Abstract
Background
Hepatitis B virus (HBV) and syphilis have been the most common co-infections that hinder treatment outcomes and increase early mortality among people living with human immunodeficiency virus (PLHIV). In this study, we aimed to determine the burden of HBV and syphilis co-infections and its impact on treatment outcomes among PLHIV in Ethiopia.
Methods
We used data from the Ethiopian Population-based HIV Impact Assessment (EPHIA), which was a household-based national survey in 2017/2018. Human immunodeficiency virus (HIV) testing was done among 19,136 participants using the national testing algorithm and 662 participants (3.50%) were HIV positives who were further tested for viral hepatitis and syphilis co-infections using HBV surface antigen and Chembio DPP syphilis assay, respectively. Viral load, CD4 count and high-sensitivity C-reactive protein (hsCRP) were done to measure HIV treatment outcomes. Descriptive statistics were used to determine the burden of co-infections and a logistic regression model to evaluate the determinants of co-infections using STATA V17.0.
Results
Overall prevalence of HBV and syphilis co-infection was 5.5% and 2.2%, respectively. HBV and syphilis (double co-infection) was 5.9%. The highest prevalence of HBV co-infection was observed among 10–19 years age group (12.9%) and male participants (7.44%) while the highest syphilis co-infection was among people aged ≥50 years (3.5%) followed by age groups 40–49 (3.3%) and 10–19 years (3.2%). Syphilis co-infection was higher among males (5.2%) compared to females (1.1%). After adjusted regression analysis, HBV co-infected PLHIV had higher odds of virologic failure (AOR (95% confidence interval (CI)) = 6.3 (4.2–14.3)), immunosuppression (CD4 count < 500 cells/mm3) (AOR (95%CI) = 2.1(1.3–4.9)) and inflammation (hsCRP >10 mg/dL) (AOR (95%CI) = 9.2(4.3–14.6)). Immunosuppression was also significantly higher among syphilis co-infected PLHIV (AOR (95%CI) = 3.4 (1.3–5.2)).
Conclusions
Burden of HBV and syphilis co-infections is high particularly among male and adolescent PLHIV and these co-infections hinder virologic and immunologic outcome in Ethiopia. Hence, the program shall enhance HBV and syphilis testing and treatment.
Keywords:
Background
Human immunodeficiency virus (HIV), hepatitis B virus (HBV) and syphilis are priority global health problems [Citation1], which are commonly called sexually transmitted infections (STIs) [Citation2]. Globally, an estimated 38.4 million people were living with HIV (PLHIV) in 2021. An estimated 0.7% of adults aged 15–49 years worldwide are living with HIV although the burden considerably varies between countries and regions [Citation3]. Hepatitis B virus infects about 296 million people globally [Citation4] while syphilis caused over 14 million illnesses in 2019 an approximately 60% increase from 8.8 million in 1990 [Citation5].
Studies have demonstrated a relationship between HIV and a number of STIs, including HBV and syphilis [Citation1,Citation2,Citation6–8]. The prevalence of HBV and syphilis co-infections was reported to be 10% and 13% in sub-Saharan Africa (SSA), respectively [Citation9,Citation10]. Studies in Ethiopia showed HIV and HBV co-infection is common among children, pregnant mothers and blood donors [Citation1,Citation6,Citation11–15]. Studies also reported the high burden of HBV and syphilis co-infection among elderly (≥50 years old) [Citation9,Citation10,Citation16,Citation17].
If not early diagnosed and treated, HBV and syphilis co-infections can cause severe complications, leading to high rates of morbidity and mortality [Citation9]. HBV and syphilis co-infections have potential risks to increase plasma HIV-1 RNA levels [Citation16]. Studies among PLHIV taking highly active antiretroviral treatment (HAART) indicated that HBV and syphilis co-infections could impact treatment outcomes and increase disease progression and fuel mortality among PLHIV [Citation7–9].
Previous studies were conducted among specific group of population including blood donors [Citation15], children [Citation17] and pregnant women [Citation13,Citation14]. Moreover, they did not address the impact of these co-infections on treatment outcome. Risk factors for the co-infections were also not thoroughly investigated. Therefore, the aim of the current study was to determine the prevalence of HBV and syphilis co-infections among PLHIV and evaluate their impact on HIV treatment outcome at community level.
