 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objectives
We aimed to investigate clinical uncertainties by characterizing the accuracy and utility of commercially available antibodies of Mycobacterium tuberculosis in the diagnostic assessment of suspected tuberculosis in high-burden countries.
Methods
We conducted a retrospective, descriptive, cohort study among participants aged ≥ 18 years with suspected tuberculosis in Nanning, Guangxi, and China. Participants were tested for M. tuberculosis infection using commercially available antibodies against Mycobacterum tuberculosis. Specificity, sensitivity, negative and positive predictive values, and negative and positive likelihood ratios of the tests were determined. Sputum specimens and bronchoalveolar lavage fluid were sent for mycobacterial culture, Xpert MTB/RIF assay, and cell-free M. tuberculosis DNA or RNA assay. Blood samples were used for IGRAs, T-cell counts (CD3 + CD4+ and CD3 + CD8+), and antibodies to tuberculosis test.
Results
Of the 1857 participants enrolled in this study, 1772 were included in the analyses, among which, 1311 were diagnosed with active tuberculosis. The specificity of antibody against 16kD for active tuberculosis was 92.7% (95% confidence interval [CI]: 89.3–95.4) with a positive likelihood ratio for active tuberculosis cases of 3.1 (95% CI: 2.1–4.7), which was higher than that of antibody to Rv1636 (90.5% [95% CI: 86.6–93.5]), antibody to 38kD (89.5% [95% CI: 85.5–92.7]), antibody against CFP-10 (82.6% [95% CI: 77.9–86.7]), and antibody against LAM (79.3% [95% CI: 74.3–83.7]). Sensitivity ranged from 15.8% (95% CI: 13.9–17.9) for antibody against Rv1636 to 32.9% (95% CI: 30.4–35.6) for antibody to LAM.
Conclusions
Commercially available antibodies against to Mycobacterium tuberculosis do not have sufficient sensitivity for the diagnostic evaluation of active tuberculosis. However, antibody against Rv1636 and 16kD may have sufficiently high specificities, high positive likelihood ratios, and correspondingly high positive predictive values to facilitate the rule-in of active tuberculosis.
HIGHLIGHTS
Existing M. tuberculosis antigens do achieve a limited sensitivity and negative predictive value to rule out a diagnosis of tuberculosis.
M. tuberculosis antigens may help to rule in a diagnosis of active or latent tuberculosis in clinical setting among the high burden tuberculosis countries.
This study is the largest retrospective, descriptive, cohort study to evaluate the clinical utilization of existing M. tuberculosis antigens integrating M. tuberculosis immunogens in patients with suspected active tuberculosis in high-burden country.
KEY MESSAGES
Existing antibodies against M. tuberculosis antigens exhibit limited sensitivity for ruling out TB.
Multiple TB antibodies against M. tuberculosis antigens may aid in identifying cases of active or latent tuberculosis.
The biggest cohort for investigating clinical applications of TB antibodies.
1. Introduction
Accurate diagnosis and prompt treatment of active tuberculosis are paramount for limiting further transmission of the tuberculosis disease, preventing progression to severe complications, and reducing mortality [Citation1–3]. However, the diagnostic evaluation of suspected tuberculosis is lengthy and may require up to 2 months. Moreover, it is costly, which causes a significant economic burden due to misdiagnoses. Thus, the diagnostic evaluation is burdensome, wherein incorrect treatment may increase adverse outcomes for patients and healthcare systems, frequently leading to significant delays in the diagnosis and treatment of other diseases before tuberculosis is eventually excluded. Consequently, precision diagnostic approaches related to tuberculosis remain a priority for public health research in high-burden countries. Over the past 2 decades, rapidly developing tuberculosis diagnosis and antibiotic susceptibility testing in molecular measurements, such as GeneXpert MTB/RIF (GeneXpert, Sunnyvale, California, USA) [Citation3,Citation4], have increased the speed and specificity of microbiological diagnosis, while molecular-based diagnostic methods do not achieve sufficient sensitivity to rule out tuberculosis [Citation5,Citation6].
Although serological tests of tuberculosis were not ecommended by World Health Organization (WHO) [Citation7], serological antibody detection for M. tuberculosis-specific antigens, which might facilitate addressal of unfulfilled clinical urgency with high diagnostic sensitivity and specificity [Citation8], were encouraged to be prioritized by WHO [Citation9]. Given that most patients are verified as cases of bacterial and/or nucleic acids-negative tuberculosis [Citation10,Citation11], such an immunodiagnostic test would be advantageous in high-burden countries. This active tuberculosis rapid test is based on the detection of immune responses to M. tuberculosis antigens rather than the direct measurement of M. tuberculosis or nucleic acids. Serological tests that measure the IgG antibodies to strongly immunogenic and highly specific M. tuberculosis antigens, such as ESAT-6 and CFP-10 antigens that are absent in the case of prior Bacille Calmette-Guérin (BCG) vaccination, might be reliable routine diagnostic procedures used for distinguishing M. tuberculosis infections and BCG vaccination [Citation12]. M. tuberculosis LAM and 38kD antigens are associated with active tuberculosis, and the antigen-specific antibody responses achieve high diagnostic specificity in the differential diagnosis between active and latent tuberculosis infections [Citation13].
