Abstract
Background
This study aimed to evaluate the redox status, antioxidant barrier, and oxidative damage to proteins, lipids, and DNA in patients with gastric cancer (GC). We are also the first to assess the diagnostic utility of redox parameters in patients with GC with respect to histopathological parameters.
Methods
Fifty patients with gastric cancer and 50 healthy controls matched for sex and age were included in the study. The antioxidant barrier, redox status, and oxidative damage products were measured in serum/plasma samples using colorimetric or spectrophotometric methods.
Results
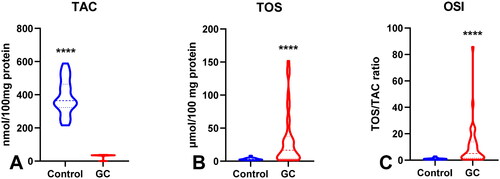
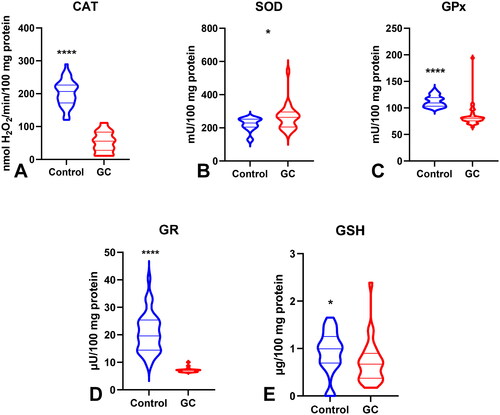
The activity of superoxide dismutase – SOD (p < 0.05) was significantly higher, whereas the activities of catalase – CAT (p < 0.0001), glutathione peroxidase – GPx (p < 0.0001), glutathione reductase – GR (p < 0.0001), and reduced glutathione – GSH (p < 0.05) were considerably lower in GC patients than in the control group. The levels of total oxidant status – TOS (p < 0.0001), oxidative stress index – OSI (p < 0.0001), advanced oxidation protein products – AOPP (p < 0.0001), ischaemia modified albumin – IMA (p < 0.01), lipid hydroperoxides – LOOH (p < 0.0001), 8-IsoProstane − 8-Iso-P (p < 0.0001), and DNA/RNA (p < 0.0001) were significantly higher, and the levels of total antioxidant capacity – TAC (p < 0.0001) and total thiols (p < 0.0001) were considerably lower in patients compared to the healthy controls. Some redox parameters are characterized by high AUC values in patients with differentiated GC according to histopathological parameters.
Conclusions
Gastric cancer is strongly linked to a systemic redox imbalance and increased oxidative damage to proteins, lipids, and DNA. Redox biomarkers are potential diagnostic indicators of gastric cancer advancement.
Key messages
Gastric cancer is associated with redox imbalance and increased oxidative damage to proteins, lipids and DNA.
Histopathological parameters of the tumour, such as size, histological type, histological differentiation grade, tumour invasion depth, presence of lymph node and distant metastasis, Lauren and Goseki classification, and presence of vascular and neural infiltration, are associated with the level of antioxidants and oxidative damage products of proteins, lipids, and DNA.
Determination of redox parameters may be useful in the assessment of the tumour progression.
1. Introduction
According to Global Cancer Observatory (GLOBOCAN) data from 2020, gastric cancer (GC) was classified as 4th most common type of cancer in the world among men and 7th most frequent cancer in women. Moreover, GC is responsible for 7.7% of all cancer deaths, places it 4th in terms of mortality among all cancers. Despite the fact that in recent decades the incidence and mortality due to gastric cancer decreased, GLOBOCAN prognosis that in 2040 the number of new cases of gastric cancer will increase from 1.09 in 2020 to over 1.8 million in 2040 and the number of deaths will come from 769,000 to 1.27 million [Citation1]). Moreover, about 95% of all gastric cancer cases are diagnosed in patients over 40 years old, but in recent years, there has been a considerable increase in the incidence of gastric cancer in people under 40 years of age [Citation2,Citation3]. Therefore, scientists have focused on the risk factors and mechanisms participating in gastric cancer development, especially in the young. It is well known that gastric carcinogenesis is closely linked to Helicobacter pylori infection, inherited cancer predisposition syndromes e.g. Lynch syndrome, juvenile polyposis syndrome (JPS), or Peutz-Jeghers syndrome; lifestyle, psychosocial environment, socioeconomic status, and dietary habits [Citation2]. Lifestyle and dietary habits such as stress, smoking, drinking alcohol, and high-fat or high-carbohydrate diets may also be responsible for the overproduction of reactive oxygen species (ROS), which leads to the development of oxidative stress in the case of an ineffective antioxidant barrier [Citation4]. Although ROS in physiological concentrations act as regulators of cellular signal transduction and energy transmission, imbalance between the generation and scavenging capability of ROS may result in cellular damage. ROS can react with proteins and lipids, transforming them into xidized forms or by damaging the structure of DNA and tumour suppressor genes that promote carcinogenesis [Citation5,Citation6]. It has been observed that oxidative stress-mediated signalling affects all features of cancer cell behaviour, such as cell cycle progression and proliferation, cell morphology and survival, apoptosis, cell-cell adhesion, cell motility, and energy metabolism. ROS, through the activation of transcription factors, including NF-κB, activator protein 1 (AP1), and hypoxia inducible factor 1α (HIF1α), regulates the expression of genes associated with cancer cell growth and survival, angiogenesis, invasion, and metastasis [Citation7].
In our previous studies focusing on the assessment of oxidative stress in colorectal, we demonstrated that CRC is associated with both enzymatic and non-enzymatic redox imbalance, as well as increased oxidative damage to proteins and lipids. Moreover, we demonstrated that many redox biomarkers can differentiate CRC patients from healthy individuals with high sensitivity and specificity [Citation8,Citation9]. We also showed that serum catalase (CAT) may be a potential non-invasive biomarker of tumour invasion depth [Citation8]. However, little is known about the clinical significance and diagnostic utility of oxidative stress/redox parameters in patients with gastric cancer. Therefore, the aim of our study was to evaluate the redox status, enzymatic and non-enzymatic antioxidants, and oxidative damage to proteins, lipids, and DNA in gastric cancer patients compared to healthy controls, as well as to assess the diagnostic utility of oxidative stress parameters using receiver operating characteristic (ROC) curve analysis. We are also the first to assess the redox parameters in gastric cancer patients with respect to histopathological classifications (TNM, Lauren’s and Goseki’s classification) and histopathological parameters such as histological type, histological differentiation grade, and presence of vascular or neural infiltration and desmoplasia.
2. Patients and methods
2.1. Study group
The study included 50 patients diagnosed with gastric cancer.were surgically treated in the 2nd Clinical Department of General and Gastroenterological Surgery Unit at the Medical University of Bialystok Clinical Hospital between 2017 and 2020. Patients were included in the study group based on a diagnosis of adenocarcinoma at any stage without the coexistence of other systemic diseases who did not receive radiotherapy or chemotherapy before the surgery. The exclusion criteria were as follows: squamous cell carcinoma and other non-epithelial neoplasms, metastases of other neoplasms to the stomach, acute inflammatory diseases, infectious and autoimmune diseases (HIV/AIDS, hepatitis, Crohn’s and Hashimoto’s disease, ulcerative colitis, rheumatoid arthritis, and psoriasis), cardiovascular diseases, metabolic diseases such as osteoporosis, type 1 diabetes, mucopolysaccharidosis and gout, and digestive, respiratory, or genitourinary system diseases. Additionally, smokers and patients taking other drugs (antibiotics, non-steroidal anti-inflammatory drugs, glucocorticosteroids, vitamins, and dietary supplements) for three months before the surgery were excluded from the study. Lack of complete medical documentation was also an exclusion criterion.
The time from cancer diagnosis to surgical resection of the tumour ranged from 2 to 4 weeks. The study material was obtained from all patients prior to surgery.
The control group comprised 50 healthy patients (selected by sex and age to match the study group) who participated in the follow-up study from January 2018 to January 2020. The control group included qualified patients with normal complete blood count (CBC) and biochemical blood test results (Na+, K+, creatinine, AST, ALT).
The study was approved by the Bioethics Committee of the Medical University of Bialystok, Poland (permission number APK-002/238/2022). After a detailed explanation of the aim of the study, all patients qualified for the study agreed to participate in the experiment. This study was conducted in accordance with the Declaration of Helsinki of the World Medical Association for ethical principles for medical research involving human subjects.
