Abstract
Background
Coronary artery disease (CAD) is associated with perioperative liver transplantation (LT) mortality. In absence of a defined risk algorithm, we aimed to test whether stress echocardiography and coronary computed tomography angiography (CCTA) could detect CAD in end-stage liver disease (ESLD) patients without previous evidence of heart disease.
Methods
LT candidates ≥30 years underwent a cardiovascular (CV) assessment through stress echocardiography. CCTA was performed in patients ≥50 years with two or more CV risk factors (e.g. diabetes, CAD family history, dyslipidaemia). Coronary angiography (CAG) was scheduled when stress echocardiography and/or CCTA were positive. Sensibility, specificity, positive and negative predictive values of stress echocardiography and CCTA were assessed by numbers of coronary revascularization (true positives) and lack of acute coronary events over a mean follow-up of 3 years (true negatives).
Results
Stress echocardiography was performed in 273 patients, CCTA in 34 and CAG in 41. Eight patients had critical coronary lesions, and 19 not-critical lesions. Sensitivity, specificity, positive and negative predictive values were 50.0%, 90.2%, 13.3% and 98.4% for stress echocardiography and 100%, 76.7%, 36.4% and 100% for CCTA. Among 163 patients who underwent LT (57.6%), 16 died and 5 had major adverse CV events over a mean follow-up of 3 years.
Conclusions
A very low prevalence of CAD in a selected population of ESLD at intermediate to high CV risk was found. A screening based on stress echocardiography and CCTA resulted in low incidence of post-LT acute coronary events in ELSD patients. CAD has no impact on mid-term survival.
Introduction
Liver transplantation (LT), the only effective end-stage liver disease (ESLD) treatment, is a procedure linked to high risk cardiovascular (CV) complications. According to the 2022 European Society of Cardiology (ESC) guidelines on CV assessment and management of patients undergoing non-cardiac surgery, the 30-day surgical risk estimate associated with LT is ≥5% [Citation1]. This latter is a broad approximation of 30-day risk of CV death, myocardial infarction (MI) and stroke.
LT may cause changes in circulating volume due to massive blood loss [Citation2], needed for transfusion [Citation3], haemodynamic instability and haemodynamic instability secondary to the ischaemia–reperfusion syndrome [Citation4]. Patients with left ventricular dysfunction, related to cirrhosis (cirrhotic cardiomyopathy) [Citation5] or with clinically relevant valvulopathies [Citation6] or history of coronary artery disease (CAD) have an increased risk of morbidity and mortality both in the peri- and post-operative phases of LT [Citation7]. Pre-existent CAD worsens the early surgical outcome [Citation8] and raises mortality after LT [Citation9]. As the demand for organ donors exceeds the supply, concomitant cardiac status is accurately evaluated prior to the LT listing in the majority of LT centres. This approach defines the effective risk of procedural and post-surgical CV events, possibly excluding severely heart affected patients.
Knowledge about CAD prevalence and its impact in ESLD remains scarce. Moreover, diagnostic tests employed to detect CAD are not always adequately referred. Data from Organ Procurement Transplant Network registry refer to 3.4% of CAD prevalence in ELSD, more frequent in non-alcohol-related (7.4%) than in alcohol-related liver diseases (2.9%). Unfortunately, methods for CAD clinical diagnosis are missing [Citation10]. A reduction in post-LT mortality and MI was reported by using coronary angiography (CAG), which was considered the gold standard technique for CAD evaluation [Citation11]. However, the impact of CV risk factors, even in absence of known CAD, is well recognized. A meta-analysis of 12 observational studies, on roughly 4800 patients, reported a fourfold higher risk of CV events at 10 years in LT recipients [Citation12].
Currently, in the absence of a standardized screening for CAD in LT candidates, most surgery units perform cardiological evaluation to identify a subclinical heart pathology (e.g. valvular disease, arrhythmias, left ventricular dysfunction, pulmonary hypertension) [Citation13]. In 2005, the American Association for the study of Liver Disease recommended screening for coronary disease in all patients scheduled for LT when main CV risk factors were present (i.e. smoking, family history of heart disease, dyslipidaemia, hypertension, diabetes – especially >50 years) [Citation14]. Thus, all possible LT candidates are usually evaluated by electrocardiogram (ECG) and baseline echocardiography.
In line with this evidence, the 2022 ESC flowchart suggests a cardiologic clinical evaluation in patients undergoing high risk non cardiac surgery (including LT), albeit suggesting provocative or imaging tests only for patients with low functional heart capacity associated with pre-existing clinical risk factors. In this context, stress echocardiography is advisable, and positive test results should indicate the need for invasive or non-invasive morphologic examinations. However, pharmacological stress echocardiography, performed either with dobutamine or dipyridamole, has given contrasting results in patients with ESLD [Citation15–18]. In these individuals, an altered response to dobutamine and dipyridamole is reported because of autonomic activation, reduced arteriolar tone and a hyperdynamic circulation related to liver failure and/or portal hypertension [Citation19].
