Abstract
Objective
The aim of this study was to compare and rank different targeted therapies or immunotherapies for advanced hepatocellular carcinoma based on efficacy.
Methods
A systematic search of the PubMed, EMBASE, and Cochrane Library databases was conducted. All systematic treatment regimens that reported comparisons with sorafenib were included in this analysis. The primary outcome measures were overall survival (OS) and progression-free survival (PFS), and other outcome measures included the objective response rate (ORR) and safety analysis according to reported treatment-related adverse events.
Results
A total of 29 RCTs involving 13376 patients were included in the analysis, including 10 single-agent therapies and 17 combination therapies. Compared with sorafenib, sintilimab plus IBI305 (HR: 0.57, 95% CI: 0.43-0.75), camrelizumab plus rivoceranib (HR: 0.62, 95% CI: 0.49-0.78), and atezolizumab plus bevacizumab (HR: 0.66, 95% CI: 0.52-0.83) ranked in the top three in terms of OS.
Conclusions
PD-1/PD-L1 inhibitors combined with anti-vascular endothelial growth factor (anti-VEGF)-targeting drugs have shown better therapeutic effects in the systematic treatment of patients with advanced hepatocellular carcinoma, and the combination of targeted and immune therapy modes should be further developed.
1. Introduction
According to the data analysis of GLOBOCAN2020, liver cancer ranks sixth in the incidence of malignancies and third in the mortality rate [Citation1]. Given the increasing incidence of liver cancer, it is estimated that in 2040, 1.4 million people will be diagnosed with liver cancer worldwide, and 1.3 million people will die from liver cancer [Citation2]. Hepatocellular carcinoma (HCC) accounts for the vast majority of primary liver cancers. Due to the concealment of HCC, some patients are in the advanced stage of unresectable disease at the time of treatment. Based on the clinical stage of HCC patients, systemic therapy may be the only option to improve survival for patients with advanced HCC or for those who are not suitable for radical surgery or local treatment [Citation3].
The systematic treatment of liver cancer progresses through the following three stages: targeted therapy, immunotherapy, and combined targeted therapy and immunotherapy. The global multicenter SHARP study [Citation4] and ORIENTAL trials [Citation5] of Asia Pacific populations confirmed the efficacy of sorafenib in advanced HCC, pioneering targeted drug therapy in patients with advanced HCC. Subsequent global multicenter, randomized controlled, noninferiority Phase III REFLECT studies showed that the efficacy of lenvatinib was nonadverse to sorafenib (mOS: 13.6 vs. 12.3 months, HR: 0.92) [Citation6], further consolidating the role of multikinase inhibitors in the first-line therapy of advanced HCC. However, the two drugs both had low response rates and serious adverse reactions in clinical practice. For example, in the REFLECT study, patients treated with lenvatinib had a higher proportion of hypertension and proteinuria, while those treated with sorafenib had more skin reactions and diarrhea, which led to a decline in quality-of-life scores for both treatments. The open-label, international multicenter, randomized controlled phase III IMbrave150 study has advanced the systemic treatment landscape. In a recently updated analysis, atezolizumab combined with bevacizumab showed consistent clinically meaningful efficacy and safety benefits over sorafenib, improving overall survival (mOS: 19.2 vs. 13.4 months, HR: 0.66) and progression-free survival (mPFS: 6.9 vs. 4.3 months, HR: 0.65) in unresectable HCC patients [Citation7].
Recently, researchers have attempted to develop systematic treatment options for advanced HCC. HIMALAYA (durvalumab vs. sorafenib) and RATIONALE301 (tislelizumab vs. sorafenib) both obtained noninferior results in OS [Citation8,Citation9], whereas the previous checkmate459 (nivolumab vs. sorafenib) did not meet the primary study endpoint [Citation10]. In combination therapy, ORIENT-32 (sintilimab + IBI305 vs. sorafenib), HIMALAYA (tremelimumab + durvalumab vs. sorafenib), and SHR-1210-310 (camrelizumab + rivoceranib vs. sorafenib) all had similar results after the success of IMbrave150[7,8,11,12], whereas COSMIC-312 (cabozantinib + atezolizumab vs. sorafenib) failed to show an extended mOS (15.4 vs. 15.5 months, HR: 0.90) [Citation11]. Patients with advanced HCC have an increasing number of potential systemic treatment options, but head-to-head comparisons between treatment options of interest are lacking. Therefore, we conducted a Bayesian network meta-analysis that combined direct and indirect comparisons to rank different treatment regimens according to efficacy by comparing their overall survival and progression-free survival, objective response rate, and treatment-related adverse events (TRAEs) with those of sorafenib.
2. Materials and methods
2.1. Protocol and registration
This study was prepared based on an expanded version of the PRISMA Statement for Reporting Systematic Reviews (PRISMA-NMA)[Citation12]. We have registered this research protocol in the PROSPERO International Prospective Register of Systematic Reviews (ID: CRD42022384274, http://www.crd.york.ac.uk/PROSPERO).
2.2. Eligibility criteria
Based on the PICOS principle of evidence-based medicine, the inclusion criteria formulated in this study were as follows: (1) P (patients)—advanced and/or not eligible for surgical or locoregional therapies; (2) I (intervention)—other targeted or immunotherapy modalities, local therapy or combination of other antineoplastic agents with sorafenib; (3) C (comparison)—sorafenib monotherapy; (4) O (outcomes)—the primary result was a comparison of overall survival (OS) and progression-free survival (PFS) with sorafenib, and the secondary result was the objective response rate (ORR) and TRAEs; and (5) S (study)—our primary objective was to select all advanced HCC randomized controlled trials using sorafenib monotherapy as a control, excluding those with second-line therapy using a placebo as a control.