2.2. Histopathological analysis
In a routine histopathological analysis, we assessed the histological type of the tumour, the grade of histological malignancy according to the World Health Organization (WHO) guidelines [Citation10], tumour stage according to the TNM classification standard of the Union for International Cancer Control [Citation11], including depth of tumour invasion (pT), presence of lymph node metastasis (pN) and distant metastasis (pM), tumour type according to Lauren’s [Citation12] and Goseki ‘s[Citation13] classification, vascular and neural infiltration, and degree of desmoplasia.
Tissue sections obtained during the surgery were fixed in 4% buffered formalin solution and embedded in paraffin at a temperature of 56 °C. The paraffin blocks were then sliced using a microtome (Microm H340) into approximately 4 μm-thick slides and stained with haematoxylin and eosin. The obtained sections were reviewed by two independent pathologists using an microscope Olympus CX22 at 200× and 400× magnifications.
2.3. Blood collection
Fasting venous blood (10 ml) was collected from all patients on an empty stomach, after overnight rest, using the S-Monovette® K3 EDTA and S-Monovette® serum collection system (Sarstedt, Germany). Immediately after collection, blood was centrifuged at 1500 × g for 10 min at +4 °C (MPW 351, MPW Med. Instruments, Warsaw, Poland) to separate plasma or serum from the erythrocytes. The top layer (plasma or serum) was then collected. 0.5 M butylated hydroxytoluene (20 μl/2 ml plasma or serum) was added to prevent sample oxidation. Until redox determinations, All samples were stored at −80 °C.
2.4. Redox assays
All reagents (unless otherwise stated) were of analytical grade and were purchased from Sigma-Aldrich (Germany) or St. Louis (MO, USA).
The absorbance and fluorescence were measured using an Infinite M200 PRO Multimode Microplate Reader (Tecan Group Ltd., Männedorf, Switzerland). Fluorescence was evaluated in 96-well black-bottom microplates. All assays were performed in duplicate. The results were standardized to 1 mg of total protein. The total protein content was determined spectrophotometrically (Thermo Scientific PIERCE BCA Protein Assay; Rockford, IL, USA).
2.4.1. Antioxidant enzymes and non-enzymatic antioxidants
CAT (EC 1.11.1.6) activity was measured using a colorimetric method by measuring the rate of hydrogen peroxide (H2O2) decomposition at 240 nm wavelength [Citation14]. One unit of catalase (CAT) activity was defined as the amount of enzyme catalyzing the decomposition of 1 mmol of H2O2 per 1 min.
GPx (EC 1.11.1.9) activity was evaluated using the colorimetric method by measuring the oxidation of NADPH (the reduced form of nicotinamide adenine dinucleotide phosphate) at 340 nm [Citation15]. One unit of glutathione peroxidase (GPx) activity was defined as the amount of enzyme catalyzing the oxidation of 1 mmol NADPH per 1 min.
GR (EC 1.8.1.7) activity was determined using a colorimetric method by measuring the decrease in NADPH absorbance at 340 nm [Citation16]. One unit of glutathione reductase (GR) activity was defined as the quantity of enzyme catalyzing the oxidation of 1 μmol NADPH per 1 min.
SOD-1 (E.C. 1.15.1.1) activity was determined colorimetrically by measuring inhibition of adrenaline oxidation at 480 nm wavelength [Citation17]. One unit of superoxide dismutase (SOD) activity was defined as the amount of enzyme that inhibited adrenaline oxidation by 50%.
The glutathione (GSH) concentration was measured colorimetrically using an enzymatic reaction with 5,5-dithiobis-(2-nitrobenzoic acid) (DTNB), NADPH, and GR [Citation18]. The absorbance of the resulting complexes was measured at a wavelength of 412 nm.
2.4.2. Redox status
Total antioxidant capacity (TAC) levels were analyzed using the colorimetric method by measuring changes in ABTS·+ (2,2′-azino-bis-3-ethylbenzothiazoline-6-sulfonate) absorbance at 660 nm [Citation19].
Total oxidant status (TOS) levels were determined using the colorimetric method by measuring the oxidation of ferrous ions to ferric ions in the presence of oxidants in the sample [Citation20].
Oxidative stress index (OSI) was calculated using the formula OSI = [TOS]/[TAC] x 100% [Citation21].
2.4.3. Oxidative damage to proteins, lipids and nucleic acids
The total thiol concentration was assessed spectrophotometrically using Ellman’s assay [Citation22,Citation23]. The absorbance was measured at 593 nm and the total thiol content was calculated from the calibration curve for reduced glutathione.
The concentration of ischaemia-modified albumin (IMA) was measured spectrophotometrically based on the exogenous cobalt (Co2+) binding facility of human serum albumin (HSA) [Citation24]. The absorbance was measured at 470 nm.
The advanced oxidation protein products (AOPP) concentration was determined colorimetrically by measuring the iodide ion oxidising capacity of the sample at 340 nm [Citation25]. Immediately before the determination, samples were diluted in 0.02 M PBS (pH 7.4, 1:5, v/v).
The concentration of lipid hydroperoxides (LOOH) was measured spectrophotometrically by the FOX-2 test using the reaction of Fe3+ ions with xylenol orange (XO) [Citation26,Citation27].
The concentration of 8-IsoProstane (8-iso-P) was assayed by ELISA using a complete set of reagents (8-Isoprostane ELISA Kit, Cayman Chemicals, Ann Arbour, MI, USA). The assay has a range of 0,8 to 500 pg/ml and a sensitivity of approximately 3 pg/ml.
The concentration of plasma DNA/RNA oxidative damage was determined according to the manufacturer’s instructions using commercial high-sensitivity ELISA kits (DNA/RNA oxidative damage ELISA Kit, Cayman Chemicals, Ann Arbour, MI, USA). The test detects all three oxidized guanine species: 8-hydroxy-2′-deoxyguanosine, 8-hydroxyguanosine, and 8-hydroxyguanine. The assay has a range of 10.3 to 3000 pg/ml and a sensitivity of approximately 30 pg/ml.
2.5. Statistical analysis
Statistical analysis was performed using GraphPad Prism 9.0 (GraphPad Software, La Jolla, CA, USA). The Shapiro–Wilk test was used to determine the normality of the distribution. For normally distributed data, Student’s t-test was used. In the case of a lack of normal distribution, the Mann-Whitney U test was used. The results are presented as median (minimum-maximum), and a value of p < 0.05 was considered statistically significant. Spearman’s correlation coefficient was used to evaluate the relationships between redox and clinicopathological parameters in the study group. Receiver Operating Characteristic (ROC) analysis was used to assess the diagnostic utility of redox biomarkers. The area under the curve (AUC) and optimal cut-off values were determined for each parameter, ensuring high sensitivity and specificity.
The number of subjects was based on our previous experiment involving 15 patients (online ClinCalc software). The variables used for the sample size calculations were concentrations of NO and AGE. The level of significance was set at 0.05 and power of study was 0.9. The ClinCalc sample size calculator provided the sample size for one group. The minimum number of patients was 38.
3. Results
3.1. Clinical characteristics
This study included 50 patients with gastric cancer. A moderately differentiated grade (G2) of the tumour was diagnosed in 40% of patients, whereas a poorly differentiated grade was present in 60% of the patients. Most of the subjects (68%) had adenocarcinoma and 32% had mucinous adenocarcinoma (). 10.0% of the patients had a pT1 stage of tumour, 30% had a pT2 stage, 34% had a pT3 stage, and 26% had a pT4 stage. The percentage of patients without lymph nodes (N0) and distant metastases (M0) was 24% and 66%, respectively. Small desmoplasia was present in 70% of the patients and diffuse in 30%. The detailed characteristics of the study groups are summarized in .
Figure 1. Gastric adenocarcinoma without mucinous component. B. Adenocarcinoma with mucinous component. H + E staining.
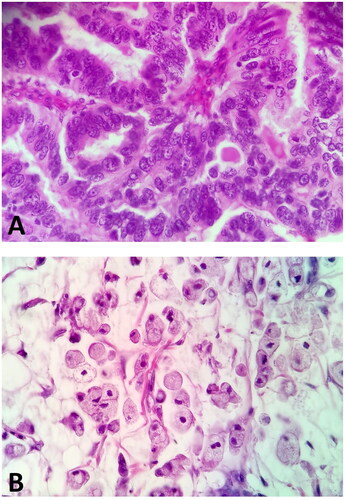
Table 1. Characteristics of study group.
