Abstract
Objective
The present study aims to investigate the clinical and histopathological features of peritoneal endometriosis (PEM) and deep infiltrating endometriosis (DIE).
Methods
A total of 100 patients with PEM and DIE admitted to Dalian Women and Children’s Hospital/Dalian Women and Children’s Medical Center between October 2018 and December 2021 were selected as the study subjects. One hundred and thirty-one PEM specimens and 37 DIE were collected, 22 cases of these patients’ eutopic endometrium were used as control (15 in PEM, seven in DIE). The present study mainly analysed the pelvic distribution, the histopathological and immunohistochemical features and peritoneal invasion of PEM and DIE.
Results
The main distribution of PEM and DIE was located in the posterior pelvic cavity (p < .001). The histopathological characteristics of different PEM forms were different: the contents of endometrioid glands, endometrioid stroma, smooth muscle, fibrous tissue and blood vessels in different lesions were statistically significant (all p < .050). Estrogen receptor (ER) of PEM and DIE was highly expressed in endometrioid glandular epithelium and endometrioid stroma, without statistical significance (p = .330/.113). Progesterone receptor (PR) was also highly expressed in endometrioid glandular epithelium and endometrioid stroma without statistical significance (p = .757/.798). Ki-67 expression of DIE in endometrioid glandular epithelium was significantly higher than that in brown and white lesions (p < .001), while its expression in the endometrioid stroma was not statistically significant in red lesions (p = .070), but higher than that in other PEM lesions (p < .001). Different morphological lesions had different invasiveness rates and depths of invasion to the peritoneum. White lesions had a deeper subperitoneal invasion level than transparent and vesicular lesions.
Conclusions
Although different morphological appearance of PEM is a degenerative process, some active brown lesions of PEM have invasive effects during the process and may further develop into DIE. PEM and DIE may be different developmental stages of the same disease.
KEY MESSAGES
In summary, PEM is a progressive disease, and its different morphological appearance reflects different stages of lesion development.
Ectopic endometrial cells have a destructive effect on the peritoneal structures; as the lesion progresses, it continuously infiltrates the subperitoneum.
PEM and DIE are different development stages of the same disease. The homology of the two lesions has yet to be explored in terms of pathogenesis.
Introduction
Endometriosis is a condition in which the endometrial tissue (including glands and/or stroma) exists outside the uterine cavity; it affects the health of >10% of women of childbearing age [Citation1]. Although endometriosis is a benign disease, its biological behaviour is similar to that of malignant tumour cells; this includes implantation, invasion and distant metastasis.
Peritoneal endometriosis (PEM) is the most frequent subtype of endometriosis; it is widely distributed in the pelvic visceral and mural peritoneum. In this disease, lesions with different morphological appearance have different clinical and pathological features [Citation2,Citation3]. The definition of deep infiltrating endometriosis (DIE) is still controversial; the traditional definition of DIE is a lesion with an infiltration depth of ≥5 mm [Citation4]. Guerriero et al. [Citation5] believed that DIE includes two parts, which infiltrate subperitoneum ≥5 mm and extrauterine adenomyoma located in rectovaginal septum (RVS), bladder and intestine. Chen et al. [Citation6] observed the appearance and morphology of the lesions, and found that most DIE lesions developed from superficial peritoneal endometriotic lesions, often co-existing with PEM. Under laparoscopy, both white lesions of PEM and DIE showed local white fibrous nodular changes.
Usually, the confirmation of PEM is casual in the operation. The most common surgical treatment to PEM is ablation or excision, the last technique is more invasive and thorough, which contains removing the lesions and the surrounding healthy issues [Citation7]. However, the choice of the best therapeutic approach for women with DIE is often challenging. Therapeutic options include medical and surgical treatment, and the decision should be dictated by the patient’s medical history, disease stage, symptom severity and personal choice. Medical therapy can control the symptoms and stop the development of pathology, keeping in mind the side effects derived from a long-term treatment and the risk of recurrence once suspended. Surgical treatment should be proposed only when it is strictly necessary (failed hormone therapy, contraindications to hormone treatment, severity of symptoms, infertility), preferring, whenever possible, a conservative approach performed by a multidisciplinary team [Citation8].
In the present study, 100 patients with PEM and DIE admitted to Dalian Women and Children’s Medical Center were prospectively investigated, along with the histopathological characteristics, hormone receptor expression and cell proliferation activity of different lesion patterns. This study also explored the correlation between PEM and DIE, in order to obtain the optimal treatment for those two.