2.3. Search strategy
From December 2022 to January 2023, we systematically searched the PubMed, EMBASE, and Cochrane Library databases by combining subject terms with free words, regardless of language type or country and time. To include the most recent research, a final search of the above database was conducted on February 5, 2023, and the retrieved data were updated. The search style and search results are recorded in detail in Table S1.
2.4. Study selection and outcomes
After searching the database, we first integrated the results retrieved from different databases and deleted the duplicate literature. Then, the first exclusion was conducted based on the title and abstract information. After the preliminary screening was completed, the full text of the remaining literature was read according to the previously established inclusion criteria, and the final studies included in this meta-analysis were determined based on this reading. The included studies reported at least one of the following: hazard ratios (HRs) and 95% confidence intervals for OS and PFS; extractable Kaplan–Meier curves if no HRs were reported; ORR based on the Response Evaluation Criteria in Solid Tumors (RECIST) or modified RECIST (mRECIST), defined as complete response plus partial response; and the number of patients with grade 3 or higher TRAEs that could be extracted from the original text.
2.5. Data extraction
After identifying the studies to be included, the two researchers reviewed the literature again and independently extracted the available information into a predeveloped standardized spreadsheet al.l disputed issues were resolved in coordination with a third researcher until consensus was reached. If necessary, the author of the original article was contacted by email. The extracted information mainly included (1) study characteristics—year, author, number of patients included, clinical trial name, and registration information; (2) baseline data—age, sex ratio, follow-up time, Child–Pugh grade, Barcelona Clinic Liver Cancer stage (BCLC), Eastern Cooperative Oncology Group performance status (PS), and hepatitis B and C information; and (3) valuable clinical endpoints—HR for OS and PFS and their 95% confidence intervals, proportion of patients in the experimental and control groups exhibiting an objective response, and proportion of patients with grade 3 or higher TRAEs. Table S2 summarizes and organizes all the extracted details.
2.6. Quality assessment
We evaluated the quality of the included randomized controlled trials using the latest version of the Cochrane Collaboration’s bias risk assessment tool (ROB 2.0) [Citation13]. Compared with ROB1, the ROB2 tool uses a more appropriate statistical approach to control bias and provides a more comprehensive and accurate assessment of the risk of bias in RCTs in five different areas: bias in randomization, bias in deviation from established interventions, bias in missing outcome data, bias in outcome measurements, and bias in selectively reported outcomes.
2.7. The geometry of the network
We show the number of studies for each intervention and the number of patients enrolled with different treatments in a network of evidence charts. Each node represents an intervention, and the lines between nodes represent direct comparisons between different interventions. The size of the nodes and the thickness of the lines represent the number of patients included in the corresponding intervention and the number of studies directly comparing the intervention, respectively. The network evidence graph of the comparison between all effect sizes is presented in Figure S1.
2.8. Statistical analysis
OS and PFS are time-to-event variables; therefore, HR and its 95% confidence intervals were used for comparison. For comparison purposes, we calculated ORs and 95% confidence intervals for the ORR and TRAEs from the extracted data. All comparative analyses are based on the common control of sorafenib. If more than two studies of a treatment model were available, we first performed a direct comparison meta-analysis using Review Manager 5.4 software to evaluate the effectiveness of the treatment. Cochran’s Q test and Higgin’s I2 test were used to evaluate the heterogeneity among the studies. If the I2 was >50% or the P value was < 0.05, the studies were considered to have significant heterogeneity, and a random-effects model was used; otherwise, a fixed-effects model was used. The network meta-analysis was performed based on a Bayesian approach with a prior distribution [Citation14]. The fixed-effects model was used because all treatments except nintedanib and HAIC + sorafenib were single studies, and we could not assess the heterogeneity parameters. Treatment measures were ranked according to the area under the SUCRA curve (from 0 to 1). If a treatment was closer to 1, it was more likely to be effective, and if it was closer to 0, it was likely to be less effective [Citation15]. To explore the heterogeneity and robustness of the model results, we conducted a subgroup analysis based on a randomized phase III trial compared with the IMbrave150 study with atezolizumab plus bevacizumab as the common control. In addition, a regression analysis of the included studies was performed, with the main covariates including sample size and the proportion of patients with BCLC stage C, Child–Pugh class A, and hepatitis B. The Bayesian network meta-analysis was conducted based on R software, version 4.2.2 (https://cran.r-project.org). The ‘gemtc’ package and ‘rjags’ package were used for the analysis, and the ‘rjags’ package was used to invoke the background JAGS software to perform the Markov Chain Monte Carlo (MCMC) simulation. In all analyses, a P value of less than 0.05 was considered statistically significant.