3.2. Antioxidant defence
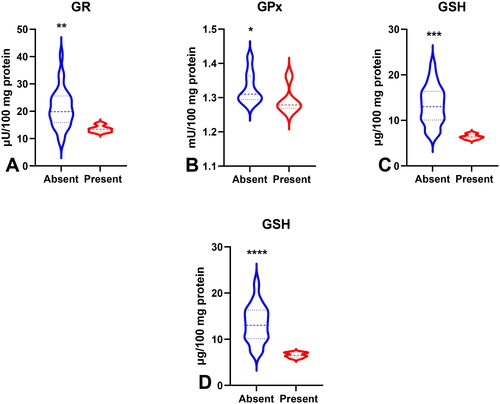
To evaluate the antioxidant barrier, we measured the activity of antioxidant enzymes (i.e. Cu,Zn-superoxide dismutase (SOD-1), catalase (CAT), glutathione peroxidase (GPx), and glutathione reductase (GR)) and the concentration of the non-enzymatic antioxidant glutathione (GSH). The activities of CAT, GPx, GR, and GSH were considerably lower, whereas SOD activity was significantly higher in the serum of patients with gastric cancer than in the control group (p < 0.0001, p < 0.0001, p < 0.0001, p < 0.05, and p < 0.05, respectively) (). CAT activity was significantly higher and SOD activity was lower in patients in the T1 + T2 stage than in those in the T3 + T4 stage (p < 0.05, p < 0.05, respectively) (). SOD activity was considerably higher in patients with distant metastasis than in those without metastasis (p < 0.05), and CAT in patients with intestinal type of gastric cancer according to Lauren’s classification compared to diffuse type (p < 0.05) and in I + II types of gastric cancer according to Goseki classification than in III + IV (p < 0.01) (, ). GPx activity was significantly higher in patients with adenocarcinoma mucinosum than in those with adenocarcinoma (p < 0.05), as well as in patients in T1 + T2 stages in comparison with T3 + T4 (p < 0.05) (, ). We observed increased GR and GSH activities in patients without lymph node metastasis compared to those with metastasis (p < 0.05, p < 0.05, respectively) (). GSH levels were also higher in patients without distant metastasis than in those without metastasis (p < 0.05) (). GPx, GR, and GSH levels were considerably higher in patients with intestinal-type gastric cancer according to Lauren’s classification than in the diffuse type (p < 0.05, p < 0.05, p < 0.05, respectively) and in patients without vascular infiltration (p < 0.05, p < 0.01, p < 0.001, respectively) ( and ). We demonstrated a significant increase in GSH activity in patients with I + II types of gastric cancer according to the Goseki classification compared to those with III + IV (p < 0.05) and in patients without neural invasion (p < 0.001) ( and Citation10).
Figure 2. Non-enzymatic and enzymatic antioxidants in patients with gastric cancer and the control group. Abbreviations: SOD: superoxide dismutase-1; CAT: catalase; GPx: glutathione peroxidase; GR: glutathione reductase; GSH: reduced glutathione. The data are presented as median (minimum - maximum). *p < 0.05, ****p < 0.0001.
3.3. Total antioxidant/oxidant status
To assess the redox status, we determined the total antioxidant capacity (TAC) and total oxidant status (TOS) and calculated the oxidative stress index (OSI) by dividing the TOS level by TAC level (TOS/TAC ratio). In the plasma of patients with gastric cancer, TAC level was significantly lower than that in the control group (p < 0.0001) (). We observed a considerable increase in TOS in the plasma of the study group compared to that in the healthy controls (p < 0.0001) (). The OSI value in patients with gastric cancer was significantly higher than that in healthy controls (p < 0.0001) (). The TOS was considerably higher in patients with G3 than in G2 (p < 0.05) (). The TOS and OSI values were also higher in patients with T3 + T4 stages than in those with T1 + T2 (p < 0.05, p < 0.01, respectively) and in patients with lymph node metastasis (p < 0.01, p < 0.05, respectively) ( ). Interestingly, we observed higher levels of TOS in patients with distant metastasis than in those without metastasis (p < 0.05) (). TAC was significantly higher in patients with intestinal-type gastric cancer according to Lauren’s classification than in those with the diffuse type (p < 0.05) ().
3.4. Oxidative damage products
To evaluate oxidative stress, we used oxidative damage products of proteins (total thiols, ischaemia-modified albumin (IMA), advanced oxidation protein products (AOPP)), lipids (lipid hydroperoxides (LOOH), 8-Isoprostane (8-Iso-P)), and DNA (DNA/RNA products). There was a statistically significant increase in IMA and AOPP in patients with gastric cancer compared to those in the control group (p < 0.01, p < 0.0001, respectively). LOOH, 8-Iso-P, and DNA/RNA concentrations were also considerably higher (p < 0.0001 and p < 0.0001, p < 0.0001, respectively) whereas total thiols were significantly lower in the plasma of the study group than in that of the control group (p < 0.0001) (). AOPP concentration was significantly higher in patients with tumours >5 cm than in those with tumours ≤5 cm (p < 0.05) ((). AOPP and LOOH levels were higher in patients with adenocarcinoma mucinosum than in those with adenocarcinoma (p < 0.01, p < 0.05, respectively) (). We demonstrated a higher concentration of total thiols in patients in G3 stage in comparison with G2 (p < 0.05), in patients with lymph node metastasis (p < 0.05), and in patients with III + IV types of gastric cancer according to the Goseki classification in comparison with I + II (p < 0.01) (). We observed a significant increase in AOPP concentration in patients with T3 + T4 stages in comparison with T1 + T2 (p < 0.05), in patients with lymph node metastasis (p < 0.05), in patients with distant metastasis (p < 0.05), and in patients with diffuse type gastric cancer according to Lauren’s classification compared to the intestinal type (p < 0.05) ().
Figure 4. Oxidative damage to proteins, lipids and DNA in patients with gastric cancer and the control group. Abbreviations: IMA: ischaemia-modified albumin; AOPP: advanced oxidation protein products; LOOH: lipid hydroperoxides; 8-Iso-P: 8-Isoprostane. The data are presented as median (minimum - maximum). **p < 0.01, ****p < 0.0001.
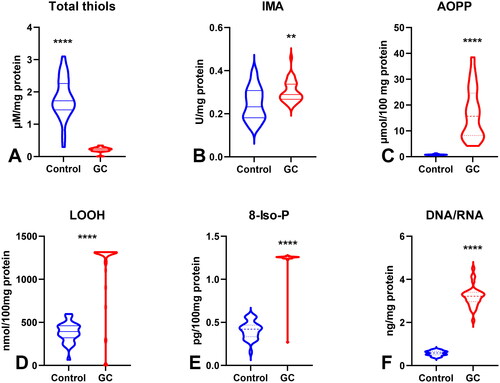
Figure 5. Comparison of AOPP concentration (A) between patients with tumours diameter ≤5cm and >5cm; AOPP (B) and LOOH (C) concentration between patients with adenocarcinoma and adenocarcinoma mucinosum; GPx (D), TOS (F) and total thiols (F) between patients with tumours in G2 and G3 stage. Abbreviations: AOPP: advanced oxidation protein products; LOOH: lipid hydroperoxides; GPx: glutathione peroxidase; TOS: total oxidant status. The data are presented as median (minimum - maximum). *p < 0.05, **p < 0.01.
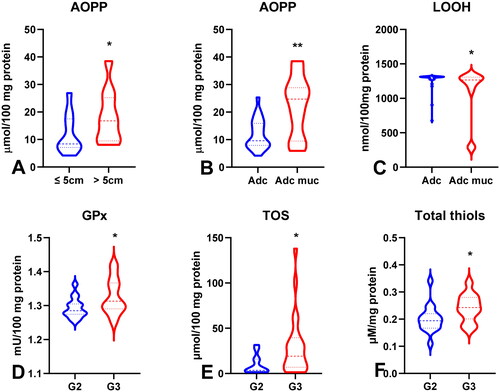
Figure 6. Comparison of SOD (a), CAT (B), GPx (C), TOS (D), OSI (E) and AOPP (F) between patients with tumours in T1 and T2 stage vs T3 and T4 stage. Abbreviations: SOD: superoxide dismutase-1; CAT: catalase, GPx: glutathione peroxidase; TOS: total oxidant status; OSI: oxidative stress index; AOPP: advanced oxidation protein products. The data are presented as median (minimum - maximum). *p < 0.05, **p < 0.01.
