Abstract
Introduction
Low-dose interleukin-2 (IL-2) regulates the homeostasis of CD4+ T cells by modulating the proportions of effector and regulatory T cells, thus reducing disease activity in patients with systemic lupus erythematosus (SLE). However, to date, no research has been carried out on the efficacy of low-dose IL-2 for treating autoimmune thyroid disease (AITD). The aim of this study was to observe the effects of IL-2 on AITD patients with concurrent SLE, and explore potential mechanism of action.
Methods
A retrospective analysis was conducted on 29 SLE patients with concurrent AITD. Among them, 11 patients were in IL-2 therapy group and 18 patients without IL-2 treatment were considered as control group. Two groups had similar disease activities and were treated with comparable regular strategy. Free triiodothyronine (FT3), free thyroxine (FT4), thyroxine(T4), triiodothyronine(T3), thyroid stimulating hormone (TSH), thyroglobulin antibody (TG-Ab), thyroid peroxidase antibody (TPO-Ab) levels and immune cell subgroups were measured.
Results
After receiving low-dose IL-2 therapy, the TG-Ab and TPO-Ab levels decreased drastically (TG-Ab p = 0.008, TPO-Ab p = 0.007), and the majority of the AITD patients became seronegative, while there was no discernible change in control group. In IL-2 group, percentage of CD4+ T cells showed a significant increase after treatment (p = 0.029), with an upward trend in the ratio of regulatory T (Treg) cells to follicular helper T (Tfh) cells (Treg/Tfh). The percentage as well as absolute count of B cells demonstrated a decreasing trend.
Conclusion
Low-dose IL-2 may downregulate the levels of TG-Ab and TPO-Ab by modulating the immune balance of Treg/Tfh and B-cells, providing new avenue for clinical treatment of AITD.
Key Messages
Low-dose IL-2 could decrease the levels of TG-Ab and TPO-Ab in AITD with concurrent SLE patients.
Low-dose IL-2 may regulate Treg/Tfh balance and B-cells to ameliorate TG-Ab and TPO-Ab.
Low-dose IL-2 provides a new avenue for AITD clinical treatment.
Introduction
Autoimmune thyroid disease (AITD) is characterized by an aberrant attack on the thyroid gland by the immune system, primarily including Graves’ disease (GD) and Hashimoto’s thyroiditis (HT) [Citation1]. The prevalence of GD and HT has been reported ranging from 21 to 120/100000/yr and 27 to 273/100000/yr respectively due to substantial geographic variation [Citation2], but the prevalence of anti-thyroid antibodies (ATAs) without clinical disease may be higher [Citation3], and is more common in women compared to men [Citation2]. Genetic susceptibility (e.g. dysfunction of immunomodulatory genes FOXP3, CD25, CD40) and some environmental factors (e.g. infections, smoking, iodine, occupational exposure at work) can cause the development of AITD [Citation4,Citation5]. Patients’ clinical manifestations differ drastically, but all of them can be detected for thyroid auto-antibodies, of which the characteristic representatives are thyroglobulin antibodies (TG-Ab) and thyroid peroxidase antibodies (TPO-Ab). Compared with abnormal secretion of thyroid hormones, subclinical thyroid disorders are more frequent. Patients can be asymptomatic and, therefore, undiagnosed and untreated, leading to critical adverse events [Citation6,Citation7]. Furthermore, autoimmunity and inflammation per se, are risk factors for thyroid cancer [Citation8]. However, current treatment for AITD is often initiated after severe destruction of thyroid function, following the onset of hypothyroidism or hyperthyroidism, and mainly relies on hormone replacement therapy or anti-thyroid drugs, ablative treatments I-radiotherapy131, or thyroidectomy. None of these treatments targets the disease process with a lack of preliminary intervention or immunomodulatory therapy [Citation9,Citation10].