Besides provocative tests, the use of coronary computed tomography angiography (CCTA) has been poorly investigated. The high spatial and temporal resolution achieved by current computed tomography allows accurate visualization of the coronary anatomy in many patients [Citation20,Citation21] and appears to be adequate for the identification of CAD in pre-LT patients [Citation22,Citation23].
Considering that the current cardiologic guidelines exclude from any functional tests the majority of ESLD patients without cardiological symptoms and without previous CV events, the aim of the present study was to explore the predictive role of a clinical protocol for the cardiological assessment of ESLD patients who were candidate to LT and were free of CV events and/or symptoms related to any CV pathology.
Methods
Studied population
From 2016 to 2021, all ESLD patients who were candidate to LT underwent cardiological examination, ECG and rest echocardiography. This protocol was submitted to the Ethical Committee ‘Milano Area 2’ Cod. Sper. 3301 and subsequently approved.
Patients with clinical history of heart disease and/or echocardiographic evidence of left ventricular dysfunction or significant valvular abnormalities were evaluated by a multidisciplinary team consisting of clinical hepatologists, transplant surgeons, anaesthesiologists and cardiologists in order to select the appropriate diagnostic and therapeutic strategy to conclude the heart study. Patients without previous cardiological history and with normal clinical assessment were ruled out according to the protocol shown in . All patients aged ≥30 years underwent pharmacological stress echocardiography either dobutamine or dipyridamole infusion; CCTA was performed in patients ≥50 years old with a negative stress echocardiography outcome, who were already diabetics (pharmacologically treated) or carried two or more CV risk factors (reported below). Risk factors that were took into consideration were hypertension (under current pharmacological treatment), smoking (current smoker or former >10 pack years), family history for CAD (CV disease occurring in first-degree relatives aged <55 for males and <65 for females) [Citation4,Citation24] and hypercholesterolaemia (under current hypocholesterolaemic treatment) [Citation25]. In the presence of abnormal stress echocardiography and/or CCTA (evidence of stenosis >50% in at least one main epicardial coronary artery) , patients underwent CAG.
Figure 1. Protocol to assess preoperatory cardiological risk in patients underwent liver transplantation. The number of patients allocated to single tests has been reported in brackets. CAD: coronary artery disease; CAG: coronary angiography; CCTA: coronary computed tomography angiography; CV-RF: cardiovascular risk factors; DM: diabetes mellitus; ECG: electrocardiogram; LT: liver transplantation; PCI: percutaneous coronary intervention; SE: stress echocardiography.
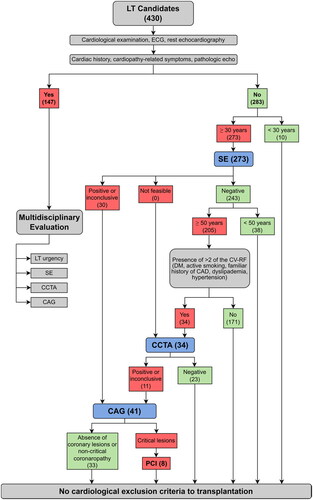
Patients were classified in four CAG-related groups: (1) patients who did not undergo CAG (NO CAG); (2) patients with a normal CAG (CAG NEG); (3) patients with CAG showing non-critical coronaropathy (CAG NCC); (4) patient with CAG showing coronary lesions that were treated by percutaneous coronary intervention (CAG PCI). In each group, patients were sorted according to their liver disease aetiology as hepatitis C virus infection, hepatitis B virus infection, alcoholic liver disease (EtOH), non-alcoholic steatohepatitis and other aetiologies. The presence of hepatocellular carcinoma (HCC) was a primary indication for LT irrespective of the severity of hepatopathy, evaluated through MELD (model for end-stage liver disease) score [Citation26], leading to a sub-stratification of patients.
All LT survivors underwent an annual cardiologic evaluation up to a mean follow-up of 3 years. The endpoints evaluated were (1) all-cause death and (2) major adverse cardiovascular events (MACE) consisting of CV death, hospitalizations for CV causes not related to coronary pathology, hospitalization for coronary revascularization.
For each of the two diagnostic tests used (stress echocardiography and CCTA), sensitivity (Sn), specificity (Sp), positive predictive value (PPV) and negative predictive value (NPV) were determined. Effective number of patients subjected to coronary revascularization was considered as true positive. The absence of MACE over a mean follow-up of 3 years was considered as true negative.
Types of CV therapy – i.e. β-blockers, renin–angiotensin system (RAS) inhibitors, calcium antagonists, anticoagulants and antiplatelets agents – as well as the prevalence of hypercholesterolaemia, hypertension and diabetes were computed and compared before and after LT in the overall population and in patients who experienced events during follow-up (deaths and/or MACE).