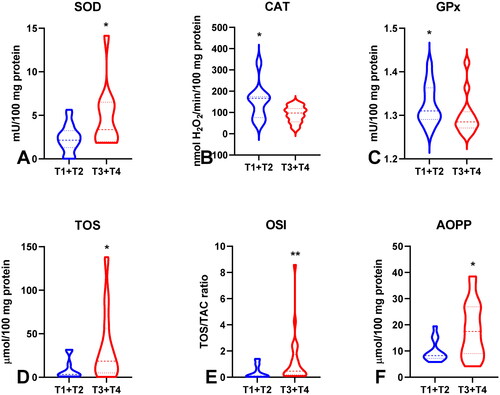
Figure 7. Comparison of GR (a), GSH (B), TOS (C), OSI (D), AOPP (E) and total thiols (F) between groups of patients without and with lymph node metastasis. Abbreviations: GR: glutathione reductase; GSH: reduced glutathione; TOS: total oxidant status; OSI: oxidative stress index; AOPP: advanced oxidation protein products. The data are presented as median (minimum - maximum). *p < 0.05, **p < 0.01.
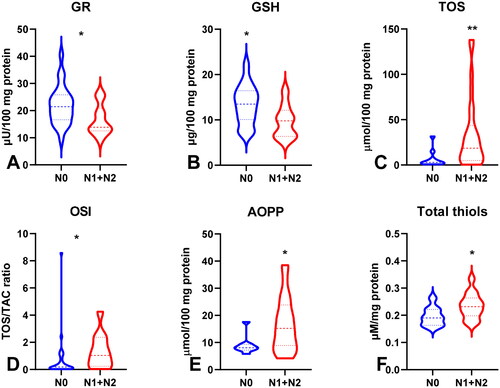
Figure 8. Comparison of SOD (a), GSH (B), TOS (C) and AOPP (D) between groups of patients without and with distant metastasis. Abbreviations: GR: glutathione reductase; GSH: reduced glutathione; TOS: total oxidant status; OSI: oxidative stress index; AOPP: advanced oxidation protein products. The data are presented as median (minimum - maximum). *p < 0.05.
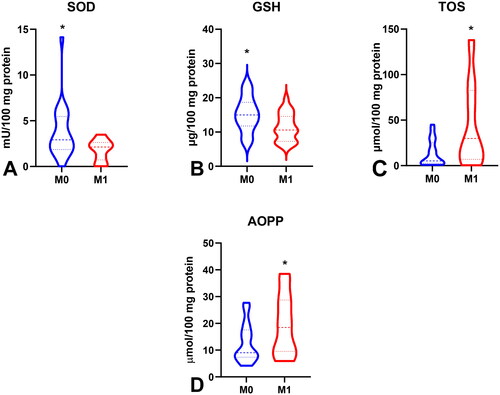
Figure 9. Comparison of CAT (a), GR (B), GPx (C), GSH (D), TAC (E) and AOPP (F) between groups of patients with intestinal and diffuse type of gastric cancer according to lauren’s classification; CAT (G), GSH (H) and total thiols between I + II and III + IV type of gastric cancer according to Goseki classification. Abbreviations: CAT: catalase; GR: glutathione reductase; GPx: glutathione peroxidase; GSH: reduced glutathione; TAC: total antioxidant capacity; AOPP: advanced oxidation protein products. The data are presented as median (minimum - maximum). *p < 0.05, **p < 0.01.
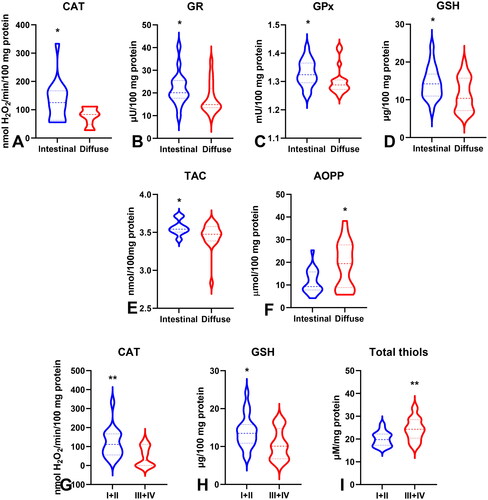
Figure 10. Comparison of GR (a), GPx (B), GSH (C) between groups of patients with absent and present vascular infiltration; GSH level (D) between groups of patients with absent and present neural infiltration. Abbreviations: GR: glutathione reductase; GPx: glutathione peroxidase; GSH: reduced glutathione. The data are presented as median (minimum - maximum). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
3.5. ROC analysis
ROC analysis was performed to assess the diagnostic value of oxidative stress biomarkers in gastric cancer (). We demonstrated that all determined redox parameters differentiate patients with GC from healthy controls to a large extent. We also demonstrated that oxidative stress biomarkers are useful for the differential diagnosis of patients with gastric cancer (). We showed a high diagnostic value of AOPP and LOOH in differentiating patients with adenocarcinoma from those with adenocarcinoma mucinosum (AUC for AOPP = 0.7725, for LOOH = 0.7022) (). GPx, TOS, and total thiols were helpful in differentiating moderately differentiated (G2) from poorly differentiated tumours (G3) (AUC for GPx = 0.7067, for TOS = 0.7464, for total thiols = 0.7279) (). We also demonstrated a very high diagnostic value of SOD (AUC = 0.7273), CAT (AUC = 0.7417), GPx (AUC = 0.6997), TOS (AUC = 0.7451), OSI (AUC = 0.7527), and AOPP (AUC = 0.7284) in differentiating patients with gastric cancer at stage pT1 + pT2 tumour invasion from those with stage pT3 + pT4 (). Parameters such as GR (AUC = 0.7460), GSH (AUC = 0.7619), TOS (AUC = 0.7964), OSI (AUC = 0.7435), AOPP (AUC = 0.7341), and total thiol (AUC = 0.7460) may be useful for differentiating patients with lymph node metastasis from those without lymph node metastasis (). SOD (AUC = 0.7190), GSH (AUC = 0.7106), TOS (AUC = 0.7105), and AOPP (AUC = 0.6889) were helpful in differentiating patients with and without distant metastasis (). We showed a high diagnostic value for CAT (AUC = 0.7531), GR (AUC = 0.7022), GPx (AUC = 0.7135), GSH (AUC = 0.6930), TAC (AUC = 0.7135), and AOPP (AUC = 0.7032) in differentiating diffuse type from intestinal type of gastric cancer according to the Lauren classification, and CAT (AUC = 0.7827), GSH (AUC = 0.7255), and total thiols (AUC = 0.7561) in differentiating I + II types from III + IV types of gastric cancer according to the Goseki classification (). Particular attention should be paid to GR, GPx, and GSH, for which AUC in the presence of vascular infiltration was 0.8933, 0.8000, and 0.9853, respectively, and GSH with AUC = 0.9875 in the presence of neural infiltration ().
Table 2. Receiver operating characteristic (ROC) analysis of oxidative stress biomarkers of gastric cancer patients and the controls.
Table 3. Receiver operating characteristic (ROC) analysis in gastric cancer patients for histological type, histological differentiation grade, depth of tumour invasion, presence of lymph node and distant metastasis.
Table 4. Receiver operating characteristic (ROC) analysis in gastric cancer patients for type of GC according to Lauren and Goseki classifications and vascular and neural infiltration.
3.6. Correlations
All statistically significant associations are shown in . We observed positive correlations between sex and AOPP (p = 0.044, R = 0.379) and LOOH levels (p = 0.045, R = 0.361).
Figure 11. Heat map of correlations between redox biomarkers and histopathological parameters in patients with gastric cancer.