Studies have shown that T-cell dysfunction is associated with disruption of immune homeostasis in thyroid tissue [Citation11,Citation12], in which regulatory T (Treg) cells play an essential role in maintaining immune homeostasis. A study indicates that the number of Treg cells in HT patients is decreased compared to healthy controls (HCs), and Treg levels are negatively correlated with anti-thyroid antibodies [Citation13].
Low-dose IL-2 has been used as a new treatment for systemic lupus erythematosus (SLE) and primary Sjogren’s syndrome (pSS) in recent years for its role in the regulation of immune homeostasis by selectively modulating the abundance of Treg cells, follicular helper T (Tfh) cells, and IL-17-producing helper T (Th17) cells to improve the disease. Hence, low-dose IL-2 may have therapeutic effect in AITD, but it has not been studied to date.
Thyroid function abnormalities and thyroid autoantibodies have frequently been described in SLE patients [Citation14]. Therefore, in this study, we performed a retrospective analysis of AITD patients with concurrent SLE who were treated with low-dose IL-2, to investigate the changes in thyroid function and related antibodies and explore its mechanism of action on autoimmune thyroid disease.
Patients and methods
Patients
A retrospective analysis was conducted on patients admitted to the Department of Rheumatology and Immunology at Peking University People’s Hospital from 2015 to 2021. A total of 11 patients with AITD who were concurrent with SLE and treated with low-dose IL-2 were included as IL-2 group in this study. In order to exclude the effect of the SLE-related treatment on antibodies, 18 SLE patients with similar disease activity and only treated with comparable regular strategy (glucocorticoid and/or immunosuppressant) without IL-2 were involved as control. All of them were diagnosed based on the 2009 SLICC/ACR SLE classification criteria and had thyroid function data before and after treatment. Inclusion criterion was positive TG-Ab or TPO-Ab and had thyroid function results for at least twice. Those patients who had been prescribed biologics were excluded. Low-dose IL-2 was injected subcutaneously at a dose of 1 million IU every other day for two weeks, followed by a two-week break. In total, patients in IL-2 group were given three cycles of IL-2 and followed up for more than three months. Glucocorticoids and immunosuppressants were adjusted by the clinician based on the response to the condition. None of the patients’ treatment regimens intensity was stepped up to higher platform during the phase of observation. The study was approved by the Institutional Ethics Committee of Peking University People’s Hospital (2015PHB210-01).
Study design
Demographics and general information, such as sex, age, disease duration, medication use and clinical manifestation were collected. Free triiodothyronine (FT3), free thyroxine (FT4), thyroxine(T4), triiodothyronine(T3), thyroid stimulating hormone (TSH), TG-Ab, TPO-Ab levels, as well as proportions of T cell subtypes (including CD4 + T cell, CD8 + T cell, Treg cell, Tfh cell), B cell and NK cell subsets were evaluated before and after the application of low-dose IL-2. Other laboratory parameters related to SLE, including complete blood count, liver and kidney function, serum albumin, immunoglobulin, complement levels, anti-nuclear antibody (ANA), anti-double-stranded DNA (anti-dsDNA) antibody, anti-nucleosome antibody (AnuA), anti-ribosomal antibody, anti-β2 glycoprotein I (anti-β2GPI) antibody, anticardiolipin antibody (ACA) and lupus anticoagulant (LAC) were also assessed from baseline to week 12. Immunological analysis of peripheral blood mononuclear cells was performed before and after treatment. Peripheral blood mononuclear cell (PMBC) was isolated from whole blood by using Lymphocyte Separation Medium (detailed procedure could be seen in Supplementary Document). Then, the proportions of cells were analyzed by flow cytometry using a FACS Aria II flow cytometer (BD Biosciences, Franklin Lake, NJ, USA) and the Flow Jo software package (Tree Star, BD Biosciences). CD4+T cells were defined as CD45+CD3+CD4+T cells, CD8+T cells were defined as CD45+CD3+CD8+T cells, Treg cells were defined as CD3+CD4+CD127-CD25+ cells; Tfh cells were defined as CD3+CD4+CXCR5+CD45RA-PD1+CCR7-cells. B cells were defined as CD45+ CD3-CD19+cells, NK cell were defined as CD45+ CD3-CD16+CD56+cells.