Statistical analysis
Age, body mass index (BMI, kg/m2), left ventricular ejection fraction (EF, %), MELD value, were expressed as mean ± standard deviation. Gender distribution, transplantation frequency, the prevalence of CV risk factors, aetiologies of liver disease, number of HCC diagnosis, number of patients treated with β-blockers, RAS inhibitors, calcium antagonists, anticoagulants and antiplatelets agents, were expressed as absolute values and percentage (%).
The number of patients undergoing pharmacological stress echocardiography, CCTA, CAG and PCI was expressed as absolute values and percentage (%). True positives are the transplanted patients who showed pre-LT abnormal results to at least one of the two non-invasive tests and required pre-LT coronary revascularization. True negatives are the transplanted patients who showed pre-LT normal results to non-invasive tests and remained free from MACE over a post-LT mean follow-up of 3 years.
The sensitivity of non-invasive tests is expressed as the percentage value of the ratio of true positives to the total number of subjects who required revascularization by PCI (true positives + false negatives).
The specificity of non-invasive tests is expressed as the percentage value of the ratio of true negatives to the total number of subjects who did not require revascularization by PCI (true negatives + false positives).
The PPV of non-invasive tests is expressed as the percentage of the ratio of true positives to the total number of subjects who presented with a positive instrumental investigation (true positives + false positives). The NPV of non-invasive tests is expressed as the percentage of the ratio of true negatives to the total number of subjects who had a negative instrumental investigation finding (true negatives + false negatives).
Statistical analysis of the prevalence of liver disease aetiologies and CV risk factors in the four CAG-related groups was performed by application of the Chi-square (χ2) test for multiple variables and subsequent post hoc custom tables analysis. Differences in number and type of pharmacological CV therapies and in prevalence of risk factors (hypercholesterolaemia, hypertension and diabetes) in overall population and in patients who experienced events during follow-up were analysed by Student’s t-test.
Distribution of MELD scores and incidence of total deaths and MACE comparing HCC and non-HCC patients were analysed by application of the Chi-square (χ2) test for multiple variables and subsequent post hoc custom tables analysis. Total mortality and MACE of LT recipients are expressed by Kaplan–Meier’s curves for the four CAG-related groups, which have been compared by the log-rank test.
Results
Among a total of 283 ESLD patients listed for LT, 163 (57.6%) received transplantation surgery during the time of the study.
Characteristics of the overall population and of the single CAD-related groups are shown in . The χ2 test for multiple variables showed no significant differences. Ten patients younger than 30 years old were positively listed for transplantation without further clinical assessment. Thus, a total of 273 stress echocardiography (96.5% of the total number of patients), 34 CCTA and 41 CAG were performed, eight of which resulted in revascularization by PCI.
Table 1. Characteristics of the overall population.
According to the non-invasive tests, the indications to CAG are shown in . CAG was performed in 41 subjects (14.5% of the 283 patients) with a mean age of 62 ± 5 years (82.9% male). The positivity to stress echocardiography was 10.6% (30 individuals) and to CCTA was 3.9% (11 individuals). Fourteen patients (4.9%) had lesion-free coronary arteries, 19 patients (6.7%) had non-critical coronary (NCC) disease and eight (2.8%) had CAD requiring revascularization.
Table 2. Indications to coronary angiography according to the non-invasive tests.
Twenty-three out of 41 CAG patients (six treated with PCI) underwent LT.
According to the pre-specified definition of true positive (CAG-PCI) and true negative (no MACE over the follow-up), reports Sn, Sp, PPV and NPV relative to non-invasive tests in patients who underwent LT. Relative to stress echocardiography, Sn was 50%, Sp was 90.2%, PPV was 13.3% and NPV was 98.4%. Pertaining CCTA, Sn was 100%, Sp was 76.7%, PPV was 36.4% and NPV was 100%. Use of dobutamine as a stressor drug was 51%. No difference in predictive powers of different stressors was found.
Table 3. Sensitivity, specificity, positive predictive value and negative predictive value relative to non-invasive tests in patients who underwent liver transplantation.
Among 163 patients who underwent LT, 16 died (9.8%) and 5 experienced MACE (3.1%) over a mean follow-up of 3 years ( and ), . In the NO CAG group, 12 patients died for non-CV causes and one experienced MACE (PCI). In the CAG NEG group, one patient died for non-CV causes and one experienced MACE (hospital admission for congestive heart failure). In the CAG NCC group, one patient died for non-CV causes and 2 experienced MACE (one CV death and one PCI). In the CAG PCI group, one patient died for non-CV causes and one experienced MACE (repeated PCI).