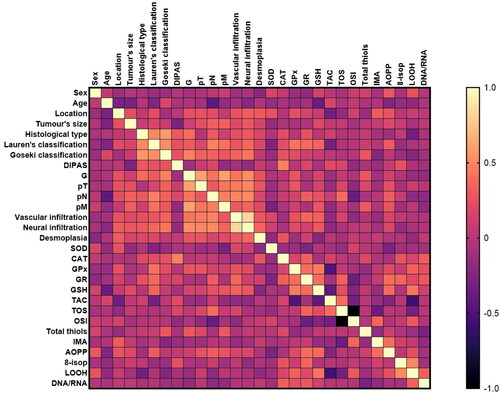
Patient age was negatively correlated with GSH activity (p = 0.018, R= −0.376). Interestingly, we observed a negative correlation between tumour location and TAC levels (p = 0.047, R= −0.388). The LOOH concentration was negatively associated with the histological type of the tumour (p = 0.024, R=-0.339). GPx (p = 0.012, R = 0.406), GR (p = 0.033, R = 0.371), GSH (p = 0.038, R = 0.341), and AOPP (p = 0.042, R = 0.299) were positively correlated, whereas TAC (p = 0.047, R=-0.315) was negatively correlated with Lauren’s classification. GPx and total thiol levels were associated with histological differentiation grade (p = 0.042, R = 0.269; p = 0.044, R = 0.285). GPx also correlated with the depth of tumour invasion (pT) (p = 0.05, R = 0.368). Moreover, the levels of GR (p = 0.042, R = 0.299), OSI (p = 0.018, R = 0.375), and AOPP (p = 0.031, R = 0.301) were positively correlated with the presence of lymph node metastasis (pN). Significant associations were also found between SOD (p = 0.05, R=-0.397), GSH (p = 0.039, R = 0.273), total thiol concentration (p = 0.036, R = 0.292), and the presence of distant metastasis. A positive correlation was observed between GR activity (p = 0.035, R = 0.355) and vascular infiltration. Moreover, there were statistically significant correlations between vascular and neural infiltration and GSH levels (p = 0.037, R = 0.359; p = 0.028, R = 0.484, respectively).
4. Discussion
Our study is the first to assess the diagnostic utility of redox biomarkers in patients with gastric cancer. We have shown a decrease in the activity of both enzymatic and non-enzymatic antioxidants, as well as increased oxidation of proteins, lipids, and DNA in the blood of GC patients. Moreover, we showed that some of the redox parameters may be helpful and may be novel non-invasive biomarkers in differentiating GC patients according to histological type, histological differentiation grade, tumour invasion depth, the occurrence of lymph node or distant metastasis, differentiation types of gastric cancer classified by Lauren and Goseki, and the presence of vascular and neural infiltration.
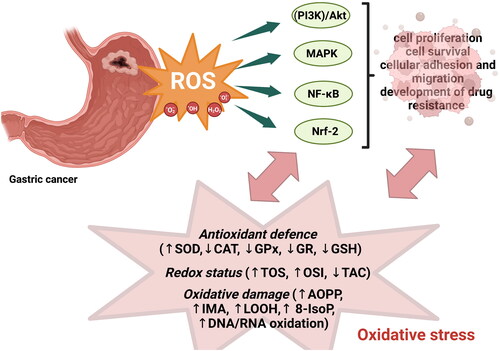
It is known that enhanced production of reactive oxygen species and, consequently, oxidative stress may be involved in cancer development via regulation of numerous signalling pathways, including phosphoinositide-3-kinase (PI3K)/Akt-regulated signalling cascades, mitogen-activated protein (MAP) kinase/Erk cascade, and the IκB kinase (IKK)/nuclear factor κB (NF-κB)-activating pathways () [Citation28]. For example, the activation of the MAPK/Erk1/2 pathway in cancer leads to increased cell proliferation; PI3K/Akt activation affects cell survival via phosphorylation and inactivation of its substrates, the pro-apoptotic proteins Bad, Bax, Bim, or FOXO transcription factors, leading to the inhibition of apoptosis, whereas activation of NF-κB, described as a’ redox-regulated sensor for oxidative stress,’ participates in tumour cell survival, regulation of cell cycle and proliferation, cellular adhesion and migration, and development of drug resistance [Citation28]. The role of H2O2 in carcinogenesis and activation of signalling pathways is often emphasized because H2O2 may be a second messenger in cellular signalling. Its high concentration in cells may induce DNA damage, inflammation (via the activation of NF-κB), and changes in cellular metabolism [Citation29]. Moreover, H2O2 can damage membranes and other organelles, leading to autophagy, mitophagy, and HIF1 activation [Citation30]. Under conditions of oxidative stress, the consequences of mitophagy are aerobic glycolysis and lactate production [Citation31]. One of the main enzymes responsible for H2O2 conversion from superoxide radicals is superoxide dismutase (SOD) considered one of the most effective intracellular enzymatic antioxidants [Citation32]. In our study, we observed significantly higher SOD activity in patients with gastric than in healthy controls, which may be due to the enhanced generation of H2O2 in GC patients. Simultaneously, SOD increase may be an adaptive reaction to ROS overproduction by gastric cancer cells [Citation33]. We also observed lower SOD activity in patients with tumours restricted to the mucosa and submucosa (T1) and muscularis propria (T2) in comparison with patients with tumours penetrating deeper layers – subserosa and serosa (T3) and adjacent structures (T4). This clearly indicates that SOD activity increases with the depth of tumour invasion. In our study, SOD levels were considerably higher in patients with distant metastasis than in those without. We concluded that SOD may be involved in the metastasis process. Moreover, studies performed on cell lines confirmed that abnormally high SOD expression can regulate cellular proliferation and metastasis, possibly by promoting cell cycle progression and inhibiting apoptosis [Citation34]. Additionally, elevated SOD levels enhance the invasive potential of cancer cells by suppressing their proliferation. Moreover, a lack of balance between the generation of superoxide radicals and H2O2 degradation in cells with high SOD levels may induce the metastatic potential of cancer cells [Citation35]. The effect of overproduction of ROS, including H2O2 is counterbalanced by the activity of antioxidant enzymes, such as CAT, GPx, and GR. Catalase is one of the major enzymes responsible for H2O2 detoxification and conversion to water and oxygen. One molecule of catalase can neutralise over six million molecules of H2O2 per minute; therefore, it is considered one of the most catalytically active enzymes [Citation36]. In this study, catalase activity was significantly lower in patients with gastric cancer than in the control group. It may be concluded that decreased CAT levels predispose to the development of cancer as a result of increased levels of H2O2 [Citation37]. This theory is confirmed by studies carried out on mice, in which it was demonstrated that at 9 months of age, female catalase-deficient mice spontaneously develop mammary tumours, which can be prevented by the addition of vitamin E to their diet [Citation38]. Japanese preclinical studies have demonstrated that therapy with catalase (via intravenous and intraperitoneal injection of catalase) in rodent animal models is sufficient to almost completely prevent tumour recurrence and metastasis [Citation39,Citation40]. Interestingly, we observed higher CAT activity in patients in T1 + T2 stages in comparison with T3 + T4, in patients with intestinal type of gastric cancer compared to diffuse type, and in I + II types of gastric cancer according to Goseki classification than in III + IV. The differences in CAT activity occurred in the groups of patients divided according to histological features, clearly indicating that the depth of tumour invasion and the histological structure of the tumour showed a strong association with CAT activity and consequently H2O2 detoxification. The intestinal type of gastric cancer is defined by tumour cells arranged in tubular or glandular formations, and is often linked to intestinal metaplasia. Moreover, this type of GC is associated with lymphatic or vascular invasion, and exhibits a longer course and better prognosis [Citation41]. The diffuse type arises from the gastric mucosa and is linked to gastritis, and the tumour cells lack adhesion and infiltrate the stroma as single cells or small groups. Intracellular mucus can shift the nucleus of cells to form signet-ring cell carcinomas. This type of cancer generally occurs in the entire stomach and is characterized by a shorter duration and worse prognosis than the intestinal type [Citation42]. Thus, CAT activity seems to be strongly associated with the histological structure of the tumour, and its lower activity observed in the diffuse type may be a factor indicating a worse prognosis for patients with GC. The Goseki histological classification is based on two cancer features: the degree of tubular differentiation and the degree of mucus production by cancer cells [Citation13]. Therefore, differences in CAT activity between patients with I + II and III + IV may indicate that the degree of tubular differentiation and mucus production is associated with the detoxification of hydrogen peroxide by catalase. Other basic components of the antioxidant barrier are glutathione peroxidase, glutathione reductase, and non-enzymatic antioxidant – reduced glutathione. Glutathione metabolism is a key element in antioxidative defense mechanisms and protects cells from oxidative stress by reducing disulphide bonds of cytoplasmic proteins to cysteines and is oxidized to glutathione disulphide (GSSG) [Citation43]. Maintaining the redox state of critical protein sulfhydryl groups is crucial for DNA repair. GPx catalyzes the breakdown of H2O2 to H2O and lipid hydroperoxides (ROOH) to alcohols, whereas GR reduces GSSG and refills the GSH pools [Citation44]. GPx participates in the conversion of H2O2 at low ROS levels, unlike catalase, which is involved in H2O2 conversion at high ROS level [Citation45]. This may be explained by the fact that GPx has a much higher affinity for H2O2 probably because of the different values of the Michaelis-Menten constant (Km) of CAT and GPx. The Km for GPx (Km = 1 × 10−6 M) is considerably lower than the Km for CAT (Km = 2.4 × 10−4 M), which suggests that CAT scavenges H2O2 efficiently at higher concentrations [Citation46]. We observed that the activity of GPx and GR and the concentration of the non-enzymatic antioxidant GSH decreased in patients with gastric cancer. The decrease in all components of the antioxidant barrier (GPx, GR, and GSH) in GC patients clearly shows exhaustion of antioxidant reserves in GC patients and predisposes them to redox imbalance and oxidative damage [Citation8]. GPx activity, similar to CAT activity, was significantly increased in patients in the T1 + T2 stage compared to those in the T3 + T4 stage. GR and GSH activities were increased in patients without lymph node metastasis compared to those with metastasis, whereas GSH activity was also higher in patients without distant metastasis than in those without metastasis. GPx, GR, and GSH levels were considerably higher in patients with intestinal-type gastric cancer according to Lauren’s classification than in the diffuse type and in patients without vascular infiltration. We demonstrated a significant increase in GSH activity in patients with I + II types of gastric cancer according to the Goseki classification compared to III + IV and in patients without neural invasion. Our observations clearly indicate that depletion of the antioxidant barrier manifested by a decrease in enzyme activity intensifies with the depth of tumour invasion, development of metastasis, and vascular and neural invasion, as well as the presence of more aggressive and worse prognosis types of gastric cancer according to the Lauren and Goseki classification.