Statistical analyses
All statistical data were calculated and analyzed by IBM SPSS Statistics 25 (IBM SPSS Software, Inc., Chicago, USA) and GraphPad Prism 8.0 software (GraphPad Software, Inc., San Diego, CA, USA). Continuous data were analyzed by t-test or Mann-Whitney U Test according to their normal distribution, and presented as mean ± standard deviation or median with quartiles. Categorical data was tested by Chi-square test and represented as percentage. p < 0.05 was considered significant.
Result
Baseline characteristics of IL-2 group patients and control group
Eleven SLE patients with concurrent AITD were included in the IL-2 group and eighteen patients were admitted in control group. Their baseline demographic, clinical characteristics are presented and its results of variance analysis with control group were shown in . Baseline laboratory parameters between IL-2 and control group were shown in Table S1. In IL-2 group, there were all young or middle-aged women, and their disease durations of SLE ranged from 2 to 39 years. AITD occurred before (36.4%), in the same year as (18.2%), or after (45.5%) the diagnosis of SLE. All patients had positive thyroid antibody test outcomes, but damage degree of thyroid function and its manifestations were variable. One patient (9.09%) presented as hyperthyroid at onset and switched to hypothyroid after treatment with iodine 131. Three (27.27%) of them had undergone thyroxine replacement therapy during their disease. All patients were on concomitant steroids (prednisone 5–40 mg/day) and hydroxychloroquine (HCQ, 400 mg/day) treatments. Regarding current immunosuppressive agents, four were on cyclosporin A (CsA, 50–150mg/day), three were on mycophenolate mofetil (MMF, 1000–2000 mg/day), one was on cyclophosphamide (CTX, 50 mg every other day), one was on tacrolimus (TAC, 1.5 mg/day) and one was on azathioprine (AZA, 25 mg/day). Compared with IL-2 group, significant differences could be observed between the two groups in terms of the disease duration of AITD, while other data did not reveal a remarkable difference.
Table 1. Baseline characteristics of patients with autoimmune thyroid disease with concurrent systemic lupus erythematosus.
Changes in clinical parameters after IL-2 treatment
Eleven patients received IL-2 therapy and their symptoms were alleviated following IL-2 and additional immunosuppressants in 3 months of follow-up. As demonstrated in , the patients’ IgA, IgG, IgM, anti-dsDNA antibody, anti-β2GPI antibody and anti-ribosomal antibody dramatically decreased before and after therapy. In addition, we observed a significant rise in C4 compared to the baseline.
Table 2. Changes in laboratory parameters before and after IL-2 treatment.
The effect of IL-2 treatment on thyroid function and antibodies compared to the control group
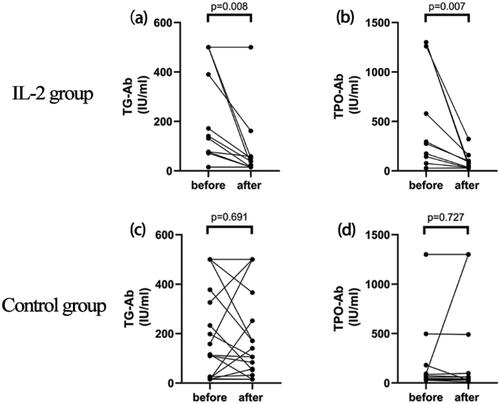
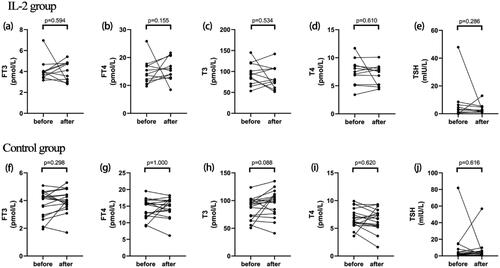
After lose-dose IL-2 treatment, there was no discernible difference in FT4, FT3, T3, T4, and TSH levels. However, ninety percent of patients showed a significant reduction in TG-Ab and TPO-Ab after receiving low-dose IL-2 therapy. As shown in and there was a remarkable decline in TG-Ab from 140.70 (76.8, 500) IU/ml to 38.5(15, 58) IU/ml (p = 0.008), and in TPO-Ab from 276 (75,1260) IU/ml to 49.2 (32,100.5) IU/ml (p = 0.007). Among these results, eight patients become seronegative for TG-Ab and four patients for TPO-Ab, respectively. On the contrary, FT4, FT3, T3, T4, TSH, TG-Ab and TPO-Ab in control group did not exhibit significant changes before or after conventional treatment .