Methods
Study design
In 2017/2018, Ethiopia conducted the Ethiopian Population-based HIV Impact Assessment (EPHIA), which was a nationwide household (HH) survey among urban dwellers. In the EPHIA study, HBV and syphilis testing were done for people diagnosed positive for HIV. CD4 count, viral load and high-sensitivity C-reactive protein (hsCRP) tests were also done to measure treatment outcome among PLHIV [Citation18,Citation19] ().
Figure 1. (A) Sampling and sample size determination and (B) detection of recent and long-term infection-HIV-1 LAg Avidity plus VL algorithm.
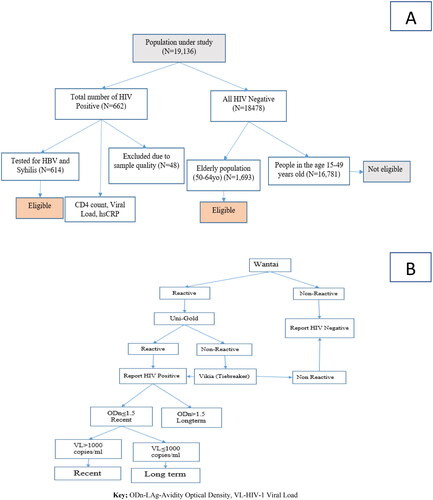
Criteria for inclusion of subjects 15–64 years: resides in selected HH or spent the night there the night before the survey, per the above definitions, and self-reported age 15–64 years, and for those between 18 and 64, is able and willing to provide written informed consent. For adolescents 15–17 years old, able and willing to provide written assent and parent/guardian able and willing to provide written informed consent/permission.
Sampling and sample size determination
EPHIA used a two-stage, age-stratified and cluster sampling approach. The sampling frame accounted all HHs in urban areas based on the 2007 Population and Housing Census in Ethiopia. The study considered 17,339 enumeration areas (EAs) consisting of 3,025,379 HHs and 11,862,821 individuals (i.e. an average of 175 HHs and 684 individuals per EA).
The first stage selected 395 EAs using a probability proportional to population size of the regions, including nine administrative regions (Tigray, Afar, Amhara, Oromia, Ethiopian Somali, Benishangul-Gumuz, SNNPR, Gambella and Harari) and two city administrations (Addis Ababa, and Dire Dawa). In the second stage, a sample of HHs was randomly selected from each EA using an equal probability method, 30 HH per EA and the actual number per EA ranging from 15 to 60.
The sample size was calculated to provide a national estimate of viral load suppression (VLS) with assumed prevalence of VLS = 50% at 95%CI of ±5%. Accordingly, the target sample size was 18,139 for adults (15–49 years old) and 1777 for elderly (50–64 years old). We finally collected data from 19,136 participants with response rate of 96.1% [Citation20]. Details about the sampling sample size determination were published (EPHIA_Report_280820_Web.pdf (columbia.edu)). Among those, 662 study participants were HIV positive who were part of this analysis to determine prevalence of HBV and syphilis co-infections ().
Data collection
Field laboratory and questionnaire data were collected on mobile tablet devices using a programmed application in Open Data Kit (ODK). Interview was administered to people aged ≥15 years old that included demographic characteristics, sexual and reproductive health, marriage, male circumcision, sexual activity, HIV/acquired immune deficiency syndrome (AIDS) knowledge and attitudes, the HIV testing and treatment history. Adolescent questionnaire (for children aged 12–14 years old), contained questions from the adult questionnaire, which included sexual activity, demographic characteristics, exposure to HIV prevention programs and HIV-related risk behaviours. The questionnaire was administered in the most common five languages used in Ethiopia (Amharic, Oromiffa, Tigrigna, Afarigna and Somaligna).
Laboratory testing
Blood sample collection, transport and storage
Whole blood was collected in two ethylenediaminetetraacetic acid (EDTA)-coated test tubes of 3–5 mL each, and plasma was extracted using centrifugation at 2000 revolutions per minute (RPM). Two separate aliquots of the plasma were taken and, one was sent to the regional site for testing while the other to the national reference laboratory for storage at −80 °C. The samples were labelled and stored in temperature-controlled boxes at the field level, transported to a testing laboratory.
HIV testing
HIV testing and counselling (HTC) was conducted in the selected HHs using the Ethiopian national HIV testing algorithm for rapid-testing: Wantai (Beijing Wantai Biological Pharmacy Enterprise Co., Ltd., Beijing, China) as the first test for screening, then Uni-Gold (HIV 1/2™, Trinity Biotech Plc., Wicklow, Ireland) as confirmatory assay. Finally, Vikia (HIV 1/2, bioMérieux, SA, Marcy-l’Etoile, France) was used as a tiebreaker ().