Highly specific M. tuberculosis antigens can be used as strongly immunogenic antigens, which presents an opportunity to develop serological tests with higher sensitivity and specificity for the rapid detection of bacteria- or nucleic acid-negative patients with tuberculosis within clinical settings. Over the past 2 decades, evidence from prior studies indicates that several promising candidates’ immunogenic antigens have been identified and the adjustment of existing serological tests with these antigens is practicable [Citation14–17]. A large number of commercial antibody diagnosis tests have been developed and evaluated [Citation13,Citation18,Citation19], however, the diagnostic accuracy of commercial immunological tests based on specific antigen and antibody reactions varies widely, and no large-scale clinical estimation of this diagnostic approach has been performed in clinical practice. Thus, we aimed to evaluate the clinical utilization of existing M. tuberculosis 16kD, 38kD, LAM, CFP-10, and Rv1636 antigens, and the bacteriological specificity of each antigen in patients with suspected active tuberculosis in The Fourth People’s Hospital of Nanning.
2. Materials and methods
2.1. Study design and participants
We conducted a retrospective, descriptive, cohort study based on prospectively collected and analysed data from clinical practice in Nanning, China. The inclusion criteria were as follows: (i) admitted to The Fourth People’s Hospital of Nanning between March 2021 and February 2022, (ii) presence of clinical findings and chest radiographic features that suggested pulmonary infection. Venous blood was sampled within the first 24 h after admission from admitting to TB clinics. (iii) underwent examinations for tuberculosis with subsequent hospitalisation for treatment, and a follow-up of at least 2 months, and (iv) age > 18 years. Out-patients and inpatients lost to follow-up were excluded, and duplicate examinations were only counted once. A three-month follow-up visit was carried out to evaluate the reaction to anti-tuberculosis therapies in light of symptoms and radiographic evidence.
Research involving human subjects complied with all relevant national regulations, institutional policies and is in accordance with the tenets of the Helsinki Declaration (as revised in 2013), and has been approved by The Fourth People’s Hospital of Nanning Ethics Committee.
2.2. Data collection
All participants were followed up for at least 3 months, with data collected on epidemiological, clinical, test results, and final diagnoses. Data were collected from hospital electronic medical records.
2.3. Diagnostic categories
All cases were investigated in routine clinical practice by an infectious disease specialist, along with the follow-up in this setting. The clinical diagnoses of all participants were confirmed by more than five infectious disease physicians specializing in tuberculosis. Diagnoses of all patients were divided into seven groups (). The seven groups were as follows. 1 A: smear-suggestive of tuberculosis, 1B: culture-positive tuberculosis, 1 C: nuclei acid-positive tuberculosis, 1D: histopathology-suggestive of tuberculosis, 2: latent pulmonary tuberculosis, 3: nontuberculosis mycobacteria, and 4: community acquired pneumonia.
2.4. Laboratory procedure
Blood samples (5 mL) were collected from all cases in blood collection tubes without anticoagulants. The tubes were centrifuged at 3,500 rpm for 10 min at 25 °C using a TD5–2 centrifuge (BEILI, Beijing, China, http://www.yinghuajiamei.com/). M. tuberculosis-specific antibodies were detected using the Tuberculosis Antibody (IgG) Detection Kit (protein chip assays) (Puruikang, Shenzhen, China, http://szprk.com/) and analysed using the SMART 80 automatic chemiluminescence immunoassay analyser (KEYSMILE, Chongqing, China, https://www.key-smile.com/) according to the manufacturer’s instructions.
Twenty microliters of CD3-FITC/CD8-PE/CD45-PerCP/CD4-APC antibody solutions (BD Biosciences) were added to BD Trucount tubes, followed by fifty microliters EDTA-anticoagulated whole blood. The solution was mixed by vortexing and incubated for 15 min at 20–25 °C, protected from the light. Subsequently, 450 µl FACS lysing solution was added and mixed well. The solution was incubated for another 10 min at 20–25 °C, protected from the light. CD3, CD4, and CD8 cells were counted using BD FACSVia or BD FACSCalibur Flow Cytometer (BD Biosciences). The reference interval for CD3 cell is 690–2540/µl, CD4 cell is 410–1590/µl, and CD8 cell is 190–1140/µl.
More details of the methods are in the Supplemental Material.
