintenance treatment for Crohn's disease. Clin Gastroenterol Hepatol. 2006;4(10):1248–1254. doi: 10.1016/j.cgh.2006.06.025.
- Nanda KS, Cheifetz AS, Moss AC. Impact of antibodies to infliximab on clinical outcomes and serum infliximab levels in patients with inflammatory bowel disease (IBD): a meta-analysis. Am J Gastroenterol. 2013;108(1):40–47; quiz 48. doi: 10.1038/ajg.2012.363.
- Ben-Horin S, Chowers Y. Review article: loss of response to anti-TNF treatments in Crohn's disease. Aliment Pharmacol Ther. 2011;33(9):987–995. doi: 10.1111/j.1365-2036.2011.04612.x.
- Billiet T, Papamichael K, de Bruyn M, et al. A matrix-based model predicts primary response to infliximab in Crohn's disease. J Crohns Colitis. 2015;9(12):1120–1126. doi: 10.1093/ecco-jcc/jjv156.
- Li Y, Pan J, Zhou N, et al. A random Forest model predicts responses to infliximab in Crohn's disease based on clinical and serological parameters. Scand J Gastroenterol. 2021;56(9):1030–1039. doi: 10.1080/00365521.2021.1939411.
- ten Hove T, van Montfrans C, Peppelenbosch MP, et al. Infliximab treatment induces apoptosis of lamina propria T lymphocytes in Crohn's disease. Gut. 2002;50(2):206–211. doi: 10.1136/gut.50.2.206.
- Onodera T, Goseki N, Kosaki G. [Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. 1984;85:1001–1005.
- Fu M, Yu L, Yang L, et al. Predictive value of the preoperative prognostic nutritional index for postoperative progression in patients with pancreatic neuroendocrine neoplasms. Front Nutr. 2022;9:945833. doi: 10.3389/fnut.2022.945833.
- Zencirkiran Agus H, Kahraman S. Prognostic nutritional index predicts one-year outcome in heart failure with preserved ejection fraction. Acta Cardiol. 2020;75(5):450–455. doi: 10.1080/00015385.2019.1661139.
- Zhang J, Xiao X, Wu Y, et al. Prognostic nutritional index as a predictor of diabetic nephropathy progression. Nutrients. 2022;14:3634. doi: 10.3390/nu14173634.
- Best WR, Becktel JM, Singleton JW, et al. Development of a Crohn's disease activity index. National cooperative Crohn's disease study. Gastroenterology. 1976;70(3):439–444. doi: 10.1016/S0016-5085(76)80163-1.
- Vermeire S, Van Assche G, Rutgeerts P. C-reactive protein as a marker for inflammatory bowel disease. Inflamm Bowel Dis. 2004;10(5):661–665. doi: 10.1097/00054725-200409000-00026.
- Mosli MH, Zou G, Garg SK, et al. C-Reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: a systematic review and meta-analysis. Am J Gastroenterol. 2015;110(6):802–819; quiz 820. doi: 10.1038/ajg.2015.120.
- Chang S, Malter L, Hudesman D. Disease monitoring in inflammatory bowel disease. World J Gastroenterol. 2015;21(40):11246–11259. doi: 10.3748/wjg.v21.i40.11246.
- Lewis JD. The utility of biomarkers in the diagnosis and therapy of inflammatory bowel disease. Gastroenterology. 2011;140(6):1817.e2–1826.e2. doi: 10.1053/j.gastro.2010.11.058.
- Zhou Y, Xu ZZ, He Y, et al. Gut microbiota offers universal biomarkers across ethnicity in inflammatory bowel disease diagnosis and infliximab response prediction. mSystems. 2018;3:e00188–e00217. doi: 10.1128/mSystems.00188-17.
- Li L, Chen R, Zhang Y, et al. A novel model based on serum biomarkers to predict primary non-response to infliximab in Crohn's disease. Front Immunol. 2021;12:646673. doi: 10.3389/fimmu.2021.646673.
- Magro F, Rodrigues-Pinto E, Santos-Antunes J, et al. High C-reactive protein in Crohn's disease patients predicts nonresponse to infliximab treatment. J Crohns Colitis. 2014;8(2):129–136. doi: 10.1016/j.crohns.2013.07.005.
- Hibi T, Sakuraba A, Watanabe M, et al. C-reactive protein is an indicator of serum infliximab level in predicting loss of response in patients with Crohn's disease. J Gastroenterol. 2014;49(2):254–262. doi: 10.1007/s00535-013-0807-0.
- Mirili C, Yılmaz A, Demirkan S, et al. Clinical significance of prognostic nutritional index (PNI) in malignant melanoma. Int J Clin Oncol. 2019;24(10):1301–1310. doi: 10.1007/s10147-019-01461-7.
- Wang Z, Zhao L, He S. Prognostic nutritional index and the risk of mortality in patients with hypertrophic cardiomyopathy. Int J Cardiol. 2021;331:152–157. doi: 10.1016/j.ijcard.2021.01.023.
- Stam F, van Guldener C, Schalkwijk CG, et al. Impaired renal function is associated with markers of endothelial dysfunction and increased inflammatory activity. Nephrol Dial Transplant. 2003;18(5):892–898. doi: 10.1093/ndt/gfg080.
- Gecse KB, Brandse JF, van Wilpe S, et al. Impact of disease location on fecal calprotectin levels in Crohn's disease. Scand J Gastroenterol. 2015;50(7):841–847. doi: 10.3109/00365521.2015.1008035.