CD4 count and viral load testing
CD4 count measurement was done for PLHIV in the field using CD4 Analyzer (Pima™, Abbott Molecular Inc., Chicago, IL, formerly Alere). HIV-1 VL (HIV RNA copies per mL) was measured from the plasma using Roche (COBAS® AmpliPrep/COBAS® TaqMan® HIV-1 Test, Roche Diagnostics, Indianapolis, IN).
ARV detection
For the purpose of classifying recent and long-term infections and identifying whether people are taking HAART, qualitative screening for antiretrovirals (ARVs) was conducted from dried blood spot (DBS) using high-resolution liquid chromatography, which was coupled with tandem mass spectrometry. Testing for ARVs was performed at the Division of Clinical Pharmacology of the Department of Medicine (University of Cape Town in South Africa).
HBV and syphilis testing
Blood samples were tested for HBV surface antigen (HBsAg) using a testing device Determine (Alere Medical Co., Ltd., Chiba, Japan), One Step Strip HBsAg test which had a specificity of 99.9% and sensitivity of 99.5% [Citation21]. Moreover, samples were also tested for syphilis using a strip Chembio Dual Path Platform (DPP) Syphilis Assay (Hauppauge, New York, NY) that had a sensitivity 98.8% and specificity 99.4% [Citation22].
Recency testing
Recent infection was estimated based on the number of PLHIV as recent with the HIV-1 VL algorithm plus LAg Avidity as well as ARV detection in blood. This was a technique approved by The World Health Organization (WHO) and obtained using the formula recommended by the WHO Incidence Working Group [Citation23]. Accordingly, the assay performance characteristic of a mean duration of recent infection was (MDRI = 130 days) ().
Statistical analysis
Statistical analysis was performed using STATA V17.0 (StataCorp, College Station, TX). Descriptive statistics were used to provide summary measures (frequencies, means, median and IQR). Multivariable logistic regression model was conducted to evaluate determinants of HBV and syphilis co-infection and assess their impact on HIV treatment outcome. Predictor variables with a significant level <0.2 in bivariable analysis were considered for inclusion of the multivariable regression (i.e. adjusted odds ratio). Each of the outcome variables was adjusted for each and statistical significance was determined at p value of <.05.
Predetermined cutoff values were used to characterize viral load, CD4 count and hsCRP. Briefly, virologic failure was defined if viral load >1000 copies/mL, immunosuppression if CD4 count of <500 cells/mm3 [Citation24,Citation25], and disease progression or inflammation if hsCRP >10.00 mg/dL [Citation26–28]. We further classified the CD4 count, viral load and hsCRP in to different ranges to identify specific ranges and link with the different co-infections to further characterize treatment outcome.
Results
Demographic characteristics
A total of 19,136 study participants included in the EPHIA study, of whom about two-third of the study participants were female (). Of the total, 662 were HIV positives, means the proportion of HIV positives was 3.5% (95%CI = 3.2–3.7%) among sampled urban dwellers in Ethiopia with significant difference in between male (1.9%) and female (4.1%) participants (). All HIV positives were eligible for HBV and syphilis testing, but 48 had sample quality problems, so 614 HIV positives were finally included in the co-infection analysis. Among the HIV positive population, a quarter of the study participants were from Oromia (24.3%) region followed by Amhara (19%) and Addis Ababa (14%).
Table 1. Demographic characteristics among people living with HIV in Ethiopia (2017/2018).
Table 2. Burden of HIV, HBV and syphilis co-infection among PLHIV in Ethiopia (2017/2018).
HBV and syphilis co-infection among HIV positive people
Overall prevalence of HBV among PLHIV was 5.5%. The co-infection was 7.8% among males and 4.8% among females. The highest prevalence of HBV co-infection was observed among the age group 10–19 years (12.9%). HBV co-infection was also higher among never married (8.8%) and divorced/widowed (7.1%). The lowest wealth quintile had the highest HBV co-infection (7.8%). Double-HBV-syphilis co-infection was 5.9% (, ).
Figure 2. (A) Overall prevalence of HIV, HBV and syphilis co-infection in urban Ethiopia and (B) HIV, HBV and syphilis co-infection disaggregated by region.