3. Results
3.1. Included studies
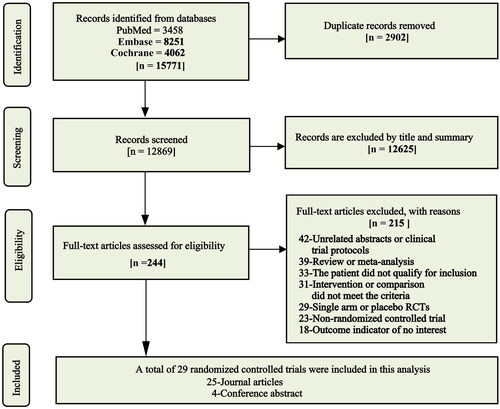
A total of 29 randomized controlled trials involving 13376 patients were included in this analysis [Citation6–11, Citation16–38], of which 4 were conference abstracts (). All studies were published between 2013 and 2022, the median or mean age of patients was 53 to 72 years, and the vast majority of patients had BCLC C stage and good liver function classified as Child–Pugh A. Cheng et al.’s [Citation35] and Abou-Alfa et al.’s [Citation8] studies were three-arm studies, while the other 27 studies were all two-arm studies. Eleven studies reported 10 monotherapy regimens compared to sorafenib, including targeted monotherapy and immunotherapy: nivolumab, donafenib, nintedanib (2), lenvatinib, dovitinib, linifanib, brivanib, sunitinib, durvalumab, and tislelizumab. Nineteen studies reported 17 different combination therapies compared with sorafenib, including targeted dual-drug combinations, immune dual-drug combinations, targeted and immune combinations, and sorafenib with local treatment or other antitumor agents: cabozantinib + atezolizumab, atezolizumab + bevacizumab, sintilimab + IBI305, SIRT + sorafenib, TACE + sorafenib, HAIC + sorafenib(3), sorafenib + pravastatin, sorafenib + GEMOX, sorafenib + doxorubicin, bevacizumab + erlotinib, sorafenib + resminostat, sorafenib + tegafur–uracil, sorafenib + erlotinib, tigatuzumab + sorafenib, tremelimumab + durvalumab, camrelizumab + rivoceranib, and SBRT + sorafenib. The included relevant studies and extracted details are shown in and Table S2.
Table 1. Baseline information from the included studies.
3.2. Risk of bias within the studies
Based on the tools (ROB 2.0) recommended by the Cochrane Collaboration, we assessed the quality of 25 studies. A bias assessment of allocation concealment was difficult due to the insufficient information available in most studies (Figure S2).
3.3. Meta-analysis of direct comparison
Two studies [Citation26, Citation29] compared the efficacy of nintedanib and sorafenib, and three studies [Citation22, Citation30, Citation32] compared the efficacy of HAIC + sorafenib and sorafenib. We extracted and analyzed the indicators of interest. Compared with sorafenib, nintedanib showed no significant difference in OS and PFS (OS, HR: 0.91, 95% CI: 0.64-1.30; PFS, HR: 1.26, 95% CI: 0.87-1.81). In addition, HAIC combined with sorafenib did not appear to show an advantage in OS compared to sorafenib alone, but combined therapy may improve the ORR (OS, HR: 0.87, 95% CI: 0.61-1.24; ORR, OR: 2.64, 95% CI: 1.53-4.56) (Figure S3).
3.4. Results of network meta-analysis and ranking of treatments
3.4.1. Overall survival
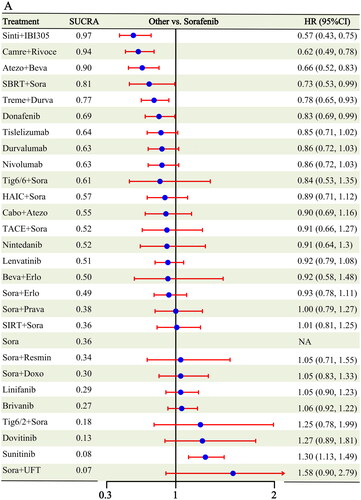
A total of 28 RCTs [Citation6–11, Citation16–23, Citation25–38] containing 27 different interventions reported OS information compared to sorafenib (Figure S1); six different interventions showed survival benefits beyond sorafenib: sintilimab + IBI305 (HR: 0.57, 95% CI: 0.43-0.75), camrelizumab + rivoceranib (HR: 0.62, 95% CI: 0.49-0.78), atezolizumab + bevacizumab (HR: 0.66, 95% CI: 0.52-0.83), SBRT + sorafenib (HR: 0.73, 95% CI: 0.53-0.99), tremelimumab + durvalumab (HR: 0.78, 95% CI: 0.65-0.93), and donafenib (HR: 0.83, 95% CI: 0.69-0.99). In addition, except sunitinib, which had a worse OS (HR: 1.30, 95% CI: 1.13-1.49), the remaining 20 interventions showed no significant difference compared to sorafenib (). Sintilimab + IBI305 had the highest probability of being ranked 1 (0.53), followed by camrelizumab + rivoceranib (0.23) and atezolizumab + bevacizumab (0.11) (Figure S4 and Table S3). The cumulative probability of different treatments compared with sorafenib was ranked according to the area under the SUCRA curve, which showed that sintilimab + IBI305 (0.97), camrelizumab + rivoceranib (0.94), and atezolizumab + bevacizumab (0.90) ranked first, second and third, respectively (Figure S5). League tables of all pairwise comparison results are presented in Table S4.
