Abstract
Introduction
Distal radius fracture (DRF) is a common injury in the upper extremities. Blood flow restriction (BFR) has been proven to be effective in improving function in low-load training, which is suitable for post-op rehabilitation. We explored the effectiveness and safety of BFR therapy in DRF patients who underwent surgery.
Materials and methods
Thirty-five patients were randomly assigned to either the BFR or the regular training (RT; no BFR therapy) groups. All patients completed the same 4-week postoperative rehabilitation program, including anti-inflammatory treatments, strengthening and range of motion (ROM) training. In the BFR group, the pressure was 120 mmHg in strengthening training course. Pain, circumferences of wrists and forearms, ROM, muscle strength, and D-dimer levels were evaluated at weeks 0, 2, and 4. Radius union scoring system (RUSS) was measured at weeks 4 and 12. Finally, wrist functionality (Cooney modification) was evaluated at week 12.
Results
The BFR group had significantly decreased pain levels compared with the RT group (p < 0.01, effect size= 2.33, −2.44 at weeks 2 and 4). Swelling was effectively relieved in both groups. The wrist swelling was less in the BFR group (p < 0.01, effect size = −2.17 at week 4). The isometric strength of wrist extension (p < 0.01, effect size = 1.5, 3.02 at weeks 2 and 4), flexion (p < 0.01, effect size = 1.33, 2.53 at weeks 2 and 4), and functionality significantly increased in the BFR group (p < 0.01, effect size = 2.80 at week 12). No risk of VT in the BFR group was found. BFR did not threaten bone healing.
Conclusions
In patients with DRF who underwent corrective surgery, BFR therapy effectively relieved pain and swelling, increased muscle strength and wrist function, and had no additional risks for bone healing and VT.
KEY MESSAGES
BFR therapy can significantly reduce pain, strengthen muscles, and improve function.
BFR therapy did not significantly improve passive ROM, and further research is needed to determine its ability to reduce swelling.
BFR therapy is safe and effective for DRF patients after ORIF, but requires individualized protocols and frequent assessments. Further research is needed for other orthopedic surgeries.
Introduction
Distal radius fracture (DRF) is the most common fracture of the upper extremities seen in the emergency department, accounting for one-sixth of fractures treated in the United States [Citation1]. Open reduction and internal fixation (ORIF) is often performed to retain wrist function [Citation2]. While surgery provides the opportunity for early mobilization, good outcomes are highly dependent on the post-operative rehabilitation [Citation3]. Due to pain and inflammation, and for fixation stability, early rehabilitation utilizes low-load training (mostly passive mobilization and low-load active movement) [Citation4]. Although low-load active training facilitates a good passive range of motion (ROM), it is not ideal for maintaining muscle strength. The American College of Sports Medicine (ACSM) [Citation5] recommends performing 70% maximum voluntary isometric contraction (MVIC) or one repetition maximum for effective muscle strengthening. However, this load is extremely high for patients following fracture surgery as it could cause bone union, fixation failure, or significant discomfort.
Blood flow restriction (BFR) is a technique in which a compression bandage is placed on the limb to reduce blood flow and venous blood pooling, inducing a hypoxic milieu, creating an ischemic and anoxic condition and inducing localized cellular and hormonal changes that contribute to muscle hypertrophy [Citation6,Citation7]. Under these conditions, the muscle can obtain a training effect similar to that of high-load but in low-load conditions. Previous research have demonstrated the efficacy of BFR in promoting muscle hypertrophy and strength gains [Citation6,Citation8]. These studies have primarily focused on athletes [Citation9,Citation10], and individuals undergoing conventional treatment for musculoskeletal conditions, including knee osteoarthritis (OA) [Citation9] and arthroscopic surgery, such as anterior cruciate ligament (ACL) reconstruction [Citation9–11]. However, literature focusing on BFR and muscle atrophy as well as on further evaluation of the safety of BFR in patients following a fracture surgery is needed [Citation12]. Delayed active movement is crucial to fracture stability, but fracture management and post-operative rehabilitation should be balanced. A previous study reported BFR as a safe and effective postoperative treatment for patients with DRF [Citation13]. However, the sample size used in that study was small, only nine patients [Citation13]. Furthermore, BFR safety was evaluated using radiographic measures that focuses on bone healing but does not consider the risk of venous thrombosis (VT), a possible consequence of the reduced blood flow [Citation14]. This study aimed to answer the following questions: (i) what is the efficacy of BFR on a large sample of patients with DRF following ORIF surgery, and (ii) is it safe to use BFR in post-operative patients in terms of effect on blood coagulation. The primary outcome was the wrist strength and wrist function after treatment, and the secondary outcomes included pain severity, limb circumference, ROM, bone healing and status of blood coagulation.