2.5. Statistical analysis
A statistical power analysis was conducted to calculate the sample size based on the minimum positive rates of antibody against 38kD between the culture-confirmed tuberculosis subgroup (19%) and the community-acquired pneumonia subgroup (10%); 105 participants were required at the 5% significance level (two-tailed) with 80% power. Sensitivity, specificity, positive and negative predictive values, and positive and negative likelihood ratios with 95% confidence intervals (CIs) were calculated using the 2 × 2 contingency table diagnostic test based on the pathogenicity and histopathology diagnosis as the reference standard. Relative sensitivity and specificity with 95% CIs, derived from the diagnostic test post-estimation procedure, were calculated using the delta method. To evaluate diagnostic performance of multiple antigens, logistic regression was modeled. Statistical analyses were conducted using MedCalc statistical software (version 15.8). Differences were considered statistically significant at p < 0.05.
3. Results
3.1. Demographic and clinical characteristics
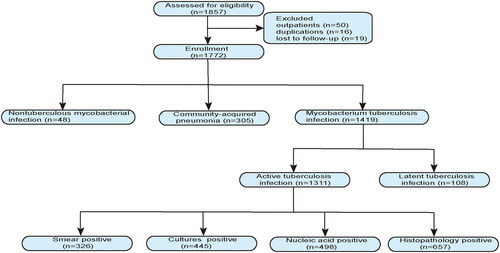
Between 1 June 2021 and 31 May 2022, 1857 patients with suspected tuberculosis were included in the study. The inclusion and exclusion criteria are shown in the flow diagram (). Finally, 85 patients were excluded, resulting in a cohort of 1772 patients.
Figure 1. Flow diagram of the participants in included in this study. Active tuberculosis: microbiology etiological or histopathology diagnosis, clinical findings, and chest radiographic features suggestive of tuberculosis. Patients successfully treated with anti-tuberculosis therapy. Latent pulmonary tuberculosis: stable chest radiographic outcome, bacteriologically and molecular biology negative, and no clinical evidence of active tuberculosis. Patients had a history of tuberculosis infection and were cured. Nontuberculous mycobacteria: microbiological culture or molecular biological identification of nontuberculous mycobacteria. Community acquired pneumonia: etiology of M. tuberculosis and nontuberculous mycobacteria negative, exclusion of tumor, tracheobronchitis, pulmonary embolus, non-infectious pulmonary disease, and clinical findings and chest radiographic features typical community acquired pneumonia.
The demographic, clinical, and laboratory data of the final cohort of the study participants are summarized in . The median age of the cohort was 58 years (interquartile range: 45–68). More than half of the patients were male, and half of them were from rural areas. The most prevalent infectious comorbidity was human immunodeficiency virus (HIV) infection, which was reported in 264 (15%) participants, and the most frequent non-infectious comorbidity was hypertension, which was reported in 343 (19%) patients. Cough, which was reported in 1214 (69%) of 1772 cases, was the most common clinical sign.
Table 1. Demographics and clinical characteristics of patients.
A total of 1311 (74%) patients had a final diagnosis of active tuberculosis (categories 1 A-D, ). Of these, 326 (25%) were diagnosed with smear-positive tuberculosis, 445 (34%) with culture-positive tuberculosis, 498 (38%) with nucleic acid-positive tuberculosis, 657 (50%) with histopathologically-suggestive of tuberculosis, smear and cultures positive (n = 239), smear and nucleic acid positive (n = 212), cultures and nucleic acid positive (n = 328), and at least one of smear, cultures, or nucleic acid positive (n = 1269). All patients with culture-positive tuberculosis underwent drug resistance phenotype testing based on M. tuberculosis isolates, and 132 of the 498 patients with nucleic acid-positive tuberculosis underwent antibiotic resistance genotyping based on M. tuberculosis nucleic acid molecules. Of the 132 patients, 21 (16%) were identified with isoniazid resistance (mainly katG 315 isoniazid mutations) and 18 (14%) were identified with rifampicin resistance (most were located in rpoB 529-533). Moreover, 9 (7%) patients had multiple resistance to M. tuberculosis infection. Only 48 (3%) of the 1772 patients were diagnosed with nontuberculous mycobacterial infections (category 3). The remaining 305 (17%) of the 1772 patients, were ultimately diagnosed with community-acquired pneumonia (category 4).
Table 2. Predefined diagnostic criteria for case definitions and categories.