Figure 2. Kaplan–Meier’s curves relative to event-free survival in patients underwent liver transplantation. Patients were divided among the four CAG-related groups as follows: NO CAG: patients not subjected to coronary angiographies; CAG – NEG: negative coronary angiographies; CAG – NCC: non-critical coronaropathy; CAG – PCI: critical coronaropathy requiring revascularization. Censored patients are detailed by markings on the respective curves.
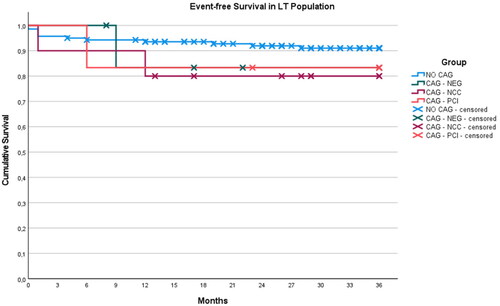
Table 4. Major adverse cardiac events (a) and all-cause mortality (b).
The majority of ESLD patients included in the present study had severe signs of liver dysfunction (high MELD score ()). Among the transplanted patients, 90 (55.2%) were harbouring HCC at listing: MELD score in HCC and non-HCC patients was not significantly different (11.5 ± 5.0 vs. 17.5 ± 6.3). Among HCC patients, 10 (11.1%) died and 2 (2.2%) experienced MACE, whereas among non-HCC, six (8.2%) died and 3 (4.1%) experienced MACE. Also in these latter, there were no statistical differences.
Comparing pre- and post-LT CV risk factors incidence, a significant increment of both diabetes (52.8%, p < .001) and hypertension (35.7%, p < .001) was observed. No changes in hypercholesterolaemia incidence were shown. In the group of patients who died (total mortality) and those who experienced MACE, postoperative increases in both diabetes (25.3%, p < .005) and hypertension (28.1%, p < .001) were observed.
Changes in medical therapies after LT are worth mentioning: β-blockers were discontinued in a high percentage of patients (72.1%, p < .001). Conversely, there were no significant changes in the adherence to antihypertensive therapy with RAS inhibitors and calcium channel blockers (8.3%), and to diuretics (22.6%). After LT, the majority of patients received antiplatelet therapy by acetylsalicylic acid (ASA) as antithrombotic prophylaxis after hepatic vessel reconstruction, a therapy that was not common pre-LT (92.0%, p < .001). Moreover, a small percentage of patients underwent clopidogrel administration (5.1%) due to hepatic arterial stenosis in the majority of cases. Only patients who had a subsequent coronary revascularization (n = 3) received dual antiplatelet therapy (DAPT). In particular, in CAG PCI patients, DAPT was administered only for one month and then clopidogrel was discontinued in all patients regardless of remaining time to transplantation. Before LT, anticoagulation therapy (low molecular weight heparin and/or oral anticoagulants) was administered in a relatively small percentage of patients (16.4%) primarily as prophylaxis in case of pre-LT portal thrombosis and it was found virtually unchanged in the first year after LT, but in part with different clinical indication (eight patients, 4.9%, had a new diagnosis of atrial fibrillation in the follow-up). Relevant differences in treatment strategies were observed in patients who experienced events compared to the general population.
No fatal acute coronary events were reported during the follow-up and total survival did not differ among the four examined groups ( and (b)).
Discussion
A pre-LT cardiological evaluation that relays on a combined use of non-invasive functional tests and imaging techniques is able to identify CAD patients and to reduce the risk of CV mortality and MACE after LT, in an ESLD population without known CV disease. In particular, patients with definite CAD who underwent PCI did not show differences in CV mortality and MACE at 3 years follow-up compared to patients without evidence of CAD.
Despite a high frequency of CV risk factors, the prevalence of identified critical CAD was relatively low (2.8%) in our ESLD population without known heart disease compared to previous studies in patients within the same age (57 ± 10 years) [Citation27,Citation28]. The most recent documents on cardiologic evaluation in ESLD patients scheduled for LT [Citation8,Citation29] reported extreme variability on CAD prevalence (between 3% and 30%) in patients with previous CAD and/or known heart disease. On the contrary, prior to the application of the proposed protocol, which was aimed at identifying CAD and at evaluating deaths and CV events after LT, the CAD prevalence was zero (because of exclusion of patients with previous cardiological history) in an ESLD population without known CV disease. Moreover, related literature included CAD patients independently of specific diagnosis and clinical setting (i.e. non-critical and critical CAD, single and multivessel, prior MI, prior PCI or coronary artery bypass graft – CABG) and no data on diagnostic tests for the assessment of residual ischaemia in CAD patients were reported [Citation30].