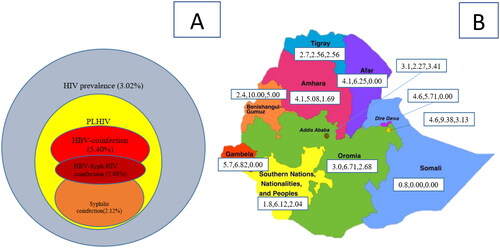
The overall prevalence of syphilis co-infection was 2.2%, it was higher among male (5.2%) compared to 1.1% among female. The co-infection was higher among people aged ≥50 years (3.5%), with multiple sexual partners (4.0%), with no viral suppression (3.3%), with CD4 count <500 cells/mm3 (2.9%) and those who were HAART-naive (4.2%) (, ).
HIV, HBV and syphilis co-infection disaggregated by region
HBV and syphilis co-infections were heterogeneous among the different regional administrations in the country. The highest prevalence of HBV co-infection was observed in Benishangul-Gumuz, Harari and Oromia regions with 10.0%, 9.4% and 6.7%, respectively. The highest prevalence of syphilis co-infection was also in Benishangul-Gumuz, Addis Ababa and Harari with 5.0%, 3.4% and 3.1%, respectively ().
Risk factors of HBV and syphilis co-infection
The odds of HBV co-infection among HIV positives were higher among males (AOR (95%CI) = 2.7 (1.7–4.0)), adolescents (AOR (95%CI) = 5.22 (2.03–11.43)) and syphilis positives (AOR (95%CI) = 2.4 (1.1–6.8)). The odds of syphilis co-infection were also higher in those with CD4 count <500 cells/mm3 (AOR (95%CI) = 2.5 (1.5–11.7)) and HAART-naive (AOR (95%CI = 3.1 (1.2–10.2) ().
Table 3. Predictors of HBV and syphilis co-infections among PLHIV in Ethiopia (2017/2018).
Impact of HBV and syphilis co-infection on HIV treatment outcome
Higher rates of HIV virologic failure (44.1%), immune suppression 44.3% and inflammation (67.6%) were observed among HBV co-infected people compared to the respective values of 28.8%, 38.3% and 18.6% among HBV negatives (, ). In other words, being HBV coinfected was associated with high virologic failure (AOR (95%CI) = 6.3 (4.2–14.3)), risk immunosuppression (AOR (95%CI) = 2.1 (1.3–4.9)) and occurrence of inflammation (AOR (95%CI) = 9.2 (4.3–14.6)).
The rates of HIV virologic failure (46.2%), immunosuppression (39.1%) and inflammation (30.8%) were also significantly higher among syphilis positive patients compared to their respective values of 29.3%, 15.4% and 21.13% among the syphilis negatives (, ).
Figure 3. Treatment outcome disaggregated by HBV and syphilis status among PLHIV in Ethiopia. (A) Treatment outcome disaggregated by HBV status and (B) treatment outcome disaggregated by syphilis status.
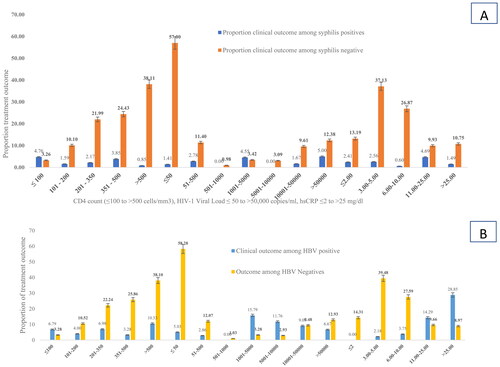
Table 4. Impact of co-infections on treatment outcome among PLHIV in Ethiopia (2017/2018).
Discussion
In this study, we have documented the burden of the two sexually transmitted co-infections (i.e. HBV and syphilis) and evaluated their impact on HIV treatment outcome. The co-infection rate of HBV and syphilis was 5.5% and 2.2% among people living with HIV in Ethiopia with significant variation in prevalence across regional states. The odds of HBV co-infection were higher among males, adolescents and syphilis positives, while syphilis co-infection was higher in patients with CD4 count <500 cells/mm3 and those who did not initiate HAART, p < .05. Both HBV and syphilis co-infections were significantly associated with increased virologic failure, immune suppression and inflammation, p < .05.