3.2. Diagnostic characteristics of antibodies against to M. tuberculosis in active tuberculosis
Results of antibodies reactivity to multiple M. tuberculosis antigens, including CFP-10, 16kD, LAM, Rv1636, and 38kD, were available for 1772 participants. The diagnostic sensitivity, specificity, negative and positive predictive values, and negative and positive likelihood ratios are summarized in . The sensitivity of antibody against CFP-10 was 31.9% (95% CI: 29.4–34.5%) for active tuberculosis diagnoses, giving a positive predictive value (PPV) of 88.8% (95% CI: 85.5–91.5%) and a positive likelihood value (PLV) of 1.83 (95% CI: 1.42–2.37) for active tuberculosis cases. The specificity of antibody reactivity to CFP-10 was 82.6% (95% CI: 77.9–86.7%) for active tuberculosis cases, giving a negative predictive value (NPV) of 22.0% (95% CI: 19.6–24.5%) and a negative likelihood value (NLV) of 0.82 (95% CI: 0.77–0.88) for patients with active tuberculosis. The sensitivity of antibody against LAM was 32.9% (95% CI: 30.4–35.6%) for active tuberculosis diagnoses, giving a PPV of 87.3% (95% CI: 84.0–90.1%) and a PLV of 1.60 (95% CI: 1.26–2.01) for active tuberculosis cases. The specificity of antibody reactivity to LAM was 79.3% (95% CI: 74.4–83.7%) for active tuberculosis cases, giving a NPV of 21.6% (95% CI: 19.2–24.1%) and a NLV of 0.85 (95% CI: 0.79–0.91) for patients with active tuberculosis. The sensitivity of antibody against to 38kD was 17.7% (95% CI: 15.7–19.9%) for active tuberculosis diagnoses, giving a PPV of 87.9% (95% CI: 83.3–91.6%) and a PLV of 1.69 (95% CI: 1.19–2.39) for active tuberculosis cases. The specificity of antibody against to 38kD was 89.5% (95% CI: 85.5–92.7%) for active tuberculosis cases, giving a NPV of 20.2% (95% CI: 18.1–22.4%) and a NLV of 0.92 (95% CI: 0.88–0.96) for patients with active tuberculosis. The sensitivity of antibody against 16KD was 16.7% (95% CI: 10.2–25.1%) for active tuberculosis diagnoses, giving a PPV of 78.3% (95% CI: 56.3–92.5%) and a PLV of 1.30 (95% CI: 0.52–3.26) for active tuberculosis cases. The specificity of antibody reactivity to 16KD was 87.2% (95% CI: 72.6–95.7%) for active tuberculosis cases, giving a NPV of 27.4% (95% CI: 19.8–63.2%) and a NLV of 0.96 (95% CI: 0.83–1.11) for patients with active tuberculosis. The sensitivity of antibody reactivity to Rv1636 was 15.8% (95% CI: 13.9–17.9%) for active tuberculosis diagnoses, giving a PPV of 87.8% (95% CI: 82.8–91.6%) and a PLV of 1.66 (95% CI: 1.15–2.40) for active tuberculosis cases. The specificity of antibody against Rv1636 was 90.5% (95% CI: 86.6–93.5%) for active tuberculosis cases, giving a NPV of 20.0% (95% CI: 17.9–22.1%) and a NLV of 0.93 (95% CI: 0.89–0.97) for patients with active tuberculosis.
Table 3. Diagnostic accuracy of commercially available probe of Mycobacterium tuberculosis antigens for diagnosis of active tuberculosis.
The sensitivity and specificity of antibody against LAM antigen was superior to those of Rv1636; the relative sensitivity was 2.09 (95% CI: 1.76–2.48, p < 0.0001), and the relative specificity was 2.17 (95% CI: 1.38–3.50, p = 0.0004). Compared with antibody against Rv1636, CFP-10 tended to outperform the relative sensitivity (2.02 [95% CI: 1.71–2.40], p < 0.0001) and the relative specificity (1.83 [95% CI: 1.14–2.98], p = 0.008). No significant differences were observed between antibodies against to LAM and CFP-10. Compared with antibody against 38kD, the relative sensitivity (1.27 [95% CI: 1.07–1.52], p = 0.0061) of 16kD was outperformed, whereas no significant differences were observed in the relative specificity.