Figure 1. Changes in thyroid function between groups and before/after IL-2 therapy. Changes in free triiodothyronine (a), free thyroxine (b), triiodothyronine(c), thyroxine (d), thyroid stimulating hormone (e) before and after initiating IL-2 treatment. Changes in free triiodothyronine (f), free thyroxine (g), triiodothyronine (h), thyroxine (i), thyroid stimulating hormone (j) before and after regular treatment in control group.
The effects of IL-2 treatment on cell subsets
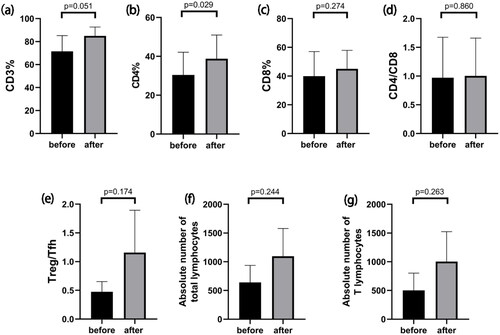
To further explore the mechanism involved in the reduction of antibody levels by low-dose IL-2 therapy, we analyzed the cell subsets of the patients before and after treatment, as illustrated in . Percentage of CD4+ T cells increased significantly after treatment (38.78 ± 12.18% vs 30.42 ± 11.71%, p = 0.029). Furthermore, we observed a rising trend in percentage of Treg from 6.8 ± 3.13% to 12.17 ± 3.70%, though not reaching statistical significance (p = 0.606). The same result was also seen for Treg/Tfh ratio, from 0.47 ± 0.18 to 1.15 ± 0.74 (p = 0.174). At the same time, the absolute number of total lymphocytes (640.33 ± 296.76 to 1095.33 ± 483.08) and the absolute number of T lymphocytes (502.00 ± 300.04 to 1002.67 ± 520.80) showed an upward trend after treatment, while the percentage of B cells decreased from 14.20 ± 13.88% to 1.33 ± 1.10% (77.33 ± 74.30/µl to 11.67 ± 10.07/µl for absolute counts).
Figure 3. Changes in T cell subpopulations after IL-2 use. (a–g) Changes in the percentage of CD3+T cells (a), percentage of CD4+T cells (b), percentage of CD8+T cells (c), the ratio of the percentage of CD4+T cells to the percentage of CD8+T cells (d), ratio of Treg cells to Tfh cells (e), the absolute number of total lymphocytes(f), the absolute number of T lymphocytes(g) before and after initiating IL-2 treatment.
Discussion
This was a retrospective study to explore the clinical and immunological effects of low-dose IL-2 treatment in individuals with AITD with concurrent SLE, which revealed a previously unidentified new role for IL-2 in AITD. Even though thyroid-related antibodies were present in all patients, thyroid function varied among patients. After three cycles of low-dose IL-2 treatment, all the patients showed a decrease in TG-Ab or TPO-Ab antibody levels. Levels of immunoglobulin, complement and some antibodies were also decreased. Therefore, IL-2 therapy may present as a potential novel therapeutic approach for patients with AITD.