Materials and methods
Study design
Before conducting this prospective randomized controlled study, ethical approval was obtained from the Ethical Research Committee at the First Affiliated Hospital of Chongqing Medical University, and the study was registered in the Chinese Clinical Trial Registry (ChiCTR2300069469). The treatment allocations were randomly assigned using a sealed opaque envelope in which two papers, labeled ‘1’ and ‘2’ were contained. Each patient picked one piece of paper to be assigned to the BFR or regular therapy (RT) group then returned the paper to the envelope Data collection and analysis were blinded to group allocation.
Patient selection
A pilot study was conducted to calculate the required sample size (eight patients in each group). As the primary outcome of this trial was strength, the sample size was calculated by the strength of wrist extension and flexion (ratio of the unhealthy side/healthy side) after four weeks of training. A two-sample design was used, with an assumption of α = 0.05 and 70% power(1-β). The sample size was calculated using the mean and standard deviation (SD) of wrist flexor and extensor strength results (wrist flexor strength: BFR group 0.7834 ± 0.0695, RT group 0.5213 ± 0.0689; wrist extensor strength: BFR group 0.7856 ± 0.1817, RT group 0.5054 ± 0.0598). G Power was used to calculate the sample size. Based on the results, 17 patients were required, in each group. After accounting for a 10% drop-out rate, 40 patients were required to ensure the power of the study. Patients >18 years old who underwent ORIF surgery for DRF were recruited to this study 3–7 days post-operatively. The DRF fracture type is classified as type A (including A2 and A3), which is an extra-articular fracture based on AO (Arbeitsgemeinschaft für Osteosynthesefragen/Association of the Study of Internal Fixation) classification [Citation15]. Patients with peripheral vessel disease, deep vein thrombosis (DVT), diabetes, severe cardio-pulmonary disease (obvious difficulty in breathing when performing strength training, Borg CR10 scale score > 5) [Citation16], or other conditions (mental illness or sensitive to pain etc.) that could influence completing the trial were excluded. A member of the research group enrolled participants, while another member assessed enrolled patients to exclude some who did not meet inclusions and exclusions and then assigned qualified participants to the trialAll patients signed informed consent statements.
Experimental procedure
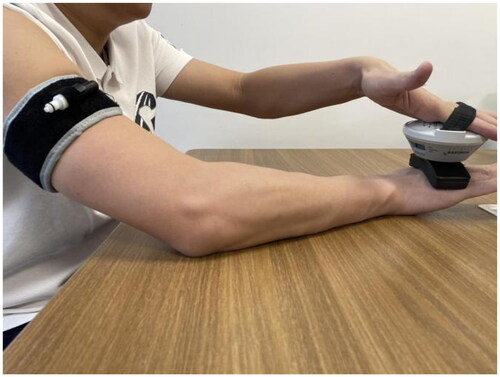
The trial was conducted over a 4-week period. Pre-trial baseline data of both arms included measurements of pain level (visual analog scale, VAS score) [Citation17]; circumference of wrists and 5 centimeters below the cubital crease; isometric strength (MVIC) of gripping (SAEHAN50111-7, Korea) [Citation18,Citation19], pinching (SAEHAN5011-7, Korea) [Citation20], and wrist flexion and extension (hand-held dynamometer, microFET2 America) [Citation21]; ROM of wrist flexion, extension, radial flexion, ulnar flexion, pronation and supination; and D-dimer (D-D). Gripping and pinching MVIC was evaluated by asking participants to maximally grip (and pinch the equipment (three times (five seconds for each maximal contraction with a one-minute break between each repetition). The mean value of these measurements was used in the analyses. The shoulder was perpendicular to the ground and the elbow was fully extended when testing grip strength. The wrist flexion and extension MVIC was acquired by using a hand-held dynamometer ( Participants were asked to maximally flex and extend three times (five seconds for each maximal contraction with a one-minute break between each repetition). The tolerance (mean value of the three attempts) was recorded. The palm was downward when testing extension strength and upward when testing flexion strength. The wrist was in a neutral position (0˚) with no angle changes during strength testing. ROM was assessed by a joint protractor ( All the above parameters were measured again in weeks 2 (mid-term records) and 4 (final records). The overall wrist function was assessed at the 12-week follow-up using the Cooney modification (modification of Green and O’Brie), a widely used and reliable scoring system for assessing wrist function [Citation22].The level of bone healing was evaluated using the radius union scoring system (RUSS) [Citation23], an indicator of bone healing, by observing the callus formation on X-rays in weeks 4 and 12. RUSS is a reliable method for a standardized assessment of radiographic union of DRF treated non-surgically or with ORIF [Citation23]. The circumference was defined as the difference between the affected and unaffected arm, measured in cm, to indicate the variation of limb swelling. As individuals may have different ROM limits, the ROM was defined as the difference between the two sides. These differences were used in the analyses, and the lower scores of these two parameters indicate a better condition. As individuals have different levels of strength, the pure strength data were not comparable between patients. Strength data of the operation sides were expressed as a proportion of the strength of the individual healthy sides to show the individualized variation, and higher scores indicate a better outcome.