3.3. Diagnostic characteristics of antibodies against to M. tuberculosis in rural populations
Given the substantial tuberculosis burden among rural populations (rural vs. urban residents, 62% vs. 38%; p < 0.0001), separate extensive analyses were performed for the rural population subgroup. The sensitivity of antibody against CFP-10 was 41.5% (95% CI: 36.7–46.5%) for active tuberculosis, giving a PPV of 76.1% (95% CI: 70.0–81.6%) and a PLV of 1.27 (95% CI: 0.99–1.63) for active tuberculosis cases. The specificity of antibody against CFP-10 was 67.3% (95% CI: 59.5–74.4%) for active tuberculosis cases, giving a NPV of 31.4% (95% CI: 26.6–36.6%) and a NLV of 0.87 (95% CI: 0.76–0.99) for patients with active tuberculosis. The sensitivity of antibody against LAM was 41.3% (95% CI: 36.5–46.2%) for active tuberculosis, giving a PPV of 72.7% (95% CI: 66.5–78.4%) and a PLV of 1.06 (95% CI: 0.85–1.33) for active tuberculosis cases. The specificity of antibody against to LAM was 61.1% (95% CI: 53.2–68.7%) for active tuberculosis cases, giving a NPV of 29.3% (95% CI: 24.5–34.5%) and a NLV of 0.96 (95% CI: 0.83–1.11) for patients with active tuberculosis. The sensitivity of antibody reactivity to Rv1636 was 19.4% (95% CI: 15.7–23.6%) for active tuberculosis, giving a PPV of 73.2% (95% CI: 63.8–81.2%) and a PLV of 1.08 (95% CI: 0.74–1.59) for active tuberculosis cases. The specificity of antibody reactivity to Rv1636 was 82.1% (95% CI: 75.3–87.7%) for active tuberculosis cases, giving a NPV of 28.9% (95% CI: 24.8–33.2%) and a NLV of 0.98 (95% CI: 0.90–1.07) for patients with active tuberculosis. The sensitivity of antibody reactivity to 38kD was 19.2% (95% CI: 15.5–23.3%) for active tuberculosis, giving a PPV of 70.9% (95% CI: 61.5–79.2%) and a PLV of 0.97 (95% CI: 0.67–1.40) for active tuberculosis cases. The specificity of antibody reactivity to 38kD was 80.3% (95% CI: 73.3–86.1%) for active tuberculosis cases, giving a NPV of 28.3% (95% CI: 24.2–32.7%) and a NLV of 1.01 (95% CI: 0.92–1.10) for patients with active tuberculosis. The sensitivity of antibody against 16kD was 29.7% (95% CI: 25.3–34.4%) for active tuberculosis, giving a PPV of 84.6% (95% CI: 77.6–90.1%) and a PLV of 2.19 (95% CI: 1.44–3.32) for active tuberculosis cases. The specificity of antibody against 16kD was 86.4% (95% CI: 80.2–91.3%) for active tuberculosis cases, giving a NPV of 32.9% (95% CI: 28.4–37.6%) and a NLV of 0.81 (95% CI: 0.74–0.89) for patients with active tuberculosis.
The sensitivity and specificity of antibody against LAM were better than those of Rv1636; the relative sensitivity was 2.13 (95% CI: 1.62–2.82, p < 0.0001), and the relative specificity was 2.17 (95% CI: 1.21–4.02, p = 0.0054). The sensitivity and specificity of antibody against LAM were superior to those of 38kD; the relative sensitivity was 2.15 (95% CI: 1.64–2.85, p < 0.0001), and the relative specificity was 2.17 (95% CI: 1.21–4.02, p = 0.0054). The sensitivity and specificity of antibody against LAM were higher than that of 16kD; the relative sensitivity was 1.39 (95% CI: 1.09–1.77, p = 0.0057), and the relative specificity was 2.33 (95% CI: 1.15–5.04, p = 0.0114). No significant differences were observed among the other M. tuberculosis antigenic probes.
3.4. Diagnostic characteristics of antibodies against to M. tuberculosis in culture-positive populations
The sensitivity of antibody against 16kD was 29.4% (95% CI: 25.2–33.9%) for culture-positive tuberculosis cases, giving a PPV of 85.6% (95% CI: 79.0–90.7%) and a PLV of 4.08 (95% CI: 2.66–6.26) for culture-positive cases. The specificity of antibody responses to 16kD was 92.7% (95% CI: 89.2–95.4%) for tuberculosis culture-positive cases, giving a NPV of 47.4% (95% CI: 43.3–51.5%) and a NLV of 0.76 (95% CI: 0.71–0.81) for patients with positive tuberculosis culture. The sensitivity of antibody against 38kD was 19.1% (95% CI: 15.5–23.0%) for culture-positive tuberculosis cases, giving a PPV of 72.6% (95% CI: 63.6–80.4%) and a PLV of 1.82 (95% CI: 1.25–2.66) for culture-positive cases. The specificity of antibody against 38kD was 89.5% (95% CI: 85.5–92.7%) for tuberculosis culture-positive cases, giving a NPV of 43.1% (95% CI: 39.2–47.0%) and a NLV of 0.90 (95% CI: 0.85–0.96) for patients with positive tuberculosis culture. The sensitivity of antibody against FP10 was 40.4% (95% CI: 35.8–45.1%) for culture-positive tuberculosis cases, giving a PPV of 77.2% (95% CI: 71.3–82.4%) and a PLV of 2.33 (95% CI: 1.78–3.05) for culture-positive cases. The specificity of antibody against CFP10 was 82.6% (95% CI: 77.8–86.7%) for tuberculosis culture-positive cases, giving a NPV of 48.7% (95% CI: 44.3–53.1%) and a NLV of 0.72 (95% CI: 0.66–0.79) for patients with positive tuberculosis culture. The sensitivity of antibody against LAM was 38.2% (95% CI: 33.6–42.9%) for culture-positive tuberculosis cases, giving a PPV of 72.9% (95% CI: 66.7–78.5%) and a PLV of 1.85 (95% CI: 1.44–2.37) for culture-positive cases. The specificity of antibody against LAM was 79.3% (95% CI: 74.3–83.7%) for tuberculosis culture-positive cases, giving a NPV of 46.8% (95% CI: 42.4–51.2%) and a NLV of 0.78 (95% CI: 0.71–0.85) for patients with positive tuberculosis culture. The sensitivity of antibody against Rv1636 was 19.5% (95% CI: 15.9–23.5%) for culture-positive tuberculosis cases, giving a PPV of 75.0% (95% CI: 66.1–82.5%) and a PLV of 2.06 (95% CI: 1.39–3.05) for culture-positive cases. The specificity of antibody against Rv1636 was 90.4% (95% CI: 86.6–93.5%) for tuberculosis culture-positive cases, giving a NPV of 43.5% (95% CI: 39.6–47.4%) and a NLV of 0.89 (95% CI: 0.84–0.94) for patients with positive tuberculosis culture.