Evaluating the redox status based on the level of single antioxidants may be difficult; hence, we assessed the biomarkers reflecting the resultant antioxidant/oxidant capacity (TAC, TOS, and OSI) in patients with gastric cancer. TAC involves the cumulative effect of all antioxidants (both enzymatic and non-enzymatic), whereas TOS enables the evaluation of all oxidants present in blood samples [Citation47]. (TOS/TAC ratio) reflects the ratio between the pro-oxidants and antioxidants. In our study, we observed significantly lower TAC levels and higher TOS and OSI values in gastric cancer patients than in healthy individuals. These results indicate a weakened antioxidant barrier caused by overproduction of free radicals in patients with gastric cancer. Moreover, a high OSI level indicates that the oxidation processes outbalance antioxidant protection. Additionally, a shift in the redox balance to the oxidation side results in a higher sensitivity to oxidative stress and a greater tendency towards oxidative damage development. Unsurprisingly, we found higher TOS levels in patients with poorly differentiated tumours (G3) than in those with moderately differentiated tumours (G2). TOS and OSI were also increased in patients with tumours penetrating deeper layers (T3 and T4) of the stomach, as well as in patients with lymph node metastasis. The TOS was considerably increased in patients with distant metastasis. These observations suggest that oxidative stress is a key driver of the malignant transformation observed in primary tumours, and our observations indicate that oxidative stress may enhance their metastatic potential. Piskounova et al. demonstrated that increased ROS production is essential for enabling and sustaining a highly metastatic phenotype in cancer [Citation48].
Oxidation processes are essential for aerobic life; however, ROS overproduction is harmful, and their insufficient removal by antioxidant defense systems leads to protein and DNA oxidation and lipid peroxidation, which may take part in the multistage carcinogenesis process. Proteins are the main point of action of ROS in biological systems because of their abundance and high rate constants. Modification of protein structure occurs as a consequence of ROS action, which may include oxidation of the side chains of almost all types of amino acids, chain fragmentation, cross-linked protein aggregates, and disorder of the secondary and tertiary structures [Citation49]. The most commonly assessed markers of protein oxidation are total thiols, ischaemia-modified albumin (IMA) and advanced oxidation protein products (AOPP). Thiols belong to a group of organic compounds containing sulfhydryl groups that participate in the control of antioxidant defence mechanisms. Thiols are considered powerful antioxidants because they can act as electron acceptors by reducing unstable free radicals [Citation50]. We showed a decreased concentration of total thiols in patients with GC compared to that in healthy controls. Moreover, total thiol levels were increased in poorly differentiated tumours (G3), in patients with lymph node metastasis, and in types III and IV GC according to the Goseki classification. Abnormalities in thiol/disulphide homeostasis may play a role in gastric carcinogenesis, and a higher level of oxidative stress may lead to an advanced and more aggressive disease course [Citation51]. We also found higher levels of IMA and AOPP in patients with GC. IMA is a marker of ischaemia and oxidative stress resulting from tissue hypoxia. Scientists also describe IMA as a marker of inflammation because it is well known that ischaemia initiates a proinflammatory reaction leading to ROS production, and consequently, oxidative stress, which may also induce malignant transformation [Citation52]. AOPP are dityrosine-containing crosslinked protein products generated mainly by chlorinated oxidants such as hypochloric acid and chloramines arising from myeloperoxidase activity [Citation53]. Moreover, AOPPs induce oxidative ignition of neutrophils, monocytes, and T lymphocytes, leading to the upregulation of dendritic cells and activation of the NF-κB signalling pathway, which enhances the generation of pro-inflammatory cytokines but also increases free radical formation [Citation8]. In this study, AOPP concentration was significantly higher in patients with a tumour diameter >5 cm, in tumours penetrating the subserosa and serosa (T3) and adjacent structures (T4), in patients with adenocarcinoma mucinosum, with lymph node and distant metastasis, as well as in patients with diffuse-type GC. It is not surprising that AOPP increased with an increase in tumour diameter and with worse prognosing types of GC because oxidized proteins tend to form aggregates resistant to degradation by proteolytic enzymes, and their accumulation in cells may result in a gradual loss of structure and function impairment. This confirms that abnormalities in protein breakdown processes are involved in cancer progression [Citation54].
Uncontrolled ROS generation results in lipid peroxidation and DNA oxidation. Lipid peroxidation may cause decreased fluidity and increased permeability of the membrane, as well as damage to membrane proteins, but is also responsible for the production of a complex range of reactive carbonyl compounds, ketones, and alkanes [Citation55]. The main products of lipid peroxidation are lipid hydroperoxides, which are formed as a result of fatty acid oxidation. At physiological levels, LOOH shows beneficial effects: it increases cellular adaptive responses and increases tolerance against oxidative stress via the modulation of antioxidants. However, their uncontrolled production finally causes the initiation and execution of ferroptosis – iron-dependent programmed cell death caused by oxidative stress and lipid peroxidation [Citation56]. Ferroptosis is involved in cell proliferation, senescence, and differentiation. LOOH are considered more stable compounds than free radicals, and their determination in serum could be useful for predicting oxidative stress in tissues [Citation57]. In our study, we observed an increased level of LOOH in patients with gastric cancer. However, these markers are of limited value because they lack sensitivity or specificity; therefore, we also assessed other markers of oxidative stress, 8-Iso-P, which are more stable and specific products of lipid peroxidation than LOOH. 8-Iso-P is a prostaglandin belonging to the F2 isoprostane class that is generated in vivo by the free radical-catalyzed peroxidation of arachidonic acid and cell membrane phospholipids [Citation58]. 8‑isoprostane is considered as a gold standard of oxidant injury and is formed when endogenous antioxidants are exhausted; hence, it is not surprising that we showed significantly higher levels of this compound in GC patients because levels of enzymatic and non-enzymatic antioxidants were also decreased in our study. To date, the role of 8-Iso-P in carcinogenesis has not been defined, but it has been postulated that it may be a risk factor for cancer [Citation59]. Lipid peroxidation is strongly associated with DNA damage because this process generates aldehydes as secondary products, such as trans-4-hydroxy-2-nonenal (HNE) and malondialdehyde (MDA), which may result in the production of promutagenic oxidatively generated and exocyclic DNA adducts in human cells. However, the main source of oxidative damage to DNA is ROS and includes breakage of strands, DNA base alterations, DNA-protein crosslinks (single and double strands), exchange of genes between two identical sister chromatids, subsequent formation of clastogenic factors, modifications in the nitrogenous base structures, and impairment of tumour suppressor genes and protooncogenes, leading to the transformation of normal cells into cancer cells [Citation60]. We observed increased levels of DNA/RNA products in our study, which confirmed the enhanced oxidation of DNA/RNA in patients with gastric cancer.