The low stress echocardiography sensitivity we found in our study is in line with data reported in previous studies in ESLD patients with a reduced response to pharmacological stimuli evoked by both dobutamine or dipyridamole [Citation17–19,Citation31]. This is a phenomenon due to autonomic activation, reduced arteriolar tone and a hyperdynamic circulation related to liver failure and/or portal hypertension [Citation31]. Moreover, the choice of pharmacological stressor-agent is determined by clinical factors linked to the ESLD. In patients with oesophageal varices, dobutamine administration is not recommended due to the need of discontinuing β-blockers, whereas dipyridamole cannot be administered in patients with asthma or suspected cerebral arteriopathy. Thus, stress echocardiography is not fully reliable, unless supported by other diagnostic methods. The use of different forms of provocative testing, such as those involving physical exertion (exercise echocardiography, ECG or myocardial scintigraphy), is not widely applicable in ESLD patients (less than 1%) [Citation29], as they often have a low exercise capacity.
According to the proposed protocol, the additional use of CCTA (as a non-invasive approach) was justified by the known high sensitivity. Specifically, CCTA sensitivity and the NPV were very high, whereas specificity and PPVs were relatively low. CCTA does not clearly discriminate the extent (critical or non-critical) of the CAD, but identifies the absence of coronary atherosclerosis [Citation1,Citation32]. According to the CAT-CAD trial (computed tomography as the first-choice diagnostics in high pre-test probability of coronary artery disease), CCTA might reduce the number of non-actionable coronary angiographies in patients with indications to CAG. According to this trial, CCTA resulted to be an effective and safe, non-invasive, outpatient-based and cost-effective alternative to CAG for patients with a high clinical odds of obstructive CAD [Citation33–35]. Furthermore, in the case of intermediate lesions, CCTA-derived fractional flow reserve can assess flow limitation across coronary stenosis with high diagnostic accuracy and good correlation to invasive fractional flow reserve [Citation36,Citation37]. Based on this finding, the assessment of the fractional flow reserve by CCTA may represent an add-on value in the evaluation of CV risk in patients selected to LT.
The low PPV for stress echocardiography and CCTA increases the need of CAG to exclude the presence of CAD in patients with a priori low probability of disease. This explains the high prevalence of normal angiography and non-critical disease (n = 33) in patients assessed through CAG. Nonetheless, the effective identification of patients free from CAD (true negatives) by the non-invasive tests can be extremely relevant in reducing the risk of LT-related cardiac events, and therefore the risk of ‘over-using’ CAG to identify true positives.
Owing to reasons related to the inherited nature of protocol, the population undergoing CCTA had a higher theoretical CV risk related to a higher prevalence of arterial hypertension, hypercholesterolaemia, smoking, diabetes and family history of CV disease. The prevalence of the main CV risk factors was as follows: diabetes mellitus (32.2%), hypertension (34.3%), dyslipidaemia (4.6%) and active smoking (29.3%). These data are in line with those reported in the literature, with diabetes ranging from 25% to 33% and hypertension ranging from 31% to 43%, thus indicating that the CV risk factor prevalence in our cohort can be representative of the general ELSD population [Citation38,Citation39].
It is worth mentioning that the prevalence of risk factors in patients with critical coronary disease (CAG PCI) was higher than the population included in the other groups without critical CAD, as shown in . Diabetes was found in 50%, hypertension in 75%, dyslipidaemia in 25% and active smoking in 25% of CAG PCI patients, thus suggesting a pathogenetic effect of these risk factors on coronary atherosclerosis progression in ESLD population. According to the aetiology of liver disease, the CAD prevalence is generally distributed in ESLD patients. Indeed, among CAG PCI patients, 37.5% presented an EtOH aetiology. No correlation was found with MELD score. In those patients who experienced events, the incidence of new-onset diabetes and hypertension post-LT was similar to the one belonging to the general population. Since modifications in the therapy were similar in all four groups after LT, we hypothesized that this was not a bias influencing outcome at 3 years.
According to the most recent 2022 ESC protocol for the cardiological assessment of patients to be referred for non-cardiac surgery [Citation1], LT is listed as a high-risk surgical procedure. It is suggested that high-risk non-cardiac surgery candidates should only be evaluated by provocative (e.g. stress echocardiography) or advanced non-invasive imaging tests (e.g. CCTA) if they have a low functional class (≤4 METS, metabolic equivalent of tasks) and/or the presence of clinical factors such as heart failure, history of CAD, history of stroke or transient ischemic attack , renal insufficiency (GFR < 60 mL/min), diabetes mellitus on insulin therapy. This approach is consistent with our design protocol, which was hypothesized in 2016. However, in our studied population, no patients had severe clinical factors proposed by the ESC guidelines, thus, hypothetically none of the 283 screened patients would have undergone any provocative or non-invasive imaging tests and therefore the identification of subjects with CAD would have not been possible. For this reason, the ESC protocol might be useful for the evaluation of CV risk in patients undergoing high-risk abdominal surgery, although it does not appear adequate to screen ESLD patients. Indeed, LT cannot be equated to a generic abdominal operation since the correct identification of the patient for the organ allocation must be considered in addition to the intrinsic surgical risk for the patient. In the last years and in agreement with our hypothesis, several surgeons have proposed pre-LT cardiological evaluation protocols which require an extensive diagnostic approach based on provocative testing [Citation40–44].