The sensitivity of antibodies against 16kD and CFP10 is increased in populations with positive tuberculosis culture. The relative sensitivity for detecting antibody against to 16kD and CFP10 was 1.31 (95% CI: 1.05–1.61, p = 0.0103) and 1.26 (95% CI: 1.06–1.51, p = 0.0075), respectively. There were no significant differences between antibody against to the other M. tuberculosis antigens. The specificity for detecting antibodies against to antigens 16kD, 38kD, CFP10, LAM, and Rv1636 were no significant differences between active tuberculosis and culture-positive tuberculosis cases.
3.5. Effect of CD4+ T-cell on the detection of antibodies against to M. tuberculosis
Among the 1311 patients with active tuberculosis, 773 (59%) had normal levels of CD4+ T-cell, 520 (40%) had low levels, and the remaining 18 (1%) did not have any available CD4+ T-cell count. Of the 305 patients with community-acquired pneumonia, 131 (43%) had normal levels of CD4+ T-cell, 168 (55%) had low levels, and the remaining 7 (2%) did not have flow cytometry results. The sensitivity, specificity, positive predictive and negative values, and positive and negative likelihood ratios for active tuberculosis in patients with normal and low levels of CD4+ T-cell are summarized in . A significant difference in sensitivity (30.0% [95% CI: 26.8–33.4%] vs. 37.3% [95% CI: 33.1–41.6%], p = 0.025) was observed in the normal levels of CD4+ T-cells and low levels subgroup for antibody against LAM antigen; however, no significant difference in other antigenic probes was observed.
Table 4. an analysis of the diagnostic accuracy of commercially available probes for the diagnosis of active tuberculosis using Mycobacterium tuberculosis antigens.
4. Discussion
To the best of our knowledge, this is the largest observational cohort study that characterized clinical practice to evaluate and compare the diagnostic properties of antibodies against M. tuberculosis antigens, including 16kD, 38kD, CFP-10, LAM, and Rv1636, in the assessment of suspected active tuberculosis. In accordance with previous findings on different antigens [Citation13, Citation17, Citation19], antigen-specific antibodies contribute to the immune response to infection and defence against M. tuberculosis. Most cases of possible tuberculosis in high-burden countries are active tuberculosis [Citation12]. Similar to previous findings, the diagnostic sensitivity for active tuberculosis was low for all antibodies against M. tuberculosis antigens in our study. Our study suggests that the low sensitivity and negative likelihood ratios of procurable and antibodies against M. tuberculosis antigen probes indicate that a negative result cannot rule out a diagnosis of active tuberculosis. However, considering that antigen CFP-10 is a secreted protein of pathogenic mycobacteria in the early stages of infection [Citation20] and is absent in nontuberculous mycobacteria or BCG vaccine, it had a high specificity for diagnosing active tuberculosis. Furthermore, the specificity of antibodies against 16kD, 38kD, and Rv1636 increased to approximately 90–93% in patients with latent tuberculosis and nontuberculous mycobacterial infections. The high specificity of antibodies against 16kD, 38kD, and Rv1636 provided high positive likelihood ratios, validating the rule-in of active tuberculosis. Thus, a positive antibody against 16kD, 38kD, and Rv1636 results may contribute to ensuring a confident diagnosis of active tuberculosis in the differential diagnosis in a condition such that latent tuberculosis and nontuberculous mycobacterium have a low prevalence. However, antibodies against M. tuberculosis antigens are used in clinical practice to evaluate the possibility of active tuberculosis but not for possible tuberculosis.