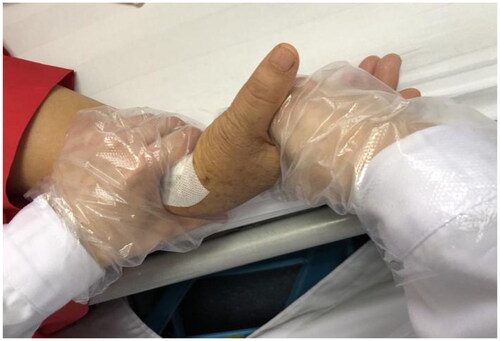
On postoperative days 3–7, pain management (use of NSAIDs and ice packs after training), swelling control (compression therapy for the forearm twice a day), and a physical therapy (ultrasound wave and low-frequency electricity therapy) were applied to control inflammation symptoms. In addition, full active ROM of the shoulder and elbow and gentle passive ROM of wrists and forearms were performed (). The wrist was passively mobilized until the patient reported pain (VAS < 4), not exceeding half of the full ROM. The wrist was helf in this position for five seconds then returned to the neutral position. The entire training session for passive ROM lasted approximately 15 min. Strength training included griping, pinching, and isometric contraction of wrist flexion and extension. All were conducted under a 20% MVIC load condition, which is an effective and safe intensity for muscle strengthening [Citation24]. Each strengthening course for different activities, such as gripping, pinching and wrist flexion was conducted in four sets of seventy-five repetitions each (30–15–15–15, respectively in each of the four sets, 5 s of contraction for each repetition) per trial [Citation25], with a 30-second break between each set, and a one-minute break between different strengthening courses [Citation26].The equipment used for assessment was also used for strength training.
Treatments in week 2 were similar to those of week 1, except for the range of passive mobilization for the wrists and forearms, which was gradually increased (50%-75% ROM), and isotonic contraction of wrist flexion and extension, which were performed in an anti-gravity direction (ROM of active mobilization was the limit of active ROM) (). In the final 2 weeks of the trial, in addition to the treatments described, advanced passive ROM training of the wrists and forearms was conducted and isotonic training in an anti-resistance direction (a resistant force of 20% MVIC, which has been proven to be safe and effective intensity for muscle strengthening, especially at the early phase after surgery [Citation24], and the ROM of active mobilization was the limit of active ROM) () was conducted [Citation24]. Four sets of seventy-five repetitions of anti-gravity and anti-resistance strength training for flexion and extension of the wrist were conducted with a 30-second break between each set and a one-minute break between wrist flexion and extension.
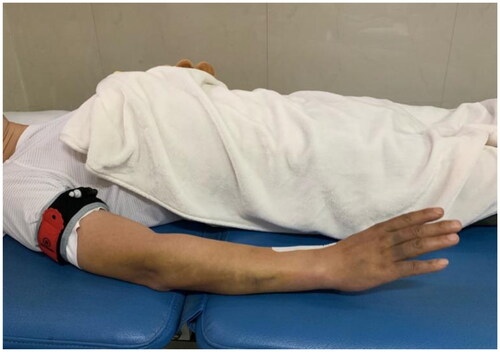
In terms of the rehabilitation treatment, the only difference between the patient groups was the additional application (five times/week for 4 weeks) of BFR therapy in the BFR group. During the BFR therapy, a bandage (5 cm width, B-strong, America) was tied on the upper one-third of the arm and worn throughout each 20-min session, with pressure set at 120 mm Hg () [Citation24,Citation27], a moderate pressure value, inducing 40%-80% arterial occlusion pressure, which is the recommended range for BFR exercise [Citation27].
Statistical analysis
Data-analysis was conducted using SPSS version 26 (IBM Corp., Armonk, NY, USA). VAS scores were compared between groups. Circumferences of wrists and forearms were measured within each group to determine changes over time, and between the healthy and surgery sides. Differences between these pairs of measurements were analyzed. Change scores of circumferences of wrists, forearms and ROM, strength, D-D data and Green and O’Brien Score for wrist functionality, and RUSS score between groups were evaluated. All statistical analyses were performed using a one-way ANOVA. The effect size of the primary outcome was calculated. Statistical significance was set at p < 0.05.