These results should be interpreted within the context of potential limitations: (1) despite the number of patients included in our protocol, this study was not powered ‘a priori’ for the outcome explored (this is a non-randomized control study). The total number of patients evaluated is not enough to define the real prevalence of CAD in ESLD patients, but the extensive characterization of patients can overcome this drawback in this peculiar clinical setting; (2) in relation to the results observed, the use of CCTA would be appropriate, when clinically possible, in all ESLD subjects screened for LT, regardless of the risk factors; (3) the lack of sensitivity found by SE is compensated by the high sensitivity of CCTA, although the positive predictive power remains low for both methods. The clinical consequence is the relatively high number of patients positive for both methods who were sent for CAG and in whom critical CAD requiring revascularization was not detected (CAG NEG group); (4) although our study did not evaluate costs associated with diagnostic procedures in relation to potential clinical benefits, this specific cardiologic diagnostic work-up seems justifiable because it increases the probability of LT uncomplicated outcome. While LT is one of the most expensive surgical and medical procedure and the proposed protocol is time and cost demanding, this diagnostic strategy, in which the identification of true negative subjects was the main goal, allows reducing the risk of perioperative and postoperative CV events.
In conclusion, the application of the present protocol in a ESLD population without evidence of previous heart disease demonstrated a reduction in the incidence of post-LT acute coronary events in patients with a theoretic high CV risk. This justifies the usefulness of two non-invasive methods to identify CAD, namely, a functional and an imaging test. The lack in sensitivity found by SE is compensated by the high sensitivity demonstrated by CCTA, although the PPV remains low for both techniques. The CAG without evidence of significant coronary lesions, even after positivity of stress echocardiography and/or CCTA and/or in the presence of CV risk factors, indicates that a single non-invasive method is not sufficient to identify CAD in ESLD patients. Conversely, application of both methods allows a more correct identification of subjects without CAD (true negatives).
Author contributions
LM and NB conceived and drafted the manuscript and conducted the cardiological tests. AF, FC, LD and FS performed the cardiological tests. NB analysed the data. MV, AZ, MR and SC drafted and critically reviewed the manuscript. BA, LC, EC, CD, MFD, AF, MAI, PL, CM, FP, EQ, CS, GT, CV and PV took care of pre- and post-transplantation management and performed liver transplantation. All the authors approved the version to be published. All authors agree to be accountable for all aspects of the work.
Ethical approval
Protocol is submitted to the Ethical Committee ‘Milano Area 2’ Cod. Sper. 3301.
Consent form
Relative to provocative testing, all patients signed an informed consent and the work was carried out in accordance with the Helsinki Declaration.
Disclosure statement
All the authors declare no conflict of interest or have no financial disclosure.
Additional information
Funding
References
- Halvorsen S, Mehilli J, Cassese S, et al. 2022 ESC guidelines on cardiovascular assessment and management of patients undergoing non-cardiac surgery. Eur Heart J. 2022;43(39):1–11. doi: 10.1093/eurheartj/ehac270.
- Lee MS. Diagnostic yield of coronary angiography in asymptomatic orthotopic liver transplantation candidates. Cardiovasc Revasc Med. 2022;35:59–63. doi: 10.1016/j.carrev.2021.03.004.
- Lekerika N, Gutierrez Rico RM, Arco Vazquez J, et al. Predicting fluid responsiveness in patients undergoing orthotopic liver transplantation: effects on intraoperative blood transfusion and postoperative complications. Transplant Proc. 2014;46(9):3087–3091. doi: 10.1016/j.transproceed.2014.10.005.
- Palanisamy AP, Nadig SN, Chedister GR, et al. Use of intra-aortic counterpulsation in cardiogenic shock post-liver transplantation. Clin Transplant. 2017;31(7). doi: 10.1111/ctr.13002.
- Lee SS. Cardiac abnormalities in liver cirrhosis. West J Med. 1989;151(5):530–535.
- Tarantini G, Nai Fovino L. TAVR versus SAVR in patients with severe aortic stenosis and concomitant end stage liver disease: when less is more. Catheter Cardiovasc Interv. 2020;96(4):956–957. doi: 10.1002/ccd.29291.
- Johnston SD, Morris JK, Cramb R, et al. Cardiovascular morbidity and mortality after orthotopic liver transplantation. Transplantation. 2002;73(6):901–906. doi: 10.1097/00007890-200203270-00012.