When compared to individuals with a clinical diagnosis of active tuberculosis, tuberculosis culture positive patients had slightly higher sensitivity for detecting antibody responses to 16kD and CFP10. CFP10 is an important expressed protein of M. tuberculosis that is one of the proteins in culture filtrates and is expressed and released by M. tuberculosis in the early period of infection. We speculate that some of active tuberculosis individuals are negative during the latter stages of mycobacterial infection, rather than the early phase of infection because of receiving a durable previous antituberculosis treatment. In contrast to and in disagreement with prior studies [Citation13,Citation17,Citation19], the present study has shown decreased sensitivity of antibodies responses to M. tuberculosis antigens. This discordance may be due to discrepancies in the enrolled participants. Previously published material suggested that immunocompromised or immunodeficient patients, including HIV infection, older individuals, and tumors, were excluded [Citation13,Citation17,Citation19]. Furthermore, healthy individuals were enrolled as controls. Low level of sensitivity for detecting antibody against to M. tuberculosis antigens may account to the T cell depletion or decreased in the immunocompromised or immunodeficient patients. Our findings of the specificity for detecting antibody against M. tuberculosis antigens are in line with previous studies [Citation13,Citation17,Citation19].Interferon-γ release assays (IGRAs) are used as a diagnostic criterion for latent tuberculosis infection [Citation12]. IGRAs achieved a diagnostic specificity of 83.6% (95% CI: 79.0–87.6) [Citation12] when active tuberculosis was ruled out, compared with 79.3–92.7% specificity of M. tuberculosis antigen probes in this diagnostically challenging subgroup of patients with latent infection who frequently suffer from community-acquired pneumonia. More active tuberculosis cases arise because of the high prevalence of latent tuberculosis in high-burden countries (Theo Citation21]. Nevertheless, similar to our study, the diagnostic specificity for latent tuberculosis has been found to be low for IGRAs and most existing M. tuberculosis antigens. However, the specificity of antibody against 16kD increased to 92.8% (95% CI: 89.3–95.4) and that of Rv1636 increased to 90.5% (95% CI: 86.6–93.5) in patients with latent tuberculosis infection based on a critical prerequisite for the exclusion of active tuberculosis. Thus, a positive antibody against 16kD and Rv1636 result may facilitate the diagnosis of latent tuberculosis infection during the differential diagnosis for populations in whom active tuberculosis is ruled out.
HIV co-infection is one of the most important global risk factors for tuberculosis [Citation22], and cases of co-infection are characterized by depletion of CD4+ T cells. In addition, previous studies have suggested that M. tuberculosis-specific CD4+ T cells may be preferentially depleted after HIV infection, leading to a decrease in the diagnostic performance of immunological methods in diagnosing tuberculosis [Citation23]. Analogously, our study showed that a reduction in CD4+ cell count had adverse effects on the performance of existing M. tuberculosis antigen probes.
In summary, the present study provides compelling evidence that existing antibodies against M. tuberculosis antigens, 16kD, 38kD, CFP-10, LAM, and Rv1636, achieve limited sensitivity, thereby leading to an increase in false-negative results and missed diagnoses in tuberculosis screening during routine clinical practice. However, the higher specificity and positive likelihood ratios may help to rule in the diagnosis of active or latent tuberculosis in clinical settings among high-burden tuberculosis countries.
Ethical approval and Informed consent
This retrospective study involving human subjects complied with all relevant national regulations, institutional policies and is in accordance with the Helsinki Declaration guidelines and was approved by The Ethics Committee of The Fourth People’s Hospital of Nanning (No. [2019]39, [2020]24). In compliance with national regulations and institutional requirements, written informed consent for participation in this study was not required.
Author contributions
Conceptualization: Xiaolu Luo and Honghua Shao; Clinical validation: Jianyan Lin, Zhouhua Xie, Lemin Wen, Chaoyou Chen, and Xike Tang; Investigation and data curation: Chaojuan Liang, Xiaoxian Huang, Huidan Pan, Yue Qin, Ximing Shi, Yunhua Tang, Ying Wang, and Mingmei Zhao; Data curation and original draft preparation: Dewu Bi; Review: Xiaocheng Luo. All authors have read and agreed to the submitted version of the manuscript.
Supplemental Material
Download MS Word (58.5 KB)Acknowledgments
The authors acknowledge Jianbin Zhong for her help during data collection and initial data processing.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Epidemiological, medical, and laboratory results, as well as treatment and outcome data, were acquired from hospital computerized medical records using data collecting forms. The study’s original contributions are included in the article/Supplemental Material. Further inquiries should be made to the correspondence.
Additional information
Funding
References
- Dosanjh DP, Hinks TS, Innes JA, et al. Improved diagnostic evaluation of suspected tuberculosis. Ann Intern Med. 2008;148(5):1–10. doi: 10.7326/0003–4819–148–5-200803040–00003.
- Liang Q, Pang Y, Yang Y, et al. An improved algorithm for rapid diagnosis of pleural tuberculosis from pleural effusion by combined testing with GeneXpert MTB/RIF and an anti-LAM antibody-based assay. BMC Infect Dis. 2019;19(1):548. doi: 10.1186/s12879–019–4166–1.
- Nicol MP, Schumacher SG, Workman L, et al. Accuracy of a novel urine test, fujifilm SILVAMP tuberculosis lipoarabinomannan, for the diagnosis of pulmonary tuberculosis in children. Clin Infect Dis. 2021;72(9):e280–288–e288. doi: 10.1093/cid/ciaa1052.