Serum TG-Ab and TPO-Ab could fluctuate up to 10% and 5–20% in the general population respectively, and they are widely accepted as markers of AITD [Citation15,Citation16]. TPO-Ab is mainly produced by plasma cells and its titer reflects the severity of lymphocyte infiltration [Citation17]. A 20-year follow-up study demonstrated that TPO-Ab positive patients with normal thyroid function have a considerably greater prevalence of overt hypothyroidism than TPO-Ab negative patients, which suggested that TPO-Ab-positive patients had more severe thyroid follicular destruction over time [Citation18]. Subclinical hypothyroidism has been linked to increased risk of heart failure, coronary artery disease events, and coronary heart disease mortality [Citation19]. Therefore, it is crucial to continue monitoring individuals with elevated antibodies and begin treatment when appropriate [Citation20]. When it comes to clinical treatment, most current conventional methods focus on using medications to maintain normal thyroid hormone levels. Matthias Schmidt et.al demonstrated that patients with HT who are taking levothyroxine showed a decline in TPO-Ab level [Citation21]. In our study, three patients in both groups had used levothyroxine during the course of the disease, but these patients in the control group presented no improvement of TPO-Ab as well. In addition, one study proposed that rituximab (RTX) therapy mediated specific decreases in TPO-Ab within one year follow-up [Citation22]. Therefore, we did not include the patient who had ever treated with RTX so as not to interfere with the judgment of the role of IL-2. However, from the standpoint of the development of autoimmune diseases, it is still necessary to administer proper medications to control the underlying autoimmune response. From this aspect, IL-2 may be an effective treatment option as an immunomodulatory drug.
With regard to AITD, lymphocyte infiltration in the thyroid parenchyma is a common clinical feature in both HT and GD patients [Citation1]. Although the mechanism of AITD is undetermined, it is presently believed to be related to both immunological disorders and genetic predisposition. Compared to HC, the imbalance of Th17 and Tregs contributes to thyroid autoimmunity and the severity of AITD [Citation23]. The proportion of Th17 cells is higher in AITDs, especially in the HT subgroup, while the proportions of Treg cells, Foxp3 mRNA expression, and Treg/Th17 cell ratio were all considerably lower in GD and HT [Citation24,Citation25]. At the same time, a negative correlation was detected between the level of anti-TPO antibodies and the percentage of CD4+CD25high T cells [Citation26]. Depletion of CD4+ CD25+ Tregs exacerbated the prevalence and severity of HT in BALB/c mouse animal model [Citation27], while the transfer of CD4+CD25+Tregs could inhibit the development of HT [Citation28]. Monica Marazuela et.al proposed an alternative viewpoint that the expression of Foxp3+ cells in inflamed thyroid tissue was not diminished, and in most cases, they were unable to downregulate the autoimmune response and tissue damage [Citation29]. The results obtained from Zha’s research indicated that activating the Tregs’ function would probably be a more effective approach to the treatment of AITD than increasing the Tregs’ number only [Citation30]. A similar result was observed by Glick and his colleagues [Citation31]. No differences were found in the frequency of Tregs between AITD and HC, and AITD patients’ Tregs were less effective in preventing Teff cell proliferation [Citation31].
Meanwhile, an increase in circulating Tfh17 cells and the percentage of PD1+Tfh was associated with higher TG-Ab and TPO-Ab levels in HT patients [Citation32]. Zhang et al. showed that the expression of Tfh and related factors was increased in thyroid tissues in GD [Citation33]. Both studies indicated that Tfh cells were essential for the pathophysiological processes underlying AITD.