- Darstein F, Hoppe-Lotichius M, Vollmar J, et al. Pretransplant coronary artery disease is a predictor for myocardial infarction and cardiac death after liver transplantation. Eur J Intern Med. 2018;51:41–45. doi: 10.1016/j.ejim.2017.12.001.
- Schoening WN, Buescher N, Rademacher S, et al. Twenty-year longitudinal follow-up after orthotopic liver transplantation: a single-center experience of 313 consecutive cases. Am J Transplant. 2013;13(9):2384–2394. doi: 10.1111/ajt.12384.
- Kwong AJ, Ebel NH, Kim WR, et al. OPTN/SRTR 2020 annual data report: liver. Am J Transplant. 2022;22(Suppl. 2):204–309. doi: 10.1111/ajt.16978.
- Kutkut I, Rachwan RJ, Timsina LR, et al. Pre-liver transplant cardiac catheterization is associated with low rate of myocardial infarction and cardiac mortality. Hepatology. 2020;72(1):240–256. doi: 10.1002/hep.31023.
- Madhwal S, Atreja A, Albeldawi M, et al. Is liver transplantation a risk factor for cardiovascular disease? A meta-analysis of observational studies. Liver Transpl. 2012;18(10):1140–1146. doi: 10.1002/lt.23508.
- Nayagam JS, Norton BC, Belete S, et al. Invasive coronary angiography as a tool in cardiac evaluation for liver transplant candidates. J Liver Transplant. 2022;7:100100. doi: 10.1016/j.liver.2022.100100.
- Murray KF, Carithers RLJr., AASLD. AASLD practice guidelines: evaluation of the patient for liver transplantation. Hepatology. 2005;41(6):1407–1432. doi: 10.1002/hep.20704.
- Doytchinova AT, Feigenbaum TD, Pondicherry-Harish RC, et al. Diagnostic performance of dobutamine stress echocardiography in end-stage liver disease. JACC Cardiovasc Imaging. 2019;12(11 Pt 1):2115–2122. doi: 10.1016/j.jcmg.2018.10.031.
- Findlay JY, Keegan MT, Pellikka PP, et al. Preoperative dobutamine stress echocardiography, intraoperative events, and intraoperative myocardial injury in liver transplantation. Transplant Proc. 2005;37(5):2209–2213. doi: 10.1016/j.transproceed.2005.03.023.
- Umphrey LG, Hurst RT, Eleid MF, et al. Preoperative dobutamine stress echocardiographic findings and subsequent short-term adverse cardiac events after orthotopic liver transplantation. Liver Transpl. 2008;14(6):886–892. doi: 10.1002/lt.21495.
- Kim MY, Baik SK, Won CS, et al. Dobutamine stress echocardiography for evaluating cirrhotic cardiomyopathy in liver cirrhosis. Korean J Hepatol. 2010;16(4):376–382. doi: 10.3350/kjhep.2010.16.4.376.
- Tsutsui JM, Mukherjee S, Elhendy A, et al. Value of dobutamine stress myocardial contrast perfusion echocardiography in patients with advanced liver disease. Liver Transpl. 2006;12(4):592–599. doi: 10.1002/lt.20651.
- Stocker TJ, Deseive S, Leipsic J, et al. Reduction in radiation exposure in cardiovascular computed tomography imaging: results from the PROspective multicenter registry on radiaTion dose estimates of cardiac CT angIOgraphy in daily practice in 2017 (PROTECTION VI). Eur Heart J. 2018;39(41):3715–3723. doi: 10.1093/eurheartj/ehy546.
- Paech DC, Weston AR. A systematic review of the clinical effectiveness of 64-slice or higher computed tomography angiography as an alternative to invasive coronary angiography in the investigation of suspected coronary artery disease. BMC Cardiovasc Disord. 2011;11:32. doi: 10.1186/1471-2261-11-32.
- Steinkohl F, Barbieri F, Senoner T, et al. Coronary atherosclerosis profile in patients with end-stage liver disease prior to liver transplantation due to alcoholic fatty liver: a coronary CTA study. Eur Radiol. 2021;31(1):494–503. doi: 10.1007/s00330-020-07037-8.
- Loffler AI, Gonzalez JA, Sundararaman SK, et al. Coronary computed tomography angiography demonstrates a high burden of coronary artery disease despite low-risk nuclear studies in pre-liver transplant evaluation. Liver Transpl. 2020;26(11):1398–1408. doi: 10.1002/lt.25869.
- Montalescot G, Sechtem U, Achenbach S, et al. ESC guidelines on the management of stable coronary artery disease: the task force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34(38):2949–3003.
- Bosner S, Haasenritter J, Becker A, et al. Ruling out coronary artery disease in primary care: development and validation of a simple prediction rule. CMAJ. 2010;182(12):1295–1300. doi: 10.1503/cmaj.100212.