- Dorman SE, Schumacher SG, Alland D, et al. Xpert MTB/RIF ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: a prospective multicentre diagnostic accuracy study. Lancet Infect Dis. 2018;18(1):76–84. doi: 10.1016/S1473–3099(17)30691–6.
- Penn-Nicholson A, Georghiou SB, Ciobanu N, et al. Detection of isoniazid, fluoroquinolone, ethionamide, amikacin, kanamycin, and capreomycin resistance by the xpert MTB/XDR assay: a cross-sectional multicentre diagnostic accuracy study. Lancet Infect Dis. 2022;22(2):242–249. doi: 10.1016/S1473–3099(21)00452–7.
- Penn-Nicholson A, Gomathi SN, Ugarte-Gil C, et al. A prospective multicentre diagnostic accuracy study for the truenat tuberculosis assays. Eur Respir J. 2021;58(5):2100526. doi: 10.1183/13993003.00526–2021.
- Ivanyi J. Serodiagnosis of tuberculosis: due to shift track. Tuberculosis (Edinb). 2012;92(1):31–37. doi: 10.1016/j.tube.2011.09.001.
- García-Basteiro AL, Cobelens F. Triage tests: a new priority for tuberculosis diagnostics. Lancet Respir Med. 2015;3(3):177–178. doi: 10.1016/S2213–2600(15)00061–2.
- Morris K. WHO recommends against inaccurate tuberculosis tests. Lancet. 2011;377(9760):113–114. doi: 10.1016/s0140–6736(11)60005–6.
- Blakemore R, Nabeta P, Davidow AL, et al. A multisite assessment of the quantitative capabilities of the xpert MTB/RIF assay. Am J Respir Crit Care Med. 2011;184(9):1076–1084. doi: 10.1164/rccm.201103–0536OC.
- Cohen KA, El-Hay T, Wyres KL, et al. Paradoxical hypersusceptibility of drug-resistant Mycobacterium tuberculosis to β-lactam antibiotics. EBioMedicine. 2016;9:170–179. doi: 10.1016/j.ebiom.2016.05.041.
- Whitworth HS, Badhan A, Boakye AA, et al. Clinical utility of existing and second-generation interferon-gamma release assays for diagnostic evaluation of tuberculosis: an observational cohort study. Lancet Infect Dis. 2019;19(2):193–202. doi: 10.1016/S1473–3099(18)30613–3.
- Wu X, Yang Y, Zhang J, et al. Comparison of antibody responses to seventeen antigens from Mycobacterium tuberculosis. Clin Chim Acta. 2010;411(19–20):1520–1528. doi: 10.1016/j.cca.2010.06.014.
- Chan ED, Heifets L, Iseman MD. Immunologic diagnosis of tuberculosis: a review. Tuber Lung Dis. 2000;80(3):131–140. doi: 10.1054/tuld.2000.0243.
- Comella-del-Barrio P, Molina-Moya B, Gautier J, et al. Diagnostic performance of the fujifilm SILVAMP TB-LAM in children with presumptive tuberculosis. J Clin Med. 2021;10:1914. doi: 10.3390/jcm10091914.
- Gennaro ML. Immunologic diagnosis of tuberculosis. Clin Infect Dis. 2000;30(Suppl 3):S243–S6. doi: 10.1086/313868.
- Hao X, Bai J, Ding Y, et al. Characterization of antibody response against 16kD and 38kD of M. tuberculosis in the assisted diagnosis of active pulmonary tuberculosis. Ann Transl Med. 2020;8(15):945. doi: 10.21037/atm-20–5476.
- Hu Y, Liu M, Hu H, et al. Accuracy of multitarget indirect enzyme-linked immunoassay assay for detection of tuberculosis antibody. Ann Transl Med. 2021;9(23):1731. doi: 10.21037/atm-21–5598.
- Wang S, Wu J, Chen J, et al. Evaluation of Mycobacterium tuberculosis-specific antibody responses for the discrimination of active and latent tuberculosis infection. Int J Infect Dis. 2018;70:1–9. doi: 10.1016/j.ijid.2018.01.007.
- Adams S, Ehrlich R, Baatjies R, et al. Predictors of discordant latent tuberculosis infection test results amongst South african health care workers. BMC Infect Dis. 2019;19(1):131. doi: 10.1186/s12879–019–3745–5.
- Vos T, Abajobir AA, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet. 2017;390(10100):1211–1259. doi: 10.1016/S0140–6736(17)32154–2.
- Esmail H, Riou C, Bruyn ED, et al. The immune response to Mycobacterium tuberculosis in HIV-1-coinfected persons. Annu Rev Immunol. 2018;36:603–638. doi: 10.1146/annurev-immunol-042617–053420.
- Scriba TJ, Tameris M, Smit E, et al. A phase IIa trial of the new tuberculosis vaccine, MVA85A, in HIV- and/or Mycobacterium tuberculosis infected adults. Am J Respir Crit Care Med. 2012;185(7):769–778. doi: 10.1164/rccm.201108–1548OC.