It is well established that IL-2 can promote the generation, survival and functional activity of Treg cells [Citation34]. Low-dose IL-2 has the advantage of selectively promoting Treg cells while suppressing effector T cells, such as Tfh and Th17 cells, to improve immunological homeostasis [Citation35]. Its function has been confirmed in the treatment of autoimmune diseases such as SLE [Citation36], rheumatoid arthritis [Citation37], and active idiopathic inflammatory myopathies [Citation38]. IL-2 (1 million IU every 8 h for 5 consecutive days, followed by 9 days of rest until 1 year) was previously utilized in combination with lanreotide to treat medullary thyroid cancer in cases of thyroid disease [Citation39]. However, some researchers demonstrated that IL-2 might account for thyroiditis [Citation40]. In reality, IL-2 production by mitogen-induced peripheral blood mononuclear cells in GD patients was noticeably lower than that in controls [Citation41]. However, studies on the effect of IL-2 on AITD disease are still lacking. Our research demonstrated that CD4+T cells were significantly increased, with an upward trend of Treg/Tfh in response to low-dose IL-2 therapy. This is consistent with some studies above. In addition, we observed a decline for percentage of B cell, which is similar with a study of active idiopathic inflammatory myopathies, demonstrating noteworthy decreases of both proportion and number of B cells at week 12 [Citation38].Therefore, we hypothesize that low-dose IL-2 may exert an immunomodulatory effect in AITD by affecting immune homeostasis, thereby reducing antibody production. However, our study did not monitor any significant difference in Treg/Tfh after treatment, which may be due to the small sample size.
Low-dose IL-2 may present as a novel therapeutic strategy that differs from the earlier standard therapy for AITD. In the past, typical AITD treatment was delayed until thyroid dysfunction, but IL-2 could provide an earlier stage therapeutic option. Upon effective intervention, disease progression may be hindered when the titers of antibodies decrease. This would improve patients’ quality of life when also lessening the financial burden of healthcare on society.
Admittedly, there are several limitations in this study. The small sample size may not reflect the entire disease population, and the timing of follow-up rechecks for thyroid function and antibodies are heterogeneous. The disease state of SLE may also affect the control of autoimmune homeostasis. Therefore, the results of this preliminary analysis must be confirmed in a randomized controlled trial with a larger sample size.
Conclusions
Low-dose IL-2 may downregulate the levels of TG-Ab and TPO-Ab by modulating the immune balance of Treg/Tfh, which plays a certain role in the treatment of autoimmune thyroid disease. This provides a new avenue for clinical treatment of autoimmune thyroid disease.
Authors’ contributions
JH was involved in the conception and design. NW and YJ performed data analysis. NW, BH, YJ, GC and KZ were involved in the interpretation of the data. NW and YL drafted the paper, revised it critically for intellectual content. JH and YJ contributed to the final approval of the version to be published. All authors agree to be accountable for all aspects of the work.
Informed consent
Informed consent was obtained from all individuals included in this study.
Supplemental Material
Download MS Word (742.6 KB)Acknowledgments
We would like to thank all the patients, study investigators, and staff who were involved with this study.
Disclosure statement
The authors report no declarations of interest.
Data availability statement
The data that support the findings of this study are available from the corresponding author, Jing He, upon reasonable request.
Additional information
Funding
References
- Antonelli A, Ferrari SM, Corrado A, et al. Autoimmune thyroid disorders. Autoimmun Rev. 2015;14(2):1–9. doi:10.1016/j.autrev.2014.10.016.
- McLeod DS, Cooper DS. The incidence and prevalence of thyroid autoimmunity. Endocrine. 2012;42(2):252–265. doi:10.1007/s12020-012-9703-2.
- Pearce SH, Leech NJ. Toward precise forecasting of autoimmune endocrinopathy. J Clin Endocrinol Metab. 2004;89(2):544–547. doi:10.1210/jc.2003-032142.
- Lee HJ, Li CW, Hammerstad SS, et al. Immunogenetics of autoimmune thyroid diseases: a comprehensive review. J Autoimmun. 2015;64:82–90. doi:10.1016/j.jaut.2015.07.009.
- Benvenga S, Elia G, Ragusa F, et al. Endocrine disruptors and thyroid autoimmunity. Best Pract Res Clin Endocrinol Metab. 2020;34(1):101377. doi:10.1016/j.beem.2020.101377.
- Cooper DS, Biondi B. Subclinical thyroid disease. Lancet. 2012;379(9821):1142–1154. doi:10.1016/s0140-6736(11)60276-6.
- Biondi B, Cooper DS. Subclinical hyperthyroidism. N Engl J Med. 2018;378(25):2411–2419. doi:10.1056/NEJMcp1709318.