Results
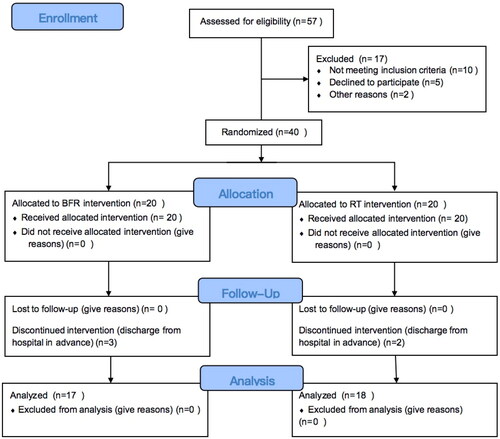
In total, 40 patients were recruited in the present study, while five patients (three in the BFR group and two in the RT group) failed to complete the whole trial due to personal issues. Therefore, the data for 35 patients (17 males and 18 females; mean age 46 years, range 23–72 years) were included in the final analysis (n = 17 in the BFR group, n = 18 in the RT group; ( All 35 patients completed the trial without complications. All patients were recruited 3–7 days after surgery, and there were no significant differences in time period between the operation and trial start, or in baseline pain levels between the two groups (p = 0.42 and 0.935, respectively). However, the midterm and final VAS scores were significantly lower in the BFR group (week 2, 0.65 ± 0.79; week 4, 0.06 ± 0.24) compared to scores in the RT group (week 2, 2.50 ± 0.62; week 4, 1.28 ± 0.67) (p < 0.01) (). The wrist and forearm circumference measurements of the operated arm significantly decreased in both groups at the end of the trial compared to measurements at baseline (p < 0.01) (Supplementary Material). In the BFR group, the affected wrist’s circumference at week 4 was significantly greater than that of the unoperated arm (p < 0.01), but no significant difference was found in the forearm circumference between the operated and unoperated arms (Supplementary Material). Similar results were found in the RT group (Supplementary Material). Regarding the change in scores for the wrist and forearm circumference, the wrist circumference decreased significantly in the BFR group at week 4 (BFR, 0.40 ± 0.44; RT, 1.51 ± 0.57) (p < 0.01), but no significant change occurred in forearm circumference in the RT group at week 4 (). No differences for the parameters of strength could be observed; however, at weeks 2 and 4, the wrist extension (week 2: BFR, 0.49 ± 0.11, RT, 0.34 ± 0.09; week 4: BFR, 0.72 ± 0.08, RT,0.52 ± 0.05) and flexion strength (week 2: BFR, 0.50 ± 0.10, RT, 0.38 ± 0.08; week 4: BFR, 0.70 ± 0.06, RT, 0.52 ± 0.08) were significantly greater than in the BFR group than in the RT group (all p < 0.001). The effect sizes of wrist extensor (week 2, effect size = 0.40; week 4: effect size = 0.67) and flexor strength (week 2, effect size = 0.32; week 4: effect size = 0.64) were high at weeks 2 and 4 ().
Table 1. Patient demographic data.
Table 2. Visual analog scale (VAS) scores of the level of pain on the operated side at 0, 2, and 4 weeks after surgery.
Table 3. Differences in the wrist and forearm circumferences (cm) changes on the operated side between the BFR and RT group.
Table 4. Strength proportion of the operated side (compared to the healthy side) of the BFR and RT groups at weeks 0, 2, and 4.
No significant differences were observed for any measurement of passive ROM between the groups (). At week 12, the wrist function in the BFR group significantly improved compared to that of the RT group (BFR, 87.35 ± 4.00; RT, 73.61 ± 5.64) and the effect size (0.68) was very large (p < 0.01) ().
Table 5. Range of motion (ROM) of the BFR and RT groups at weeks 0, 2, and 4.
Table 6. Cooney modification of the BFR and RT groups at week 12.
No significant differences were detected between the BFR and RT groups in D-D blood levels or in RUSS scores throughout the study period (Supplementary Material).
Discussion
Postoperative management of DRF plays a crucial role in the functional recovery of patients, as it directly determines the outcome of wrist and forearm function. Postoperative pain and mobilization fear can lead to muscle atrophy and decreased ROM following surgery, limiting the ability to perform daily activities. We compared 4 weeks of BFR and RT treatments in DRF patients following ORIF surgery and found that patients in the BFR group had significantly improved functionality compared to patients in the RT group. Moreover, the safety of the BFR treatment was validated by evaluating the change in D-D blood levels and callus formation.
Pain
The ability of BFR to significantly relieve pain in sports-related injuries is well documented. A study about quadriceps strengthening for patients with patellofemoral pain showed that patients undergoing BFR had a 93% greater reduction in pain (VAS scale) than those who did not [Citation28]. A previous meta-analysis concluded that the pain score was significantly reduced after BFR training [Citation29]. Similar results were found in the current study; the significant decrease in VAS scores in the BFR group at weeks 2 and 4 indicates the effectiveness of BFR on relieving pain following a DRF surgery. Previous studies compared low load with BFR groups and high load without BFR groups; hence, the reduction in pain may have also been attributed to the relatively lower load, which caused less strain and stress in the injured limb. In our study, the groups were trained using the same load, and a notable reduction in pain was consistently observed. Previous studies have reported the effects of BFR training on pain (reduces pain sensitivity) by inducing hypoalgesia and increasing circulation of opioids and endocannabinoids during low-intensity exercise [Citation30,Citation31]. These effects are more significant during low-intensity exercise with higher pressure BFR. Other mechanisms of BFR training reducing pain intensity have been reported, including the recruitment of high threshold motor units and the interaction between cardiovascular and pain regulatory system [Citation32]. In addition, BFR increases metabolism [Citation6,Citation7], reducing concentrations of localized inflammation factors faster, thus causing a decrease in pain intensity; and the pressure causes local ischemia, decreasing pain sensitivity, leading to reduced pain perception [Citation33,Citation34].Therefore, the effect of low-intensity exercise with BFR on pain reduction is significant and acceptable.