3.4.2. Progression-free survival
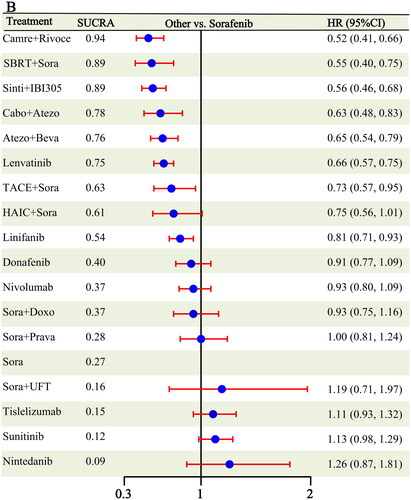
Eighteen RCTs [Citation6,Citation7, Citation9–11, Citation16–19, Citation21, Citation23, Citation25,Citation26, Citation29–31, Citation36, Citation38] reported PFS outcomes, covering 18 different treatment modalities (Figure S1). Eight treatments showed better PFS outcomes than sorafenib: camrelizumab + rivoceranib (HR: 0.52, 95% CI: 0.41-0.66), SBRT + sorafenib (HR: 0.55, 95% CI: 0.40-0.75), sintilimab + IBI305 (HR: 0.56, 95% CI: 0.46-0.68), cabozantinib + atezolizumab (HR: 0.63, 95% CI: 0.48-0.83), atezolizumab + bevacizumab (HR: 0.65, 95% CI: 0.54-0.79), lenvatinib (HR: 0.66, 95% CI: 0.57-0.75), TACE + sorafenib (HR: 0.73, 95% CI: 0.57-0.95), and linifanib (HR: 0.81, 95% CI: 0.71-0.93) (). The remaining 10 treatments did not differ from sorafenib. Camrelizumab + rivoceranib (0.43) was most likely to be ranked first, followed by SBRT + sorafenib (0.31) and sintilimab + IBI305 (0.17) (Figure S4 and Table S3). According to the aggregated SUCRA results, camrelizumab + rivoceranib had the highest likelihood of cumulatively ranking first (0.94); SBRT + sorafenib and sintilimab + IBI305 had similar results (0.89), followed by cabozantinib + atezolizumab (0.78) (Figure S5).
3.4.3. Objective response rate
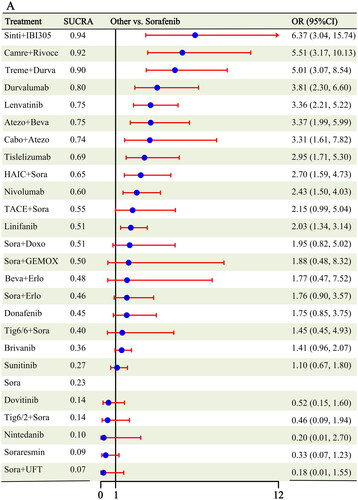
Twenty-five studies [Citation6–11, Citation16,Citation17, Citation19, Citation21,Citation22, Citation24,Citation25, Citation27–38] reported the ORR as an outcome, and after combining the same treatment measures, 25 different interventions were included in the analysis (Figure S1). Eleven interventions showed an improved ORR over sorafenib: sintilimab + IBI305 (OR: 6.37, 95% CI: 3.04-15.74), camrelizumab + rivoceranib (OR: 5.51, 95% CI: 3.17-10.13), tremelimumab + durvalumab (OR: 5.01, 95% CI: 3.07-8.54), durvalumab (OR: 3.81, 95% CI: 2.30-6.60), lenvatinib (OR: 3.36, 95% CI: 2.21-5.22), atezolizumab + bevacizumab (OR: 3.37, 95% CI: 1.99-5.99), cabozantinib + atezolizumab (OR: 3.31, 95% CI: 1.61-7.82), tislelizumab (OR: 2.95, 95% CI: 1.71-5.30), HAIC + sorafenib (OR: 2.70, 95% CI: 1.59-4.73), nivolumab (OR: 2.43, 95% CI: 1.50-4.03), and linifanib (OR: 2.03, 95% CI: 1.34-3.14) (). In addition, the ORR for the remaining 14 treatment modalities did not significantly differ from that of sorafenib. Both the Rank1 results and SUCRA cumulative ranking showed that the efficacy of sintilimab + IBI305 was superior (Rank1:0.46, Sucra:0.94), followed by camrelizumab + rivoceranib (Rank1:0.24, Sucra:0.92) and tremelimumab + durvalumab in third place (Rank1:0.13, Sucra:0.90) (Figures S4–S5 and Table S3).
3.4.4. Treatment-related adverse events ≥ grade 3
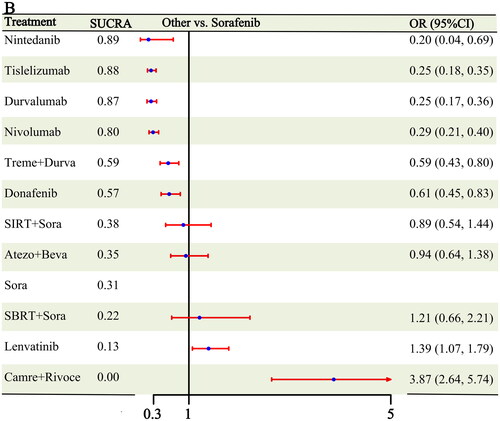
In terms of the safety analysis, 10 studies [Citation6–10, Citation17–20, Citation29] reported tertiary TRAE results or greater, covering 11 different treatment modalities (Figure S1). Six interventions showed fewer grade 3 or higher TRAEs than sorafenib: nintedanib (OR: 0.20, 95% CI: 0.04-0.69), tislelizumab (OR: 0.25, 95% CI: 0.18-0.35), durvalumab (OR: 0.25, 95% CI: 0.17-0.36), nivolumab (OR: 0.29, 95% CI: 0.21-0.40), tremelimumab + durvalumab (OR: 0.59, 95% CI: 0.43-0.80), and donafenib (OR: 0.61, 95% CI: 0.45-0.83). The safety profiles of lenvatinib (OR: 1.39, 95% CI: 1.07-1.79) and camrelizumab + rivoceranib (OR: 3.87, 95% CI: 2.64-5.74) were inferior to those of sorafenib (). Both in the Rank1 results and SUCRA cumulative ranking, nintedanib showed better efficacy (Rank1:0.55, Sucra:0.89), followed by tislelizumab (Rank1:0.20, Sucra:0.88) and durvalumab in third place (Rank1:0.20, Sucra:0.87) (Figures S4–S5 and Table S3).