- Ferrari SM, Fallahi P, Elia G, et al. Thyroid autoimmune disorders and cancer. Semin Cancer Biol. 2020;64:135–146. doi:10.1016/j.semcancer.2019.05.019.
- Ralli M, Angeletti D, Fiore M, et al. Hashimoto’s thyroiditis: an update on pathogenic mechanisms, diagnostic protocols, therapeutic strategies, and potential malignant transformation. Autoimmun Rev. 2020;19(10):102649. doi:10.1016/j.autrev.2020.102649.
- Bartalena L. Diagnosis and management of graves disease: a global overview. Nat Rev Endocrinol. 2013;9(12):724–734. doi:10.1038/nrendo.2013.193.
- McLachlan SM, Rapoport B. Breaking tolerance to thyroid antigens: changing concepts in thyroid autoimmunity. Endocr Rev. 2014;35(1):59–105. doi:10.1210/er.2013-1055.
- Smith TJ, Hegedüs L. Graves’ disease. N Engl J Med. 2016;375(16):1552–1565. doi:10.1056/NEJMra1510030.
- Hu Y, Zhang L, Chen H, et al. Analysis of regulatory T cell subsets and their expression of helios and PD-1 in patients with hashimoto thyroiditis. Int J Endocrinol. 2019;2019:5368473. doi:10.1155/2019/5368473.
- Sacks D, Baxter B, Campbell BCV, et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int J Stroke. 2018;13:612–632. doi:10.1177/1747493018778713.
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133. doi:10.1089/thy.2015.0020.
- Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): national health and nutrition examination survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489–499. doi:10.1210/jcem.87.2.8182.
- Khan FA, Al-Jameil N, Khan MF, et al. Thyroid dysfunction: an autoimmune aspect. Int J Clin Exp Med. 2015;8:6677–6681.
- Vanderpump MP, Tunbridge WM, French JM, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the whickham survey. Clin Endocrinol (Oxf). 1995;43(1):55–68. doi:10.1111/j.1365-2265.1995.tb01894.x.
- Biondi B, Cappola AR, Cooper DS. Subclinical hypothyroidism: a review. Jama. 2019;322(2):153–160. doi:10.1001/jama.2019.9052.
- Soh SB, Aw TC. Laboratory testing in thyroid Conditions - Pitfalls and clinical utility. Ann Lab Med. 2019;39(1):3–14. doi:10.3343/alm.2019.39.1.3.
- Schmidt M, Voell M, Rahlff I, et al. Long-term follow-up of antithyroid peroxidase antibodies in patients with chronic autoimmune thyroiditis (hashimoto’s thyroiditis) treated with levothyroxine. Thyroid. 2008;18(7):755–760. doi:10.1089/thy.2008.0008.
- El Fassi D, Banga JP, Gilbert JA, et al. Treatment of graves’ disease with rituximab specifically reduces the production of thyroid stimulating autoantibodies. Clin Immunol. 2009;130(3):252–258. doi:10.1016/j.clim.2008.09.007.
- Shao S, Yu X, Shen L. Autoimmune thyroid diseases and Th17/treg lymphocytes. Life Sci. 2018;192:160–165. doi:10.1016/j.lfs.2017.11.026.
- Li C, Yuan J, Zhu YF, et al. Imbalance of Th17/treg in different subtypes of autoimmune thyroid diseases. Cell Physiol Biochem. 2016;40(1–2):245–252. doi:10.1159/000452541.
- Xue H, Yu X, Ma L, et al. The possible role of CD4+CD25(high)Foxp3+/CD4+IL-17A+ cell imbalance in the autoimmunity of patients with hashimoto thyroiditis. Endocrine. 2015;50(3):665–673. doi:10.1007/s12020-015-0569-y.