Circumference
Swelling is a common symptom after surgery that can be evaluated by measuring the circumference of the limbs. No significant differences in wrist and forearm circumference were detected between groups at week 0; however, significant decreases in circumference in both groups were detected at the end of the trial, compared to the baseline, suggesting that both BFR and RT relieved swelling. A significant decrease was observed in the wrist circumference of the BFR group alone from weeks 0 to 4, indicating that wrist swelling is reduced more significantly in the BFR group compared to the RT group. A previous study reported that BFR was effective in reducing effusion in patients following ACL reconstruction surgery, and thus reducing knee joint circumference [Citation34]. However, in that study, BFR resistance training was compared with heavy-load resistance training that may generate greater effusion than the lower load employed in the BFR group. Further research is needed to clarify the efficacy and mechanism of swelling reduction.
Although the ability of BFR to reduce swelling was validated by the significant difference in wrist circumference, no difference was found in the forearm circumference after BFR, and this needs further analysis. The strengthening exercises were focused on the forearm muscles; thus, if the ability of BFR to reduce swelling is accepted, the unaffected forearm circumference may be the result of muscle hypertrophy that increases the local circumferences. However, the forearm circumference of the unoperated arm was not significantly greater than that of the operated arm at week 4, indicating that there was no obvious muscle hypertrophy.
Strength
Wrist extension and flexion strength were significantly greater following BFR than after RT. The effect sizes of wrist extensor and flexor strength were very large at weeks 2 and 4, indicating that BFR is effective to increase wrist strength. Although the muscle strength significantly increased, the forearm circumference did not indicate muscle hypertrophy. Low-resistance training with reduced blood flow to the muscle could stimulate growth hormone and insulin-like growth factor-1, facilitating muscle cell growth and hypertrophy [Citation35]. In addition, BFR recruits more fast-twitch fibers that are more pronounced in muscular adaptation [Citation36], and could also lead to muscle hypertrophy. Although muscle hypertrophy was not directly revealed by the forearm circumference in this study, possibly because of the briefness of the trial or the relatively low training load, the increase in the strength of the BFR group was significant. Multiple postoperative rehabilitation studies have reported the efficacy of BFR for muscle strengthening [Citation6,Citation37]. Postoperative swelling and pain may hinder adequate strength training performance in most patients following surgery. The significant efficacy of BFR in reducing swelling and pain post-BFR has been reported in former discussions. This may explain the increased strength without obvious muscle hypertrophy. For a muscle hypertrophy effect, a longer duration of BFR training might be required. There were no significant differences of pinching and gripping strength results, possibly due to the imposed limit for patients to extend and flex their wrists in their daily life while there is no imposed limit for gripping and pinching. Thus, gripping and pinching strength might be similar in both arms. Although high-load training is more effective than low-load training with BFR for muscle hypertrophy [Citation38], high-load training is not suitable for patients post-surgery. In contrast, BFR in the low-load condition is suitable for patients across a wide spectrum of ages and physical capacities. Relatively lower or similar loads in the BFR group were as effective for strength training as those seen in the RT group, indicating a wider acceptable load range in BFR training. Thus, BFR is the better choice for postoperative strength training.
ROM
The effect of BFR on joint ROM is unclear. A previous study found that BFR significantly increased knee ROM following ACL reconstruction [Citation34]; however, a study on resistance training reported no significant change in ROM following low-load BFR [Citation39]. This is consistent with our results. Both the low-load BFR study and our present work measured passive ROM due to safety considerations, whereas the study on BFR after ACL reconstruction measured active ROM. Muscle strength increasing via BFR therapy can directly influence active ROM, but has limited impact on passive ROM. According to these findings, BFR training may help improve active rather than passive ROM.
Function
The ability of BFR to improve function has been widely accepted [Citation40,Citation41]. Similarly, in the current study, wrist function significantly increased after BFR therapy. This is a predictable result due to the significant decrease in pain and increase in muscle strength. However, the functional assessment was conducted at the week-12 follow up, 8 weeks after discharge, when patients had not yet engaged in regular training. Nevertheless, the better maintenance of functionality in the BFR group suggests that BFR could shorten the duration of symptoms and rehabilitation.