3.5. Subgroup analyses
In this analysis, 18 studies [Citation6–11, Citation16–19, Citation21, Citation23, Citation25, Citation30, Citation34, Citation36–38] were randomized phase III trials, of which 18 studies reported OS, 14 studies reported PFS, 16 studies reported the ORR, and 8 studies reported ≥ grade 3 TRAEs. We compared these studies with the results of IMbrave150, which showed that sintilimab + IBI305 had the highest treatment rankings compared with atezolizumab + bevacizumab in terms of OS and the ORR. In terms of PFS and safety, the highest ranked treatments were camrelizumab + rivoceranib and tislelizumab. Considering the primary end point of our study, we found that sintilimab + IBI305 and camrelizumab + rivoceranib have the potential to be superior to atezolizumab plus bevacizumab in terms of OS and PFS (Figure S6).
3.6. Assessment consistency and homogeneity
The entire analysis lacked a closed-loop structure; therefore, we used a model comparison approach to detect global inconsistency by comparing the fit of the two models to determine whether the consistency assumption was reasonable and the data were consequently consistent. The deviance information criterion (DIC) was not significantly different between the consistent model and the inconsistent model (the differences were all < 5). In addition, the overall network I2 of all analysis results was less than 10%; Table S5 presents the specific comparison results of the two models. In addition, we compared the results of the direct meta-analysis with those of the network meta-analysis, including OS results and PFS results of nintedanib versus sorafenib and OS and ORR of HAIC + Sora versus sorafenib, all of which showed no between-group differences (Table S6).
3.7. Network meta-regression
Four covariates, including sample size, proportion of patients with BCLC stage C, Child–Pugh class A status, and HBV status, were analyzed by using a regression model. The 95% confidence intervals of beta values of the four covariates of all outcomes included 0. In other words, the values of all covariates included in this analysis did not affect the final analysis results. The results of the regression analysis are presented in Table S7.
4. Discussion
In recent years, many targeted and immunotherapy regimens have emerged for advanced HCC. Many small-molecule tyrosine kinase inhibitors (TKIs) and immune checkpoint inhibitors, such as monotherapy or combination therapy, continue to refresh the first-line treatment pattern of advanced HCC. New targeted monotherapies, immune monotherapies and new combination therapies have shown encouraging results. For this analysis, we pooled all randomized controlled studies comparing sorafenib with systemic therapy for the treatment of advanced HCC. These modalities include targeted or immune monotherapy and their combination or a combination of local therapies with these treatments. In general, the combination of targeted and/or immune therapy has improved efficacy. Considering the gold standard OS in advanced tumor studies, the combination treatment mode of sintilimab + IBI305, camrelizumab + rivoceranib, and atezolizumab + bevacizumab has more benefits, and these results were also replicated in the subgroup comparison with atezolizumab + bevacizumab.
In terms of single-agent targeted therapy, currently available drugs include multikinase inhibitors similar to sorafenib and inhibitors with more specific targets. After lenvatinib showed noninferiority compared to sorafenib [Citation6], donafenib, a modified form of sorafenib multikinase inhibitor, showed a statistically significant improvement in overall survival (HR: 0.83, 95% CI: 0.69-0.99) [Citation19]. In addition, other multitarget inhibitors, i.e. cabozantinib and apatinib, all showed positive results compared with placebo [Citation39,Citation40]. Moreover, drugs that selectively target MET, such as tepotinib [Citation41] and capmatinib [Citation42], and fibroblast growth factor receptor 4 (FGFR4) inhibitors, such as FGF401[Citation43] and fisogatinib [Citation44], also showed therapeutic potential. In this study, two PD-1 inhibitors, tislelizumab (RATIONALE301) and nivolumab (checkmate459), and a PD-L1 inhibitor, durvalumab (HIMALAYA), were included in this analysis. Regardless of the superiority design of the CheckMate459 trial, all three immunotherapy single agents would probably have achieved noninferiority to sorafenib [Citation8–10]. In addition, on the safety front, patients who received immunotherapy had fewer grade 3 or higher TRAEs than those who received sorafenib (tislelizumab, OR: 0.25, 95% CI: 0.18-0.35; nivolumab, OR: 0.29, 95% CI: 0.21-0.40; durvalumab, OR: 0.25, 95% CI: 0.17-0.36). Collectively, these results suggest that immune monotherapy may be a potential first-line treatment option for advanced HCC.