The overall prevalence of HBV co-infection in the current study (5.5%) was concordant with findings in various localities of Ethiopia: Addis Ababa (3.9%) [Citation29], Mekelle (5.9%) [Citation21] and Gondar (5.6%) [Citation30]. The similar prevalence of HBV among the general population (7%) [Citation31] or other population groups such as pregnant women (3.8–6%) [Citation11] and blood donors (4.7–10.9%) [Citation32] highlights that HIV infected people might not be different from other population segments with respect to the burden of HBV. Moreover, the current prevalence of HBV is lower compared to the 15% among HIV-infected people in sub-Sahara Africa, which might be due to the epidemiologic differences across countries [Citation2], and the difference might also be affected by difference in the HAART contents and effectiveness across country because certain HIV drugs such as lamivudine can reduce HBV incidence. Nonetheless, the observed magnitude of HBV is of concern and requires interventions to reduce consequences since the absolute number of people living with HIV in Ethiopia is large (N = 609,349) [Citation33].
The regional disparities in HBV co-infections that ranged from 0.0% in Somali to 10.0% in Benishangul-Gumuz was consistent with the prevalence of HIV among the general population. This could be explained by similarity with the route of transmission of HIV and HBV [Citation34]. On the hand, this study identified a relatively higher burden of HBV co-infection among male (7.8%) compared to women (4.8%) which is consistent with a previous study [Citation35]. However, this was contrary to HIV prevalence in Ethiopia, which is higher in female (4.1%) than male (1.9%) [Citation36]. This may require further investigation to answer why HBV co-infection is higher in male unlike HIV mono-infection. Furthermore, the highest prevalence of HBV co-infection among adolescents aged 10–19 years (12.90%) highlights the needs to strengthening targeted HBV vaccination and enhancing school-based interventions.
The 2.2% syphilis co-infection in our study is similar to the 2.3% national prevalence among the general population in Ethiopia [Citation13]. Other studies done in different areas of Ethiopia have also shown similar prevalence among pregnant women (2.3%) [Citation29], 2.9% [Citation13] and 3.9% [Citation21]. The prevalence of syphilis co-infection was the highest among people in the aged ≥50 years (3.5%) followed by age groups of 40–49 (3.3%) and 10–19 years (3.2%). Moreover, the higher syphilis co-infection among adolescents might be explained by recent high HIV infections, which highlight the interplay between new HIV infection with syphilis co-infections [Citation37].
The higher risk HBV co-infection among male (AOR (95%CI) = 2.7 (1.7–4.0)) is supported by similar studies in Zimbabwe [Citation35] and South Africa [Citation38]. This could be due to the fact that vaccination program for HBV was not inclusive of men. Moreover, CD4 count <500 cells/mm3 was also a risk for acquiring active syphilis (AOR = 2.45, 95%CI = 1.51, 11.71), which could be explained by syphilis reactivation as immunosuppression [Citation1]. On the other hand, syphilis infection itself was a risk for acquiring HBV (AOR (95%CI) = 2.4 (1.1–6.8)), which is also consistent with previous studies [Citation1,Citation18]. The genital ulcers associated with syphilis infection might facilitate the transmission of HBV infection [Citation16].
Co-infection of HBV with HIV complicates the clinical course, management and also adversely affect therapy for HIV infection [Citation30,Citation31]. Virologic failure was substantially higher among HBV co-infected people (44.1%) compared to the 28.8% among HBV negatives. Similarly, the 46.2% virologic failure among syphilis co-infected patients was substantially higher (AOR (95%CI) = 6.3 (4.2–14.3)) compared to the 29.3% in syphilis non-infected. This was consistent with a previous systematic review [Citation39]. This emphasizes the need for HBV and syphilis screening as part of the clinical management of HIV positives to improve virologic outcomes.
Similarly, immune suppression was higher among both HBV coinfected (AOR (95%CI) = 2.1 (1.3–4.9)), and this is consistent with previous findings [Citation2,Citation16,Citation31]. Moreover, it was also higher and syphilis co-infected patients (AOR (95%CI) = 3.4 (1.3–5.2)), and it is supported by reports in SSA [Citation40,Citation42]. Beyond the impact on treatment outcome, this study highlighted the effect of immune suppression on syphilis reactivation which was also previously reported [Citation41].
In addition, HBV positives had higher levels of hsCRP (AOR (95%CI) = 9.2 (4.3–14.6)). This may indicate the impact of HBV co-infection on the high level of inflammation which could lead to the different types of toxicities as revealed by different studies [Citation28,Citation45,Citation46].
This was the first community based nationwide study that could provide better insight about the burden of HBV and syphilis co-infections and its impact on HIV treatment outcome to the program and scientific community. However, since prevalence of HIV in the country was concentrated in urban Ethiopia (i.e. 3.0%) while it was 0.62% in rural, this study was conducted in urban area.