Safety
The status of blood coagulation should be considered in BFR training, especially in patients following orthopedic surgery. A reasonable and suitable use of BFR is not believed to pose any risks for VT [Citation42]. The D-D blood assay is a cost-effective, routine, and sensitive screening test that can help rule out DVT when the D-D concentration is <0.5 µg/mL [Citation14,Citation43,Citation44]. An elevated D-D concentration strongly and independently correlates with the incidence of stable thrombosis [Citation45]. We observed no significant change in D-D blood levels or incidence of VT in any of the patients in the BFR group, indicating that BFR does not create additional risk of VT. Previous studies have revealed similar findings, but mostly in patients following arthroscopic surgery [Citation6,Citation11,Citation37,Citation39].
Since BFR training requires active movement, fracture patients in the early postoperative phase may be at risk for delayed union or nonunion if BFR is performed too often or without good supervision and control of load and intensity. The safety of BFR training on bone healing for patients after DRF surgery was demonstrated by Sgromolo et al. [Citation13] reporting that fracture stability was maintained despite the implementation of BFR therapy. In the current study, no re-fracture occurred in either group and no significant differences in RUSS scores were found between the groups, indicating a similar level of callus formation. Moreover, no episodes of fainting or other symptoms of discomfort were recorded during the BFR training. Taken together, our results indicate that low-load incremental strength training with BFR can be performed safely in the early phase following ORIF.
Limitations
While this study provides a comprehensive picture of the effectiveness and safety of BFR training for patients following fracture surgery, it has limitations that should be acknowledged. We set the BFR pressure at 120 mm Hg but did not measure maximal limb occlusion pressure to determine individualized pressure settings during the trial. Hence, different patients may have had different reactions to the same pressure. This limitation should be addressed in future studies.
Even though the sample size was larger than that in a previous study of BFR training for DRF, the length of the trial was shorter due to some practical issues. Thus, the efficacy and safety of BFR over a longer period is unclear from our present work. Demonstrable muscle hypertrophy might require a longer duration of BFR therapy, and follow-up data obtained more than 12 weeks after surgery may be better suited to provide evidence of functional ability.
Finally, changes in circumference and muscle strength were similar between the operated and unoperated arms. Data were standardized based on the unoperated arm, and the subtle differences between the two arms were ignored, this could have resulted in data processing bias.
Conclusions
This study illustrates that BFR therapy can significantly reduce pain, strengthen muscles, and improve function. BFR did not significantly improve passive ROM, and its ability to reduce swelling requires further research. No safety issues in terms of VT risk and bone healing were observed for BFR. Therefore, it is safe and effective to apply BFR therapy as a postoperative rehabilitation therapy for DRF patients following ORIF. The BFR protocol should be tailored to the tolerance of each patient, and frequent assessments are warranted to guarantee safety and better outcomes. Future research is needed for more applications of BFR training following other orthopedic surgeries.
Authors’ contributions
All authors contributed to the study conception and design. Yi Fan: Study conception and design; drafting the article; data collection; data analysis and interpretation. Dingqun Bai: Study conception and design. Chongyuan Cheng: Data collection. Guihua Tian: Data collection; data analysis and interpretation; revising the article critically for intellectual content and final approval of the version to be published. All authors read, approved the final manuscript and agreed to be accountable for all aspects of the work.
Supplemental Material
Download MS Word (18 KB)Acknowledgments
The authors would like to thank the Orthopedics Department of The First Affiliated Hospital of Chongqing Medical University for supporting participants recruiting.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, Guihua Tian, upon reasonable request.
Additional information
Funding
References
- Petron DJ. Distal radius fractures in adults [updated 2021 Oct 21]. Available from: https://www.uptodate.com/contents/distal-radius-fractures-in-adults
- Thomas D, Zanin D. Hand rehabilitation after distal radius fracture. Hand Surg Rehabil. 2016;35:1–11. doi: 10.1016/j.hansur.2016.09.006.
- Quadlbauer S, Pezzei C, Jurkowitsch J, et al. Rehabilitation after distal radius fractures: is there a need for immobilization and physiotherapy? Arch Orthop Trauma Surg. 2020;140(5):651–663. doi: 10.1007/s00402-020-03367-w.
- Ikpeze TC, Smith HC, Lee DJ, et al. Distal radius fracture outcomes and rehabilitation. Geriat Orthopaed Surg Rehabil. 2016;7:202–205.
- American College of Sports Medicine (ACSM). Available at: https://www.acsm.org/docs/default-source/regional-chapter-individual-folders/mid-atlantic/013_marc_abstract_book.pdf?sfvrsn=38743946_2
- Filbay SR, Skou ST, Bullock GS, et al. Blood flow restriction training in clinical musculoskeletal rehabilitation: a systematic review and meta-analysis. Br J Sports Med. 2022;56(24):1465–1474.