5. Conclusions
In summary, GC development is strongly linked to an imbalance between enhanced oxidant production and antioxidants, and consequently, increased oxidative damage to proteins, lipids, and DNA/RNA. We observed a decrease in enzymatic activity (CAT, GPx, GR) as well as non-enzymatic (GSH) antioxidants, and a decrease in total antioxidant capacity in patients with gastric cancer. Moreover, TOS and OSI values were increased in patients with GC. It is well known that a weakened antioxidant barrier is ineffective in ROS removal, resulting in protein, lipid, and DNA damage and increased levels of protein oxidation products (IMA, AOPP), lipid peroxidation products (LOOH, 8-Iso-P), and DNA/RNA products. We also observed differences in oxidative stress parameters between groups of GC patients divided according to histopathological parameters, such as tumour size, histological type of the tumour, histological differentiation grade, tumour invasion depth, presence of lymph node and distant metastasis, Lauren and Goseki classification, and presence of vascular and neural infiltration. Many redox biomarkers allow for the distinction between patients with gastric cancer and healthy people with high sensitivity and specificity. Therefore, our study is an initial step towards further clinical trials focusing on the evaluation of the diagnostic utility of redox biomarkers in a larger population of patients with gastric cancer. Determination of redox parameters may be useful in the assessment of tumour progression. Evaluation of the link between the intensity of oxidative stress and survival rate of patients with gastric cancer seems appropriate.
Finally, some of the limitations of our study are worth considering. Our study was conducted on a small group of patients; hence, further research should be conducted on a larger group of patients with gastric cancer. The levels of redox biomarkers were determined only in blood samples (serum/plasma), giving our results an approximate value. The redox parameters assessed in this study are not specific to GC patients; therefore, they should be evaluated after excluding other oxidative stress-associated diseases. However, the great advantage of our research is a carefully selected group of gastric cancer patients and a control group without any coexisting diseases, and the fact that some of the determined redox parameters may be applied in non-invasive GC diagnostics. Our study may be a preliminary step for further clinical trials to evaluate redox biomarkers and their diagnostic utility in a larger population of patients with gastric cancer.
Informed consent
Informed consent was obtained from all participants involved in the study. Written informed consent was obtained from the patients (s) for publication of this paper.
Authors’ contribution
Conceptualisation, J.D., K.Z.; Methodology, J.D., M.M.; Software, J.D., K.Z., M.M., P.Ż.; Validation, J.D., K.Z., M.M., P.Ż.; Formal Analysis, J.D., M.M., A.P.; Investigation, J.D., M.M., A.Z.; Resources, K.Z., B.K., A.P.; Data Curation, J.D., M.M.; Writing – Original Draft Preparation, J.D., K.Z.; Writing – Review and Editing, J.D., M.M., A.Z., J.M-K.; Visualisation, J.D., K.Z., A.P.; Supervision, J.D., M.M.; Project Administration, J.D., M.M.; Funding Acquisition, J.D., M.M., J.M-K’.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The full data presented in this study are available upon request from the corresponding author.
Additional information
Funding
References
- Ferlay J, Ervik M, Lam F, et al. Global cancer observatory: cancer tomorrow. Lyon, France: International Agency for Research on Cancer. Available from: https://gco.iarc.fr/tomorrow/en.
- Li J. Gastric cancer in young adults: a different clinical entity from carcinogenesis to prognosis. Gastroenterol Res Pract. 2020;2020:1.
- Zaręba KP, Zińczuk J, Dawidziuk T, et al. Stomach cancer in young people-a diagnostic and therapeutic problem. Prz Gastroenterol. 2019;14(4):1–20. doi: 10.5114/pg.2019.90254.
- Jiang S, Liu H, Li C. Dietary regulation of oxidative stress in chronic metabolic diseases. Foods. 2021;10:1854. doi: 10.3390/foods10081854.
- Zińczuk J, Zaręba K, Kamińska J, et al. Association of tumour microenvironment with protein glycooxidation, DNA damage, and nitrosative stress in colorectal cancer. Cancer Manag Res. 2021;13:6329–6348. doi: 10.2147/CMAR.S314940.
- Weinberg F, Ramnath N, Nagrath D. Reactive oxygen species in the tumor microenvironment: an overview. Cancers. 2019;11(8):1191. Available from: https://pubmed.ncbi.nlm.nih.gov/31426364/.
- Sreevalsan S, Safe S. Reactive oxygen species AND colorectal cancer. Curr Colorectal Cancer Rep. 2013;9(4):350–357. doi: 10.1007/s11888-013-0190-5.
- Zińczuk, Maciejczyk Zaręba. Antioxidant barrier, redox status, and oxidative damage to biomolecules in patients with colorectal cancer. Can malondialdehyde and catalase be markers of colorectal cancer advancement? Biomolecules. 2019;9:637. doi: 10.3390/biom9100637.
- Dorf J, Zaręba K, Matowicka-Karna J, et al. May the nitrosative and carbonyl stress promote inflammation in patients with colorectal cancer? J Inflamm Res. 2022; 15:4585–4600. doi: 10.2147/JIR.S374387.
- Bosman F, Carneiro F, Hruban R, et al. WHO classification of tumours of the digestive system. Fourth Edition. France: IARC; 2010. DOI:10.1017/CBO9781107415324.004
- Amin MB. AJCC cancer staging system. 8th Edition. New York: Springer; 2017.
- Lauren P. The two histological main types OF gastric carcinoma: diffuse and so-called intestinal-type carcinoma. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31.
- Goseki N, Takizawa T, Koike M. Differences in the mode of the extension of gastric cancer classified by histological type: new histological classification of gastric carcinoma. Gut. 1992;33(5):606–612. doi: 10.1136/gut.33.5.606.
- Aebi H. [Catalase in vitro. Methods Enzymol. 1984;105]:121–126. doi: 10.1016/s0076-6879(84)05016-3.
- Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70(1):158–169.
- Mize CE, Langdon RG. Hepatic glutathione reductase. I. Purification and general kinetic properties. J Biol Chem. 1962;237:1589–1595.
- Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247(10):3170–3175.
- Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980;106(1):207–212. doi: 10.1016/0003-2697(80)90139-6.
- Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem. 2004;37(4):277–285. doi: 10.1016/j.clinbiochem.2003.11.015.
- Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38(12):1103–1111. doi: 10.1016/j.clinbiochem.2005.08.008.
- Knaś M, Maciejczyk M, Daniszewska I, et al. Oxidative damage to the salivary glands of rats with streptozotocin-induced diabetes-temporal study: oxidative stress and diabetic salivary glands. J Diabetes Res. 2016;2016:4583742. doi: 10.1155/2016/4583742.
- Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6.
- Choromańska B, Myśliwiec P, Kozłowski T, et al. Antioxidant and antiradical activities depend on adrenal tumor type. Front. Endocrinol. 2022;13:1011043. doi: 10.3389/fendo.2022.1011043.
- Klimiuk A, Maciejczyk M, Choromańska M, et al. Salivary redox biomarkers in different stages of dementia severity. J Clin Med . 2019;8:840. doi: 10.3390/jcm8060840.
- Kalousová M, Škrha J, Zima T. Advanced glycation end-products and advanced oxidation protein products in patients with diabetes mellitus. Physiol Res. 2002;51(6):597–604.
- Nourooz-Zadeh J, Tajaddini-Sarmadi J, Wolff SP. Measurement of hydroperoxides in edible oils using the ferrous oxidation in xylenol orange assay. J. Agric. Food Chem. 1995;43(1):17–21. doi: 10.1021/jf00049a005.