- Bossowski A, Moniuszko M, Dąbrowska M, et al. Lower proportions of CD4 + CD25(high) and CD4 + FoxP3, but not CD4 + CD25 + CD127(low) FoxP3+ T cell levels in children with autoimmune thyroid diseases. Autoimmunity. 2013;46(3):222–230. doi:10.3109/08916934.2012.751981.
- Wei WZ, Morris GP, Kong YC. Anti-tumor immunity and autoimmunity: a balancing act of regulatory T cells. Cancer Immunol Immunother. 2004;53(2):73–78. doi:10.1007/s00262-003-0444-1.
- Verginis P, Li HS, Carayanniotis G. Tolerogenic semimature dendritic cells suppress experimental autoimmune thyroiditis by activation of thyroglobulin-specific CD4 + CD25+ T cells. J Immunol. 2005;174(11):7433–7439. doi:10.4049/jimmunol.174.11.7433.
- Marazuela M, García-López MA, Figueroa-Vega N, et al. Regulatory T cells in human autoimmune thyroid disease. J Clin Endocrinol Metab. 2006;91(9):3639–3646. doi:10.1210/jc.2005-2337.
- Zha B, Huang X, Lin J, et al. Distribution of lymphocyte subpopulations in thyroid glands of human autoimmune thyroid disease. J Clin Lab Anal. 2014;28(3):249–254. doi:10.1002/jcla.21674.
- Glick AB, Wodzinski A, Fu P, et al. Impairment of regulatory T-cell function in autoimmune thyroid disease. Thyroid. 2013;23(7):871–878. doi:10.1089/thy.2012.0514.
- Zhao J, Chen Y, Zhao Q, et al. Increased circulating Tfh17 and PD-1(+)tfh cells are associated with autoantibodies in hashimoto’s thyroiditis. Autoimmunity. 2018;51(7):352–359. doi:10.1080/08916934.2018.1516761.
- Zhang J, Ren M, Zeng H, et al. Elevated follicular helper T cells and expression of IL-21 in thyroid tissues are involved in the pathogenesis of graves’ disease. Immunol Res. 2015;62(2):163–174. doi:10.1007/s12026-015-8647-z.
- Abbas AK, Trotta E, D RS, et al. Revisiting IL-2: biology and therapeutic prospects. Sci Immunol. 2018;3(25):1–8. doi:10.1126/sciimmunol.aat1482.
- He J, Zhang X, Wei Y, et al. Low-dose interleukin-2 treatment selectively modulates CD4(+) T cell subsets in patients with systemic lupus erythematosus. Nat Med. 2016;22(9):991–993. doi:10.1038/nm.4148.
- He J, Zhang R, Shao M, et al. Efficacy and safety of low-dose IL-2 in the treatment of systemic lupus erythematosus: a randomised, double-blind, placebo-controlled trial. Ann Rheum Dis. 2020;79(1):141–149. doi:10.1136/annrheumdis-2019-215396.
- Rosenzwajg M, Lorenzon R, Cacoub P, et al. Immunological and clinical effects of low-dose interleukin-2 across 11 autoimmune diseases in a single, open clinical trial. Ann Rheum Dis. 2019;78(2):209–217. doi:10.1136/annrheumdis-2018-214229.
- Miao M, Li Y, Huang B, et al. Treatment of active idiopathic inflammatory myopathies by Low-Dose interleukin-2: a prospective cohort pilot study. Rheumatol Ther. 2021;8(2):835–847. doi:10.1007/s40744-021-00301-3.
- Vitale G, Lupoli G, Guarrasi R, et al. Interleukin-2 and lanreotide in the treatment of medullary thyroid cancer: in vitro and in vivo studies. J Clin Endocrinol Metab. 2013;98(10):E1567–1574. doi:10.1210/jc.2013-1443.
- Martinez Quintero B, Yazbeck C, Sweeney LB. Thyroiditis: evaluation and treatment. Am Fam Physician. 2021;104:609–617.
- Eisenstein Z, Engelsman E, Weiss M, et al. Production of and response to interleukin-2 in graves’ disease. J Clin Immunol. 1988;8(5):349–355. doi:10.1007/bf00917150.