Conclusions
The rates of HBV and syphilis co-infections among HIV positive are comparable to their prevalence among the general population in Ethiopia. However, our findings highlight that the burden of these co-infections is substantially higher in male and adolescents. This calls for specific program interventions to address most affected population segments such as targeted vaccination for highly affected male and adolescents. HBV and syphilis co-infections hinder HIV virologic and immunologic outcomes. Moreover, immunosuppression and virologic failure in HIV patients can also lead to syphilis reactivation—a threat for high surge of syphilis in HIV positives with poor treatment compliance and responses. Hence, the national HIV/AIDS program shall enhance HBV and syphilis testing for PLHIV as part of the standard clinical management during the follow-up.
Author contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Ethical approval
Ethical approval was obtained from the Ethiopian Public Health Institute Scientific and Ethical Review Office (SERO) with approval number; EPHI-IRB-19111-2019. Confidentiality was respected during abstraction of data by the use of specific identification code for each enrolled patient number. Eligible study participants were identified by trained and experienced data collectors and supervisor at facility level. Written informed consent was also obtained from adults. Those aged 13–17 years old who because of being married, or having independent incomes, or have a living arrangement separate from the other family members, were considered emancipated minors. Hence, these segments of the study population (13–17) who were willing to provide written informed consent were included. Moreover, for adolescents (10–17) able and willing to provide written assent and parent/guardian able and willing to provide written informed consent/permission were included in the study. All methods were performed in accordance with the approved protocol, relevant guidelines and regulations.
Consent form
Authors and responsible authorities were informed and agreed for this publication.
Acknowledgments
The authors are grateful to the Chinese Center for Disease Prevention and Control, Zhejiang University, School of Medicine and the Ethiopian Public Health Institute for the support and facilitation of coordination. The authors would like to express their special thanks to all data collectors and the study participants.
Disclosure statement
The authors declare that they have no competing interests.
Data availability statement
Since data analysis for other objectives is ongoing, the raw data can be obtained from the first corresponding author.
Additional information
Funding
References
- Care P. Coinfections with hepatitis B and C virus and syphilis among HIV-infected clients in Southern Ethiopia: a cross-sectional study. HIV AIDS. 2017;9:1–11.
- Sun H, Sheng W, Tsai M, et al. Hepatitis B virus coinfection in human immunodeficiency virus-infected patients: a review. World J Gastroenterol. 2014;20(40):14598–14614. doi: 10.3748/wjg.v20.i40.14598.
- UNAIDS, Global AIDS Monitoring 2023. UNAIDS 2022 Guid. no. 3; 2023. p. 168 [Online]. Available from: https://indicatorregistry.unaids.org/sites/default/files/2023-global-aids-monitoring_en.pdf
- Lin C-L, Kao J-H. Hepatitis B viral factors and clinical outcomes of chronic hepatitis B. J Biomed Sci. 2008;15(2):137–145. doi: 10.1007/s11373-007-9225-8.
- Tao Y-T, Gao T-Y, Li H-Y, et al. Global, regional, and national trends of syphilis from 1990 to 2019: the 2019 global burden of disease study. BMC Public Health. 2023;23(1):754. doi: 10.1186/s12889-023-15510-4.
- Fan L, Yu A, Zhang D, et al. Consequences of HIV/syphilis co-infection on HIV viral load and immune response to antiretroviral therapy. Infect Drug Resist. 2021;14:2851–2862. doi: 10.2147/IDR.S320648.
- Gong H-Z, Hu K-R, Lyu W, et al. Risk factors for the co-infection with HIV, hepatitis B and C virus in syphilis patients. Acta Derm Venereol. 2020;100(17):1–6.
- Bagheri Amiri F, Mostafavi E, Mirzazadeh A. HIV, HBV and HCV coinfection prevalence in Iran – a systematic review and meta-analysis. PLOS One. 2016;11(3):e0151946. doi: 10.1371/journal.pone.0151946.
- Saghir SAM, Al-Hassan F, Al-Salahi OSA, et al. Frequencies of HBV, HCV, HIV, and syphilis markers among blood donors: a hospital-based study in Hodeidah, Yemen. Trop J Pharm Res. 2012;11:132–136.
- Getaneh Y, Lusida MI. Burden of HIV, HBV and syphilis among children in urban Ethiopia: community-based cross-sectional study. HIV Med. 2023;24(6):1–15.
- Khamduang W, Ngo-Giang-Huong N, Gaudy-Graffin C, et al. Prevalence, risk factors, and impact of isolated antibody to hepatitis B core antigen and occult hepatitis B virus infection in HIV-1-infected pregnant women. Clin Infect Dis. 2013;56(12):1704–1712. doi: 10.1093/cid/cit166.