- Morgul MH, Felgendreff P, Kienlein A, et al. Milan criteria in the MELD era—is it justifiable to extend the limits for orthotopic liver transplantation? World J Surg Oncol. 2020;18(1):158. doi: 10.1186/s12957-020-01932-6.
- Malik MU, Russell SD, Pustavoitau A, et al. The predictors of post-transplant coronary events among liver transplant recipients. Hepatol Int. 2016;10(6):974–982. doi: 10.1007/s12072-016-9742-5.
- Dangl M, Eisenberg T, Grant JK, et al. A comprehensive review of coronary artery disease in patients with end-stage liver disease. Transplant Rev. 2022;36(3):100709. doi: 10.1016/j.trre.2022.100709.
- Case BC, Yang M, Qamer SZ, et al. Pre-operative cardiovascular testing before liver transplantation. Am J Cardiol. 2021;152:132–137. doi: 10.1016/j.amjcard.2021.04.012.
- An J, Shim JH, Kim SO, et al. Prevalence and prediction of coronary artery disease in patients with liver cirrhosis: a registry-based matched case-control study. Circulation. 2014;130(16):1353–1362. doi: 10.1161/CIRCULATIONAHA.114.009278.
- Harinstein ME, Flaherty JD, Ansari AH, et al. Predictive value of dobutamine stress echocardiography for coronary artery disease detection in liver transplant candidates. Am J Transplant. 2008;8(7):1523–1528. doi: 10.1111/j.1600-6143.2008.02276.x.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130(24):2215–2245. doi: 10.1161/CIR.0000000000000105.
- Rudziński PN, Kruk M, Kępka C, et al. The value of coronary artery computed tomography as the first-line anatomical test for stable patients with indications for invasive angiography due to suspected coronary artery disease: CAT-CAD randomized trial. J Cardiovasc Comput Tomogr. 2018;12(6):472–479. doi: 10.1016/j.jcct.2018.08.004.
- Rudziński PN, Kruk M, Kępka C, et al. Assessing the value of coronary artery computed tomography as the first-line anatomical test for stable patients with indications for invasive angiography due to suspected coronary artery disease. Initial cost analysis in the CAT-CAD randomized trial. J Cardiovasc Comput Tomogr. 2020;14(1):75–79. doi: 10.1016/j.jcct.2019.07.008.
- Rudziński PN, Kruk M, Demkow M, et al. Efficacy and safety of coronary computed tomography angiography in patients with a high clinical likelihood of obstructive coronary artery disease. Kardiol Pol. 2022;80(1):56–63. doi: 10.33963/KP.a2021.0185.
- Cook CM, Petraco R, Shun-Shin MJ, et al. Diagnostic accuracy of computed tomography-derived fractional flow reserve: a systematic review. JAMA Cardiol. 2017;2(7):803–810. doi: 10.1001/jamacardio.2017.1314.
- Tang CX, Qiao HY, Zhang XL, et al. Functional CAD-RADS using FFR(CT) on therapeutic management and prognosis in patients with coronary artery disease. Eur Radiol. 2022;32(8):5210–5221. doi: 10.1007/s00330-022-08618-5.
- Nicolau-Raducu R, Gitman M, Ganier D, et al. Adverse cardiac events after orthotopic liver transplantation: a cross-sectional study in 389 consecutive patients. Liver Transpl. 2015;21(1):13–21. doi: 10.1002/lt.23997.
- Główczyńska R, Galas M, Witkowska A, et al. The pre-transplant profile of cardiovascular risk factors and its impact on long-term mortality after liver transplantation. Ann Transplant. 2018;23:591–597. doi: 10.12659/AOT.908771.
- Martin P, DiMartini A, Feng S, et al. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the study of liver diseases and the American Society of Transplantation. Hepatology. 2014;59(3):1144–1165. doi: 10.1002/hep.26972.
- European Association for the Study of the Liver. EASL clinical practice guidelines: liver transplantation. J Hepatol. 2016;64(2):433–485.
- VanWagner LB, Harinstein ME, Runo JR, et al. Multidisciplinary approach to cardiac and pulmonary vascular disease risk assessment in liver transplantation: an evaluation of the evidence and consensus recommendations. Am J Transplant. 2018;18(1):30–42. doi: 10.1111/ajt.14531.
- Cheng XS, VanWagner LB, Costa SP, et al. Emerging evidence on coronary heart disease screening in kidney and liver transplantation candidates: a scientific statement from the American Heart Association: endorsed by the American Society of Transplantation. Circulation. 2022;146(21):e299–e324. doi: 10.1161/CIR.0000000000001104.
- McCarthy KJ, Motta-Calderon D, Estrada-Roman A, et al. Introduction of a standardized protocol for cardiac risk assessment in candidates for liver transplant – a retrospective cohort analysis. Ann Hepatol. 2022;27(2):100582. doi: 10.1016/j.aohep.2021.100582.