- Lorenz DS, Bailey L, Wilk KE, et al. Blood flow restriction training. J Athlet Train. 2021;56(9):937–944. doi: 10.4085/418-20.
- Heitkamp HC. Training with blood flow restriction. Mechanisms, gain in strength and safety. J Sports Med Phys Fit. 2015;55:446–456.
- Ferraz RB, Gualano B, Rodrigues R, et al. Benefits of resistance training with blood flow restriction in knee osteoarthritis. Med Sci Sports Exerc. 2018;50(5):897–905. doi: 10.1249/MSS.0000000000001530.
- Wortman RJ, Brown SM, Savage-Elliott I, et al. Blood flow restriction training for athletes: a systematic review. Am J Sports Med. 2021;49(7):1938–1944. doi: 10.1177/0363546520964454.
- Lu Y, Patel BH, Kym C, et al. Perioperative blood flow restriction rehabilitation in patients undergoing ACL reconstruction: a systematic review. Orthopaed J Sports Med. 2020;8(3):232596712090682. doi: 10.1177/2325967120906822.
- Minniti MC, Statkevich AP, Kelly RL, et al. The safety of blood flow restriction training as a therapeutic intervention for patients with musculoskeletal disorders: a systematic review. Am J Sports Med. 2020;48(7):1773–1785. doi: 10.1177/0363546519882652.
- Sgromolo NM, Cancio JM, Rhee PC. Safety and efficacy of blood flow restriction therapy after operative management of distal radius fractures: a randomized controlled study. J Wrist Surg. 2020;9:345–352.
- Kruger PC, Eikelboom JW, Douketis JD, et al. Deep vein thrombosis: update on diagnosis and management. Med J Austr. 2019;210(11):516–524. doi: 10.5694/mja2.50201.
- Jayakumar P, Teunis T, Giménez BB, et al. AO distal radius fracture classification: global perspective on observer agreement. J Wrist Surg. 2016;06(01):46–53. doi: 10.1055/s-0036-1587316.
- Shariat A, Cleland JA, Danaee M, et al. Borg CR-10 scale as a new approach to monitoring office exercise training. Work. 2018;60(4):549–554. doi: 10.3233/WOR-182762.
- Sindhu BS, Shechtman O, Tuckey L. Validity, reliability, and responsiveness of a digital version of the visual analog scale. J Hand Therapy. 2011;24(4):356–364. doi: 10.1016/j.jht.2011.06.003.
- Huang L, Liu Y, Lin T, et al. Reliability and validity of two hand dynamometers when used by community-dwelling adults aged over 50 years. BMC Geriatr. 2022;22(1):580.
- Clifford MS, Hamer P, Phillips M, et al. Grip strength dynamometry: reliability and validity for adults with upper limb burns. Burns. 2013;39(7):1430–1436. doi: 10.1016/j.burns.2013.03.020.
- Brauers L, Smeets R, Feys P, et al. Test-retest reliability of static and dynamic motor fatigability protocols using grip and pinch strength in typically developing children. Eur J Pediatr. 2021;180(8):2505–2512. doi: 10.1007/s00431-021-04033-y.
- Chamorro C, Arancibia M, Trigo B, et al. Absolute reliability and concurrent validity of hand-held dynamometry in shoulder rotator strength assessment: systematic review and meta-analysis. Int J Environ Res Public Health. 2021;18(17):9293. doi: 10.3390/ijerph18179293.
- Kwok IH, Leung F, Yuen G. Assessing results after distal radius fracture treatment: a comparison of objective and subjective tools. Geriatr Orthop Surg Rehabil. 2011;2(4):155–160. doi: 10.1177/2151458511422701.
- Patel SP, Anthony SG, Zurakowski D, et al. Radiographic scoring system to evaluate union of distal radius fractures. J Hand Surg Am. 2014;39(8):1471–1479. doi: 10.1016/j.jhsa.2014.05.022.
- Loenneke JP, Wilson JM, Marín PJ, et al. Low intensity blood flow restriction training: a meta-analysis. Eur J Appl Physiol. 2012;112(5):1849–1859. doi: 10.1007/s00421-011-2167-x.
- Counts BR, Dankel SJ, Barnett BE, et al. Influence of relative blood flow restriction pressure on muscle activation and muscle adaptation. Muscle Nerve. 2016;53(3):438–445. doi: 10.1002/mus.24756.
- Fahs CA, Loenneke JP, Thiebaud RS, et al. Muscular adaptations to fatiguing exercise with and without blood flow restriction. Clin Physiol Funct Imaging. 2015;35(3):167–176. doi: 10.1111/cpf.12141.
- Credeur DP, Jones R, Stanford D, et al. Central cardiovascular hemodynamic response to unilateral handgrip exercise with blood flow restriction. Eur J Appl Physiol. 2019;119(10):2255–2263. doi: 10.1007/s00421-019-04209-3.