- Ramos JM, Toro C, Reyes F, et al. Seroprevalence of HIV-1, HBV, HTLV-1 and Treponema pallidum among pregnant women in a rural hospital in Southern Ethiopia. J Clin Virol. 2011;51(1):83–85. doi: 10.1016/j.jcv.2011.01.010.
- Kassa D, Gebremichael G, Tilahun T, et al. Prevalence of sexually transmitted infections (HIV, hepatitis B Virus, herpes simplex virus type 2, and syphilis) in pregnant women in Ethiopia: trends over 10 years (2005–2014). Int J Infect Dis. 2019;79:50–57.
- Shimelis T, Tadesse E. The diagnostic performance evaluation of the SD BIOLINE HIV/syphilis duo rapid test in Southern Ethiopia: a cross-sectional study. BMJ Open. 2015;5:1–5.
- Tafuri S, Prato R, Martinelli D, et al. Prevalence of hepatitis B, C, HIV and syphilis markers among refugees in Bari, Italy. BMC Infect Dis. 2010;10:213.
- Omatola CA, Lawal C, Omosayin DO, et al. Seroprevalence of HBV, HCV, and HIV and associated risk factors among apparently healthy pregnant women in Anyigba, Nigeria. Viral Immunol. 2019;32(4):186–191. doi: 10.1089/vim.2018.0140.
- Getaneh Y, Khairunisa S, Husada D, et al. Burden of HIV, HBV and syphilis among children in urban Ethiopia: community‐based cross‐sectional study. HIV Med. 2023;24(6):676–690. doi: 10.1111/hiv.13457.
- WHO. WHO HIV drug resistance report 2012; 2016 [cited 2016 Jan 30] [Online]. Available from: http://www.who.int/hiv/pub/drugresistance/report2012/en/
- Lau B, Sharrett AR, Kingsley LA, et al. C-reactive protein is a marker for human immunodeficiency virus disease progression. Arch Intern Med. 2006;166(1):64–70. doi: 10.1001/archinte.166.1.64.
- Ethiopian Public Health Institute. Ethiopia population-based HIV impact assessment; 2020. Final Rep. Vol. 3 [Online]. Available from: https://phia.icap.columbia.edu/wp-content/uploads/2020/11/EPHIA_Report_280820_Web.pdf
- Ikeako LC, Ezegwui HU, Ajah LO, et al. Seroprevalence of human immunodeficiency virus, hepatitis B, hepatitis C, syphilis, and co‑infections among antenatal women in a tertiary institution in South East, Nigeria. Ann Med Health Sci Res. 2014;4(6):954–958.
- Alberta Health Services’ Edmonton STI Clinic. Syphilis and HIV point‐of‐care testing pilot project. Canada: National Collaborating Centre for Infectious Diseases; 2013.
- Bwalya S, Alebachew S. Analysing regional performance and disparities in health outcomes in Ethiopia. UNDP Ethiopia No. 2; 2012.
- Humphreys E, Chang LW, Harris J. Antiretroviral regimens for patients with HIV who fail first-line antiretroviral therapy. Cochrane Database Syst Rev. 2009;2010(4):CD006517.
- Ross EL, Tanser F, Pei PP, et al. The impact of the 2013 WHO antiretroviral therapy guidelines on the feasibility of HIV population prevention trials. HIV Clin Trials. 2014;15(5):185–198.
- Vishwanath A, Quaiser S, Khan R. Role of high‑sensitivity C‑reactive protein measurements in HIV patients. Indian J Sex Transm Dis AIDS. 2016;37(2):123–128.
- Nkinda L, Patel K, Njuguna B, et al. C-reactive protein and interleukin-6 levels among human immunodeficiency virus-infected patients with dysglycemia in Tanzania. BMC Endocr Disord. 2019;19:1–8.
- Tan DHS, Rolon MJ, Figueroa MI, et al. Inflammatory biomarker levels over 48 weeks with dual vs triple lopinavir/ritonavir-based therapy: substudy of a randomized trial. PLOS One. 2019;310:1–13.
- Genetu K, Abere K, Tachbele E. Magnitudes and correlates of human immunodeficiency virus, hepatitis B virus, and syphilis among pregnant mothers attending antenatal care in Addis Ababa, Ethiopia. Infect Dis Obstet Gynecol. 2022;2022:6156613.