At present, the systemic treatment of hepatocellular carcinoma has transitioned from single-drug targeted therapy to dual immunotherapy or combined targeted and immune therapy. Among these regimens, the combination of PD-1/PD-L1 inhibitors and CTLA4 inhibitors has become the focus of current research. Blocking the CTLA4 pathway leads to increased activation of CD8-positive cells in lymph nodes and increased infiltration of activated CD8-positive T cells. In the presence of antigen-specific CD8-positive T cells in tumor tissues, blocking the PD-1/PD-L1 pathway induces antitumor immunity and enhances the antitumor activity of effector T cells [Citation45,Citation46]. In the previous CheckMate 040 study [Citation47], nivolumab plus ipilimumab demonstrated a manageable safety profile and a high ORR (approximately 30%), which led to accelerated approval as a second-line treatment for HCC in the United States. In our analysis, tremelimumab + durvalumab showed a favorable OS benefit compared with sorafenib (HR: 0.78, 95% CI: 0.65-0.93), although its efficacy was slightly lower than that of atezolizumab + bevacizumab. With respect to combined immune and targeted therapy, the combination of PD-1/PD-L1 inhibitors and anti-vascular endothelial growth factor (anti-VEGFA) targeting showed significantly better effects than sorafenib. The combination therapy of camrelizumab (anti-PD-1 antibody) + rivoceranib (high selectivity for VEGF receptor 2), atezolizumab (anti-PD-L1 antibody) + bevacizumab (anti-VEGFA antibody), and sintilimab (anti-PD-1 antibody) + IBI305 (a bevacizumab biosimilar) all significantly improved the OS of patients with advanced HCC [Citation7, Citation16,Citation17]. In combination with a multikinase inhibitor, cabozantinib + atezolizumab failed to improve median survival compared with sorafenib but showed a statistically significant difference in PFS (mPFS: 6.8 vs. 4.2 months, HR: 0.63) [Citation11]. Unfortunately, recent data from the phase III LEAP-002 study [Citation48] indicate that the primary endpoints OS and PFS of lenvatinib + pembrolizumab compared with lenvatinib did not meet the prespecified statistical significance. The combination of immunotherapy and multikinase inhibitors currently accounts for nearly half of the combined treatment programs for HCC. Although the current results seem to be unsatisfactory, this strategy still has great potential [Citation46].
Although neither SIRT + Sora nor TACE + Sora previously improved efficacy when sorafenib was combined with local therapy, it is perhaps encouraging that SBRT + Sora showed superiority over sorafenib alone when considering the primary endpoints of OS and PFS. The efficacy of HAIC + Sora remains controversial. Our results show that OS did not significantly differ between HAIC + Sora and sorafenib alone, but the combination of HAIC and sorafenib may improve the objective response. However, two recent studies have shown better OS results with either HAIC monotherapy [Citation49] or HAIC + Sora [Citation50] (LYU et al. Zheng et al.). First, these two studies were from a single center in China and mainly targeted patients with macrovascular invasion. Second, Lyu et al. used the FOLFOX regimen (fluorouracil, leucovorin, and oxaliplatin), whereas Zheng et al. used a 3cir-OFF protocol (oxaliplatin followed by 5-fluorouracil), which is different from the three studies we included in this study. Although the FOLFOX regimen has recently shown good efficacy in the treatment of HCC [Citation51], the efficacy of HAIC combined with sorafenib in patients with advanced HCC still needs to be confirmed in more multicenter studies with large samples.
Our study also has some limitations. All included studies lacked direct contrast between the interventions and sorafenib, and we obtained efficacy rankings between them based only on indirect contrast; therefore, all estimates are subject to relative uncertainty. In addition, our analysis was limited to the available reported data from all studies, and the median follow-up in some studies was less than 12 months. Therefore, long-term efficacy results were not available. We have also learned some revelatory lessons. For example, although nivolumab, durvalumab, and tislelizumab showed similar OS benefits compared to sorafenib in the recent immunotherapy monotherapy versus sorafenib trial, nivolumab was declared a failure because the study did not reach the primary endpoint. This finding may be due to the superior experimental design for testing nivolumab, whereas the experimental design for testing durvalumab and tislelizumab was noninferior. Therefore, good experimental design and endpoint setting are essential for subsequent trials of new drugs.
Overall, the combination of targeted and immunotherapy is more effective in the systemic treatment of advanced hepatocellular carcinoma, whether based on OS, PFS or ORR. Specifically, sintilimab + IBI305 and camrelizumab + rivoeranib showed efficacy similar to that of atezolizumab + bevacizumab. On the premise of safety, different treatment methods should be combined to improve the treatment effect and quality of life of patients.
Ethical approval
Ethical approval was not needed, as this study did not require the use of patient identifiers.
Author contribution
G-Z. Wang and Y-L. Zhang participated in the conception and design of this study; Y-L. Zhang, X-J. Cui and H. Xing collected and analyzed the data; H-F. Ning and G-Z. Wang contributed to interpretation of data; Y-L. Zhang contributed to drafting of the paper; P. Dong and G-Z. Wang revised it critically for intellectual content. All authors agreed to be accountable for all aspects of the work.
Supplemental Material
Download MS Word (9.1 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding authors.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):1–13. doi: 10.3322/caac.21660.
- Rumgay H, Arnold M, Ferlay J, et al. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022;77(6):1598–1606. doi: 10.1016/j.jhep.2022.08.021.
- Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi: 10.1016/j.jhep.2021.11.018.
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857.
- Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. doi: 10.1016/S1470-2045(08)70285-7.
- Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi: 10.1016/S0140-6736(18)30207-1.
- Cheng AL, Qin S, Ikeda M, et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76(4):862–873. doi: 10.1016/j.jhep.2021.11.030.
- Abou-Alfa GK, Chan SL, Kudo M, et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. J Clin Oncol. 2022;40(4_suppl):379–379. doi: 10.1200/JCO.2022.40.4_suppl.379.
- Qin S, Kudo M, Meyer T, et al. LBA36 final analysis of RATIONALE-301: randomized, phase III study of tislelizumab versus sorafenib as first-line treatment for unresectable hepatocellular carcinoma. Annals of Oncology. 2022;33: s 1402–S1403. doi: 10.1016/j.annonc.2022.08.033.