- Fasanmade AA, Adedokun OJ, Olson A, et al. Serum albumin concentration: a predictive factor of infliximab pharmacokinetics and clinical response in patients with ulcerative colitis. Int J Clin Pharmacol Ther. 2010;48(5):297–308. doi: 10.5414/cpp48297.
- Hemperly A, Vande Casteele N. Clinical pharmacokinetics and pharmacodynamics of infliximab in the treatment of inflammatory bowel disease. Clin Pharmacokinet. 2018;57(8):929–942. doi: 10.1007/s40262-017-0627-0.
- Dotan I, Ron Y, Yanai H, et al. Patient factors that increase infliximab clearance and shorten half-life in inflammatory bowel disease: a population pharmacokinetic study. Inflamm Bowel Dis. 2014;20(12):2247–2259. doi: 10.1097/MIB.0000000000000212.
- Brandse JF, Mould D, Smeekes O, et al. A real-life population pharmacokinetic study reveals factors associated with clearance and immunogenicity of infliximab in inflammatory bowel disease. Inflamm Bowel Dis. 2017;23(4):650–660. doi: 10.1097/MIB.0000000000001043.
- Eksteen B, Liaskou E, Adams DH. Lymphocyte homing and its role in the pathogenesis of IBD. Inflamm Bowel Dis. 2008;14(9):1298–1312. doi: 10.1002/ibd.20453.
- Dotan I, Allez M, Danese S, et al. The role of integrins in the pathogenesis of inflammatory bowel disease: approved and investigational anti-integrin therapies. Med Res Rev. 2020;40(1):245–262. doi: 10.1002/med.21601.
- Selby WS, Jewell DP. T lymphocyte subsets in inflammatory bowel disease: peripheral blood. Gut. 1983;24(2):99–105. doi: 10.1136/gut.24.2.99.
- Gordon AS. Some aspects of hormonal influences upon the leukocytes. Ann N Y Acad Sci. 1955;59(5):907–927. doi: 10.1111/j.1749-6632.1955.tb45990.x.
- Toft P, Svendsen P, Tønnesen E, et al. Redistribution of lymphocytes after major surgical stress. Acta Anaesthesiol Scand. 1993;37(3):245–249. doi: 10.1111/j.1399-6576.1993.tb03708.x.
- Tumpey TM, Lu X, Morken T, et al. Depletion of lymphocytes and diminished cytokine production in mice infected with a highly virulent influenza A (H5N1) virus isolated from humans. J Virol. 2000;74(13):6105–6116. doi: 10.1128/jvi.74.13.6105-6116.2000.
- Mahid SS, Minor KS, Soto RE, et al. Smoking and inflammatory bowel disease: a meta-analysis. Mayo Clin Proc. 2006;81(11):1462–1471. doi: 10.4065/81.11.1462.
- Higuchi LM, Khalili H, Chan AT, et al. A prospective study of cigarette smoking and the risk of inflammatory bowel disease in women. Am J Gastroenterol. 2012;107(9):1399–1406. doi: 10.1038/ajg.2012.196.
- Avidan B, Sakhnini E, Lahat A, et al. Risk factors regarding the need for a second operation in patients with Crohn's disease. Digestion. 2005;72(4):248–253. doi: 10.1159/000089960.
- Vermeire S, Louis E, Carbonez A, et al. Demographic and clinical parameters influencing the short-term outcome of anti-tumor necrosis factor (infliximab) treatment in Crohn's disease. Am J Gastroenterol. 2002;97(9):2357–2363. doi: 10.1111/j.1572-0241.2002.05991.x.
- Lindholm CR, Siegel CA. Are We ready to include prognostic factors in inflammatory bowel disease trials? Curr Pharm Des. 2019;25(1):64–68. doi: 10.2174/1381612825666190312113935.
- Alexdottir MS, Bourgonje AR, Karsdal MA, et al. Serological biomarkers of intestinal collagen turnover identify early response to infliximab therapy in patients with Crohn's disease. Front Med. 2022;9:933872. doi: 10.3389/fmed.2022.933872.
- van Haaften WT, Mortensen JH, Dige AK, et al. Serological biomarkers of tissue turnover identify responders to anti-TNF therapy in Crohn's disease: a pilot study. Clin Transl Gastroenterol. 2020;11(9):e00217. doi: 10.14309/ctg.0000000000000217.
- Andreou N-P, Legaki E, Dovrolis N, et al. B-cell activating factor (BAFF) expression is associated with Crohn's disease and can serve as a potential prognostic indicator of disease response to infliximab treatment. Dig Liver Dis. 2021;53(5):574–580. doi: 10.1016/j.dld.2020.11.030.
- Verstockt B, Verstockt S, Creyns B, et al. Mucosal IL13RA2 expression predicts nonresponse to anti-TNF therapy in Crohn's disease. Aliment Pharmacol Ther. 2019;49(5):572–581. doi: 10.1111/apt.15126.
- Laharie D, Mesli S, El Hajbi F, et al. Prediction of Crohn's disease relapse with faecal calprotectin in infliximab responders: a prospective study. Aliment Pharmacol Ther. 2011;34(4):462–469. doi: 10.1111/j.1365-2036.2011.04743.x.
- Ventin-Holmberg R, Eberl A, Saqib S, et al. Bacterial and fungal profiles as markers of infliximab drug response in inflammatory bowel disease. J Crohns Colitis. 2021;15(6):1019–1031. doi: 10.1093/ecco-jcc/jjaa252.