- Shiferaw MB, Tulu KT, Zegeye AM, et al. Liver enzymes abnormalities among highly active antiretroviral therapy experienced and HAART naïve HIV-1 infected patients at Debre Tabor Hospital, North West Ethiopia: a comparative cross-sectional study. AIDS Res Treat. 2016;2016:1–7. doi: 10.1155/2016/1985452.
- Gebremicael G, Tola HH, Gebreegziaxier A, et al. Incidence of hepatotoxicity and factors associated during highly active antiretroviral therapy in people living with HIV in Ethiopia: a prospective cohort study. HIV AIDS. 2021;13:329–336. doi: 10.2147/HIV.S283076.
- FMOH Ethiopia. National consolidated guidelines for comprehensive HIV prevention, care and treatment. Fmoh; 2018. p. 1–238 [Online]. Available from: file:///C:/Users/Tere/Desktop/University of South Africa (17-2017)/Documents on Cervical Cancer/July 22,2017/All Litratures/National Comprehensive HIV Care Guideline 2018.pdf
- Ethiopian Public Health Institute. HIV related estimates and projections in Ethiopia for the year 2021–2022. Addis Ababa: Ethiopian Public Health Institute; 2021.
- Liu Y, Zeng P, Wang J, et al. Hepatitis B virus infection in a cohort of HIV infected blood donors and AIDS patients in Sichuan, China. J Transl Med. 2014;12(1):1–8.
- Liu M, Li L, Zhao J, et al. Gender differences in demographic and clinical characteristics in patients with HBV-related liver diseases in China. PeerJ. 2022;10:e13828. doi: 10.7717/peerj.13828.
- The DHS Program. Ethiopia demographic and health survey; 2016 [Online]. Available from: https://dhsprogram.com/pubs/pdf/FR328/FR328.pdf
- Shegaw F, Yibeltal D, Tessema Z. Prevalence of HIV/AIDS among elderly people and associated factors in Habru Woreda, Amhara Region, Northeast Ethiopia. HIV/AIDS Res Palliat Care. 2020;12:411–423.
- Mdlalose N, Naidoo K, Yende-Zuma N, et al. High incidence and persistence of hepatitis B virus infection in individuals receiving HIV care in KwaZulu-Natal, South Africa. BMC Infect Dis. 2020;20:1–9.
- Teame G, Gebreyesus A, Tsegay E, et al. Hepatitis B and C viral coinfections and their association with HIV viral load suppression among HIV‑1 infected patients on ART at Mekelle Hospital, Northern Ethiopia. AIDS Res Ther. 2022;19:1–10.
- World Health Organization. C-reactive protein concentrations as a marker of inflammation or infection for interpreting biomarkers of micronutrient status. Vitamin and Mineral Nutrition Information System; 2014. p. 1–4 [Online]. Available from: http://apps.who.int/iris/bitstream/10665/133708/1/WHO_ NMH_NHD_EPG_14.7_eng.pdf?ua=1
- Viegas EO, Tembe N, Macovela E, et al. Incidence of HIV and the prevalence of HIV, hepatitis B and syphilis among youths in Maputo, Mozambique: a cohort study. PLOS One. 2015;10:1–15.
- Cheng Z, Lin P, Cheng N. HBV/HIV coinfection: impact on the development and clinical treatment of liver diseases. Front Med. 2021;8(October):1–15. doi: 10.3389/fmed.2021.713981.
- Wu P-Y, Chen M-Y, Hsieh S-M, et al. Comorbidities among the HIV-infected patients aged 40 years or older in Taiwan. PLOS One. 2014;9(8):e104945. doi: 10.1371/journal.pone.0104945.
- Buchacz K, Patel P, Taylor M, et al. Syphilis increases HIV viral load and decreases CD4 cell counts in HIV-infected patients with new syphilis infections. AIDS. 2004;18(15):2075–2079. doi: 10.1097/00002030-200410210-00012.
- Sadiq ST, McSorley J, Copas AJ, et al. The effects of early syphilis on CD4 counts and HIV-1 RNA viral loads in blood and semen. Sex Transm Infect. 2005;81(5):380–385. doi: 10.1136/sti.2004.012914.
- Boulware DR, Hullsiek KH, Puronen CE, et al. Higher levels of CRP, D-dimer, IL-6, and hyaluronic acid before initiation of antiretroviral therapy (ART) are associated with increased risk of AIDS or death. J Infect Dis. 2011;203(11):1637–1646. doi: 10.1093/infdis/jir134.