- Yau T, Park JW, Finn RS, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;23(1):77–90. doi: 10.1016/S1470-2045(21)00604-5.
- Kelley RK, Rimassa L, Cheng AL, et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2022;23(8):995–1008. doi: 10.1016/S1470-2045(22)00326-6.
- Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–784. doi: 10.7326/M14-2385.
- Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898.
- Dias S, Sutton AJ, Ades AE, et al. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making. 2013;33(5):607–617. doi: 10.1177/0272989X12458724.
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64(2):163–171. doi: 10.1016/j.jclinepi.2010.03.016.
- Ren Z, Xu J, Bai Y, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2-3 study. Lancet Oncol. 2021;22(7):977–990. doi: 10.1016/S1470-2045(21)00252-7.
- Qin S, Chan LS, Gu S, et al. LBA35 camrelizumab (C) plus rivoceranib (R) vs. sorafenib (S) as first-line therapy for unresectable hepatocellular carcinoma (uHCC): a randomized, phase III trial. Annals of Oncology. 2022;33: s 1401–S1402. doi: 10.1016/j.annonc.2022.08.032.
- Dawson LA, Winter K, Knox J, et al. NRG/RTOG 1112: randomized phase III study of sorafenib vs. Stereotactic body radiation therapy (SBRT) Followed by sorafenib in hepatocellular carcinoma (HCC) (NCT01730937). International Journal of Radiation Oncology Biology Physics. 2022;114(5):1057. doi: 10.1016/j.ijrobp.2022.09.002.
- Qin S, Bi F, Gu S, et al. Donafenib versus sorafenib in First-Line treatment of unresectable or metastatic hepatocellular carcinoma: a randomized, Open-Label, Parallel-Controlled phase II-III trial. J Clin Oncol. 2021;39(27):3002–3011. doi: 10.1200/JCO.21.00163.
- Ricke J, Klümpen HJ, Amthauer H, et al. Impact of combined selective internal radiation therapy and sorafenib on survival in advanced hepatocellular carcinoma. J Hepatol. 2019;71(6):1164–1174. doi: 10.1016/j.jhep.2019.08.006.
- Park JW, Kim YJ, Kim DY, et al. Sorafenib with or without concurrent transarterial chemoembolization in patients with advanced hepatocellular carcinoma: the phase III STAH trial. J Hepatol. 2019;70(4):684–691. doi: 10.1016/j.jhep.2018.11.029.
- Kondo M, Morimoto M, Kobayashi S, et al. Randomized, phase II trial of sequential hepatic arterial infusion chemotherapy and sorafenib versus sorafenib alone as initial therapy for advanced hepatocellular carcinoma: SCOOP-2 trial. BMC Cancer. 2019;19(1):954. doi: 10.1186/s12885-019-6198-8.
- Jouve JL, Lecomte T, Bouché O, et al. Pravastatin combination with sorafenib does not improve survival in advanced hepatocellular carcinoma. J Hepatol. 2019;71(3):516–522. doi: 10.1016/j.jhep.2019.04.021.
- Assenat E, Pageaux GP, Thézenas S, et al. Sorafenib alone vs. sorafenib plus GEMOX as 1(st)-line treatment for advanced HCC: the phase II randomised PRODIGE 10 trial. Br J Cancer. 2019;120(9):896–902. doi: 10.1038/s41416-019-0443-4.
- Abou-Alfa GK, Shi Q, Knox JJ, et al. Assessment of treatment with sorafenib plus doxorubicin vs sorafenib alone in patients with advanced hepatocellular carcinoma: phase 3 CALGB 80802 randomized clinical trial. JAMA Oncol. 2019;5(11):1582–1588. doi: 10.1001/jamaoncol.2019.2792.
- Yen CJ, Kim TY, Feng YH, et al. A phase I/randomized phase II study to evaluate the safety, pharmacokinetics, and efficacy of nintedanib versus sorafenib in asian patients with advanced hepatocellular carcinoma. Liver Cancer. 2018;7(2):165–178. doi: 10.1159/000486460.
- Thomas MB, Garrett-Mayer E, Anis M, et al. A randomized phase II Open-Label Multi-Institution study of the combination of bevacizumab and erlotinib compared to sorafenib in the First-Line treatment of patients with advanced hepatocellular carcinoma. Oncology. 2018;94(6):329–339. doi: 10.1159/000485384.
- Tak WY, Ryoo BY, Lim HY, et al. Phase I/II study of first-line combination therapy with sorafenib plus resminostat, an oral HDAC inhibitor, versus sorafenib monotherapy for advanced hepatocellular carcinoma in east asian patients. Invest New Drugs. 2018;36(6):1072–1084. doi: 10.1007/s10637-018-0658-x.
- Palmer DH, Ma YT, Peck-Radosavljevic M, et al. A multicentre, open-label, phase-I/randomised phase-II study to evaluate safety, pharmacokinetics, and efficacy of nintedanib vs. sorafenib in european patients with advanced hepatocellular carcinoma. Br J Cancer. 2018;118(9):1162–1168. doi: 10.1038/s41416-018-0051-8.
- Kudo M, Ueshima K, Yokosuka O, et al. Sorafenib plus low-dose cisplatin and fluorouracil hepatic arterial infusion chemotherapy versus sorafenib alone in patients with advanced hepatocellular carcinoma (SILIUS): a randomised, open label, phase 3 trial. Lancet Gastroenterol Hepatol. 2018;3(6):424–432. doi: 10.1016/S2468-1253(18)30078-5.