- Giles L, Webster KE, McClelland J, et al. Quadriceps strengthening with and without blood flow restriction in the treatment of patellofemoral pain: a double-blind randomised trial. Br J Sports Med. 2017;51(23):1688–1694. doi: 10.1136/bjsports-2016-096329.
- Li S, Shaharudin S, Kadir MR. Effects of blood flow restriction training on muscle strength and pain in patients with knee injuries: a meta-analysis. Am J Phys Med Rehabil. 2021;100(4):337–344. doi: 10.1097/PHM.0000000000001567.
- Hughes L, Patterson SD. The effect of blood flow restriction exercise on exercise-induced hypoalgesia and endogenous opioid and endocannabinoid mechanisms of pain modulation. J Appl Physiol. 2020;128(4):914–924. doi: 10.1152/japplphysiol.00768.2019.
- Hughes L, Grant I, Patterson SD. Aerobic exercise with blood flow restriction causes local and systemic hypoalgesia and increases circulating opioid and endocannabinoid levels. J Appl Physiol. 2021;131(5):1460–1468. doi: 10.1152/japplphysiol.00543.2021.
- Hughes L, Patterson SD. Low intensity blood flow restriction exercise: rationale for a hypoalgesia effect. Med Hypotheses. 2019;132:109370. doi: 10.1016/j.mehy.2019.109370.
- Song JS, Spitz RW, Yamada Y, et al. Exercise-induced hypoalgesia and pain reduction following blood flow restriction: a brief review. Phys Ther Sport. 2021;50:89–96. doi: 10.1016/j.ptsp.2021.04.005.
- Hughes L, Rosenblatt B, Haddad F, et al. Comparing the effectiveness of blood flow restriction and traditional heavy load resistance training in the post-surgery rehabilitation of anterior cruciate ligament reconstruction patients: a UK national health service randomised controlled trial. Sports Med. 2019;49(11):1787–1805. doi: 10.1007/s40279-019-01137-2.
- Takano H, Morita T, Iida H, et al. Hemodynamic and hormonal responses to a short-term low-intensity resistance exercise with the reduction of muscle blood flow. Eur J Appl Physiol. 2005;95(1):65–73. doi: 10.1007/s00421-005-1389-1.
- Saatmann N, Zaharia OP, Loenneke JP, et al. Effects of blood flow restriction exercise and possible applications in type 2 diabetes. Trends Endocrinol Metabol. 2021;32(2):106–117. doi: 10.1016/j.tem.2020.11.010.
- Erickson LN, Lucas KC, Davis KA, et al. Effect of blood flow restriction training on quadriceps muscle strength, morphology, physiology, and knee biomechanics before and after anterior cruciate ligament reconstruction: protocol for a randomized clinical trial. Phys Ther. 2019;99(8):1010–1019. doi: 10.1093/ptj/pzz062.
- Lixandrao ME, Ugrinowitsch C, Berton R, et al. Magnitude of muscle strength and mass adaptations between high-load resistance training versus low-load resistance training associated with blood-flow restriction: a systematic review and meta-analysis. Sports Med. 2018;48(2):361–378. doi: 10.1007/s40279-017-0795-y.
- Shiromaru FF, de Salles Painelli V, Silva Batista C, et al. Differential muscle hypertrophy and edema responses between high-load and low-load exercise with blood flow restriction. Scand J Med Sci Sports. 2019;29(11):1713–1726. doi: 10.1111/sms.13516.
- Clarkson MJ, Conway L, Warmington SA. Blood flow restriction walking and physical function in older adults: a randomized control trial. J Sci Med Sport. 2017;20(12):1041–1046. doi: 10.1016/j.jsams.2017.04.012.
- Rodrigues R, Ferraz RB, Kurimori CO, et al. Low-load resistance training with blood-flow restriction in relation to muscle function, mass, and functionality in women with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2020;72(6):787–797. doi: 10.1002/acr.23911.
- Bond CW, Hackney KJ, Brown SL, et al. Blood flow restriction resistance exercise as a rehabilitation modality following orthopaedic surgery: a review of venous thromboembolism risk. J Orthop Sports Phys Ther. 2019;49(1):17–27. doi: 10.2519/jospt.2019.8375.
- Saleh J, El-Othmani MM, Saleh KJ. Deep vein thrombosis and pulmonary embolism considerations in orthopedic surgery. Orthoped Clin North Am. 2017;48(2):127–135. doi: 10.1016/j.ocl.2016.12.003.
- Tritschler T, Kraaijpoel N, Le Gal G, et al. Venous thromboembolism: advances in diagnosis and treatment. J Am Med Associat. 2018;320(15):1583–1594. doi: 10.1001/jama.2018.14346.
- Halaby R, Popma CJ, Cohen A, et al. D-Dimer elevation and adverse outcomes. J Thromb Thrombolysis. 2015;39(1):55–59. doi: 10.1007/s11239-014-1101-6.