- Choromańska B, Myśliwiec P, Kozłowski T, et al. Antioxidant barrier and oxidative damage to proteins, lipids, and DNA/RNA in adrenal tumor patients. Oxid Med Cell Longev. 2021;2021:5543531. doi: 10.1155/2021/5543531.
- Liou GY, Storz P. Reactive oxygen species in cancer. Free Radic Res. 2010;44(5):479–496. doi: 10.3109/10715761003667554.
- Lisanti MP, Martinez-Outschoorn UE, Lin Z, et al. Hydrogen peroxide fuels aging, inflammation, cancer metabolism and metastasis: the seed and soil also needs ‘fertilizer. Cell Cycle. 2011;10(15):2440–2449. doi: 10.4161/cc.10.15.16870.
- Martinez-Outschoorn UE, Trimmer C, Lin Z, et al. Autophagy in cancer associated fibroblasts promotes tumor cell survival: role of hypoxia, HIF1 induction and NFκB activation in the tumor stromal microenvironment. Cell Cycle. 2010; 9(17):3515–3533. doi: 10.4161/cc.9.17.12928.
- Lisanti MP, Martinez-Outschoorn UE, Chiavarina B, et al. Understanding the ‘lethal’ drivers of tumor-stroma co-evolution: emerging role(s) for hypoxia, oxidative stress and autophagy/mitophagy in the tumor micro-environment. Cancer Biol Ther. 2010;10(6):537–542. doi: 10.4161/cbt.10.6.13370.
- Landis GN, Tower J. Superoxide dismutase evolution and life span regulation. Mech Ageing Dev. 2005;126(3):365–379. https://pubmed.ncbi.nlm.nih.gov/15664623/.
- Zińczuk J, Maciejczyk M, Zaręba K, et al. Pro-oxidant enzymes, redox balance and oxidative damage to proteins, lipids and DNA in colorectal cancer tissue. Is oxidative stress dependent on tumour budding and inflammatory infiltration? Cancers. 2020;12(6):1636. doi: 10.3390/cancers12061636.
- Liu S, Li B, Xu J, et al. SOD1 promotes cell proliferation and metastasis in non-small cell lung cancer via an miR-409-3p/SOD1/SETDB1 epigenetic regulatory feedforward loop. Front Cell Dev Biol. 2020;8:213. doi: 10.3389/fcell.2020.00213.
- Valko M, Rhodes C, Moncol J, et al. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160(1):1–40. doi: 10.1016/j.cbi.2005.12.009.
- Nishikawa M, Hashida M, Takakura Y. Catalase delivery for inhibiting ROS-mediated tissue injury and tumor metastasis. Adv Drug Deliv Rev. 2009;61(4):319–326. doi: 10.1016/j.addr.2009.01.001.
- Valko M, Leibfritz D, Moncol J, et al. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001.
- Ishii K, Zhen LX, Wang DH, et al. Prevention of mammary tumorigenesis in acatalasemic mice by vitamin E supplementation. Jpn J Cancer Res. 1996;87(7):680–684. doi: 10.1111/j.1349-7006.1996.tb00277.x.
- Nishikawa M, Hashida M. Inhibition of tumour metastasis by targeted delivery of antioxidant enzymes. Expert Opin Drug Deliv. 2006;3(3):355–369. doi: 10.1517/17425247.3.3.355.
- Nishikawa M, Tamada A, Hyoudou K, et al. Inhibition of experimental hepatic metastasis by targeted delivery of catalase in mice. Clin Exp Metastasis. 2004;21(3):213–221. doi: 10.1023/b:clin.0000037706.13747.5e.
- Zheng H, Takahashi H, Murai Y, et al. Pathobiological characteristics of intestinal and diffuse-type gastric carcinoma in Japan: an immunostaining study on the tissue microarray. J Clin Pathol. 2007;60(3):273–277. doi: 10.1136/jcp.2006.038778.
- Chen YC, Fang WL, Wang RF, et al. Clinicopathological variation of lauren classification in gastric cancer. Pathol Oncol Res 2015 221 [Internet]. 2015 [Cited. 2022;22:197–202. Available from: https://link.springer.com/article/10.1007/s12253-015-9996-6.
- Masella R, Di Benedetto R, Varì R, et al. Novel mechanisms of natural antioxidant compounds in biological systems: involvement of glutathione and glutathione-related enzymes. J Nutr Biochem. 2005;16(10):577–586. doi: 10.1016/j.jnutbio.2005.05.013.
- Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox45120403095857.
- Kirkman HN, Gaetani GF. Mammalian catalase: a venerable enzyme with new mysteries. Trends Biochem Sci. 2007;32(1):44–50. doi: 10.1016/j.tibs.2006.11.003.
- Hetarinen-Runtti P, Lakari E, Raivio KO, et al. Expression of antioxidant enzymes in human inflammatory cells. Am J Physiol - Cell Physiol. 2000;278(1):C118–C125.
- Ghiselli A, Serafini M, Natella F, et al. Total antioxidant capacity as a tool to assess redox status: critical view and experimental data. Free Radic Biol Med. 2000;29(11):1106–1114. doi: 10.1016/s0891-5849(00)00394-4.
- Piskounova E, Agathocleous M, Murphy MM, et al. Oxidative stress inhibits distant metastasis by human melanoma cells. Nat. 2015;;527(7577):186–191. Available from: https://www.nature.com/articles/nature15726. doi: 10.1038/nature15726.
- Davies MJ. Protein oxidation and peroxidation. Biochem J [Internet]. 2016;473(7):805–825. doi: 10.1042/BJ20151227.
- Kükürt A, Gelen V, Başer ÖF, et al. Thiols: role in oxidative stress-related disorders. Accent Lipid Peroxidation [Internet]. 2021. Available from: https://www.intechopen.com/state.item.id.
- Hizal M, Sendur MAN, Bilgin B, et al. Evaluation of dynamic serum thiol/disulfide homeostasis in locally advanced and metastatic gastric cancer. J Oncol Sci. 2018;4(1):1–4. doi: 10.1016/j.jons.2018.01.002.
- Ellidag HY, Bulbuller N, Eren E, et al. Ischemia-modified albumin: could it be a new oxidative stress biomarker for colorectal carcinoma? Gut Liver. 2013;7(6):675–680. doi: 10.5009/gnl.2013.7.6.675.
- Kalousová M, Zima T, Tesař V, et al. Advanced glycoxidation end products in chronic diseases - Clinical chemistry and genetic background. Mutat. Res. - Fundam. Mol. Mech. Mutagen. 2005;579(1-2):37–46.
- Ma Y, Zhang L, Rong S, et al. Relation between gastric cancer and protein oxidation, DNA damage, and lipid peroxidation. Oxid Med Cell Longev. 2013;2013:543760. doi: 10.1155/2013/543760.
- Cai F, Dupertuis YM, Pichard C. Role of polyunsaturated fatty acids and lipid peroxidation on colorectal cancer risk and treatments. Curr Opin Clin Nutr Metab Care. 2012;15(2):99–106. doi: 10.1097/MCO.0b013e32834feab4.
- Bartolacci C, Andreani C, El-Gammal Y, et al. Lipid metabolism regulates oxidative stress and ferroptosis in RAS-driven cancers: a perspective on cancer progression and therapy. Front Mol Biosci. 2021;8:706650. doi: 10.3389/fmolb.2021.706650.
- Argüelles S, García S, Maldonado M, et al. Do the serum oxidative stress biomarkers provide a reasonable index of the general oxidative stress status? Biochim Biophys Acta. 2004;1674(3):251–259. doi: 10.1016/j.bbagen.2004.06.023.
- Montuschi P, Barnes PJ, Roberts LJ. Isoprostanes: markers and mediators of oxidative stress. Faseb J. 2004;18(15):1791–1800. Available from: https://pubmed.ncbi.nlm.nih.gov/15576482/.
- Gào X, Zhang Y, Burwinkel B, et al. The associations of DNA methylation alterations in oxidative stress-related genes with cancer incidence and mortality outcomes: a population-based cohort study. Clin Epigenetics. 2019;11(1):14. doi: 10.1186/s13148-018-0604-y.
- Arfin S, Jha NK, Jha SK, et al. Oxidative stress in cancer cell metabolism. Antioxidants. 2021;10(5) [:642. https://www.mdpi.com/2076-3921/10/5/642/htm. doi: 10.3390/antiox10050642.