- Azim HA, Omar A, Atef H, et al. Sorafenib plus tegafur-uracil (UFT) versus sorafenib as first line systemic treatment for patients with advanced stage HCC: a phase II trial (ESLC01 study). J Hepatocell Carcinoma. 2018;5:109–119. doi: 10.2147/JHC.S169285.
- Ikeda M, Shimizu S, Sato T, et al. Sorafenib plus hepatic arterial infusion chemotherapy with cisplatin versus sorafenib for advanced hepatocellular carcinoma: randomized phase II trial. Ann Oncol. 2016;27(11):2090–2096. doi: 10.1093/annonc/mdw323.
- Cheng AL, Thongprasert S, Lim HY, et al. Randomized, open-label phase 2 study comparing frontline dovitinib versus sorafenib in patients with advanced hepatocellular carcinoma. Hepatology. 2016;64(3):774–784. doi: 10.1002/hep.28600.
- Zhu AX, Rosmorduc O, Evans TR, et al. SEARCH: a phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2015;33(6):559–566. doi: 10.1200/JCO.2013.53.7746.
- Cheng AL, Kang YK, He AR, et al. Safety and efficacy of tigatuzumab plus sorafenib as first-line therapy in subjects with advanced hepatocellular carcinoma: a phase 2 randomized study. J Hepatol. 2015;63(4):896–904. doi: 10.1016/j.jhep.2015.06.001.
- Cainap C, Qin S, Huang WT, et al. Linifanib versus sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized phase III trial. J Clin Oncol. 2015;33(2):172–179. doi: 10.1200/JCO.2013.54.3298.
- Johnson PJ, Qin S, Park JW, et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J Clin Oncol. 2013;31(28):3517–3524. doi: 10.1200/JCO.2012.48.4410.
- Cheng AL, Kang YK, Lin DY, et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol. 2013;31(32):4067–4075. doi: 10.1200/JCO.2012.45.8372.
- Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379(1):54–63. doi: 10.1056/NEJMoa1717002.
- Qin S, Li Q, Gu S, et al. Apatinib as second-line or later therapy in patients with advanced hepatocellular carcinoma (AHELP): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Gastroenterol Hepatol. 2021;6(7):559–568. doi: 10.1016/S2468-1253(21)00109-6.
- Ryoo BY, Cheng AL, Ren Z, et al. Randomised phase 1b/2 trial of tepotinib vs sorafenib in asian patients with advanced hepatocellular carcinoma with MET overexpression. Br J Cancer. 2021;125(2):200–208. doi: 10.1038/s41416-021-01380-3.
- Qin S, Chan SL, Sukeepaisarnjaroen W, et al. A phase II study of the efficacy and safety of the MET inhibitor capmatinib (INC280) in patients with advanced hepatocellular carcinoma. Ther Adv Med Oncol. 2019;11:1758835919889001. doi: 10.1177/1758835919889001.
- Chan SL, Schuler M, Kang YK, et al. A first-in-human phase 1/2 study of FGF401 and combination of FGF401 with spartalizumab in patients with hepatocellular carcinoma or biomarker-selected solid tumors. J Exp Clin Cancer Res. 2022;41(1):189. doi: 10.1186/s13046-022-02383-5.
- Kim RD, Sarker D, Meyer T, et al. First-in-Human phase I study of fisogatinib (BLU-554) validates aberrant FGF19 signaling as a driver event in hepatocellular carcinoma. Cancer Discov. 2019;9(12):1696–1707. doi: 10.1158/2159-8290.CD-19-0555.
- Kudo M. Scientific rationale for combination immunotherapy of hepatocellular carcinoma with anti-PD-1/PD-L1 and anti-CTLA-4 antibodies. Liver Cancer. 2019;8(6):413–426. doi: 10.1159/000503254.
- Yang C, Zhang H, Zhang L, et al. Evolving therapeutic landscape of advanced hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2023;20(4):203–222. doi: 10.1038/s41575-022-00704-9.
- Yau T, Kang YK, Kim TY, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the CheckMate 040 randomized clinical trial. JAMA Oncol. 2020;6(11):e204564. doi: 10.1001/jamaoncol.2020.4564.
- Finn RS, Kudo M, Merle P, et al. LBA34 primary results from the phase III LEAP-002 study: lenvatinib plus pembrolizumab versus lenvatinib as first-line (1L) therapy for advanced hepatocellular carcinoma (aHCC). Annals of Oncology. 2022;33: s 1401. doi: 10.1016/j.annonc.2022.08.031.
- Lyu N, Wang X, Li JB, et al. Arterial chemotherapy of oxaliplatin plus fluorouracil versus sorafenib in advanced hepatocellular carcinoma: a biomolecular exploratory, randomized, phase III trial (FOHAIC-1). J Clin Oncol. 2022;40(5):468–480. doi: 10.1200/JCO.21.01963.
- Zheng K, Zhu X, Fu S, et al. Sorafenib plus hepatic arterial infusion chemotherapy versus sorafenib for hepatocellular carcinoma with major portal vein tumor thrombosis: a randomized trial. Radiology. 2022;303(2):455–464. doi: 10.1148/radiol.211545.
- Li QJ, He MK, Chen HW, et al. Hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin versus transarterial chemoembolization for large hepatocellular carcinoma: a randomized phase III trial. J Clin Oncol. 2022;40(2):150–160. doi: 10.1200/JCO.21.00608.