Materials and methods
Patients
One hundred patients with a postoperative histopathological diagnosis of PEM and DIE who attended Dalian Women and Children’s Health Hospital/Dalian Women and Children’s Medical Center between October 2018 and December 2021 were enrolled as the study subjects. All patients were women of childbearing age (18–50, average of 36 ± 7 years) with normal menstrual cycle. Patients with a history of malignancy, autoimmune disease, premature ovarian failure and pregnancy, and patients who had taken hormone medications in the last 3 months were excluded from the present study. The operation time was within seven days after the end of menstruation.
A total of 190 specimens were obtained, including 22 specimens of eutopic endometrium (15 in PEM, seven in DIE), 131 PEM specimens and 37 DIE specimens.
This study was approved by the Medical Ethics Committee of the hospital, and all enrolled patients had signed informed consent.
Methods
Eutopic endometrium: Diagnostic curettage was performed one day before surgery, and 22 eutopic endometrial specimens were obtained.
PEM and DIE sampling and HE staining: The pelvic and abdominal cavity was fully laparoscopically explored under general anaesthesia, and the suspected PEM and DIE were photographed and recorded. The peritoneum around the lesion was clamped with non-invasive forceps to prevent artificial destruction of the peritoneal mesothelial tissue, and scissors were used to completely excise the suspicious peritoneal and subperitoneal lesions together with 0.5 cm of the surrounding tissue. The wound was electro-coagulated or sutured to close the bleeding point after stripping. The excised specimen was immediately fixed with 10% formalin solution, the contralateral peritoneal side of the specimen was stained and marked, and longitudinal sections were paraffin-embedded, serially sectioned with a 5-μm interval, stained with HE, and observed under an ordinary light microscope.
Immunohistochemistry: The primary antibody reagents estrogen receptor (ER) (clone ER SP1)/progesterone receptor (PR) (clone PR 1E2)/Ki-67 (clone 30-9) were purchased from Roche (Basel, Switzerland), and the Roche BenchMark GX automatic immunohistochemical staining system was used to complete the staining, and the clear brown particles in the nucleus were interpreted as positive.
Analysis of the clinical and pathological features
The histopathological features of lesions with different morphological appearance of PEM and DIE, including vesicular, transparent, red, brown, white and DIE, were observed under laparoscopy [Citation2,Citation3]. Content and distribution of endometrioid glands, endometrioid stroma, smooth muscle tissue, fibrous tissue and blood vessels in specimens of PEM and DIE.
HE staining: Eutopic endometrium and endometriotic lesions were all stained.
Five fields of view (×40) were randomly selected for observation under the microscope. Regarding literature reports [Citation9,Citation10], semi-quantitative determination criteria were formulated; among these, the distribution of endometrioid glands, endometrioid stroma and blood vessels were classified into three categories: sparse (+), moderate (++) and abundant (++++).
Endometrioid glands: There were four types of glands according to glandular cavity size: (1) a large gland with an obviously dilated glandular cavity with an irregular outline; (2) a medium gland with a regular structure, round shape and dilated glandular cavity; (3) a small gland with a round or elongated shape and a less dilated glandular cavity than the medium gland; (4) a micro gland with a narrow and collapsed glandular cavity that was fissured or completely closed.
Endometrioid gland distribution: (1) sparse: sporadic gland distribution or no glands; (2) moderate: scattered gland distribution limited to 1–2 glands per visual field; (3) abundant: gland distribution all over the lesion or a presence of >3 glands.
Endometrioid stroma: (1) sparse: a focal plaque with narrow margins of stromal cell aggregates that does not completely encircle the endometrioid glands; (2) moderate: a focal plaque with a wide margin of stromal cell aggregates that do not completely encircle the endometrioid glands; (3) abundant: a focal plaque with a wide margin of stromal cell aggregates that completely encircle the endometrioid glands.
Smooth muscle and fibrous tissue. Five grades were established: (1) G0: no smooth muscle or fibrous tissue in the lesion; (2) G1: the percentage of smooth muscle or fibrous tissue made up <25% of the field of view; (3) G2: the percentage of smooth muscle or fibrous tissue made up ≥25% and <50% of the field of view; (4) G3: the percentage of smooth muscle or fibrous tissue made up ≥50% and <75% of the field of view; (5) G4: the percentage of smooth muscle or fibrous tissue made up ≥75% of the field of view.
Blood vessels: (1) sparse: a sporadic vessel distribution in the lesion and surrounding tissues and a narrow lumen; (2) moderate: scattered, sparse–abundant vessel distribution and a narrow lumen; (3) abundant: a sporadic distribution of vessels in the lesion and surrounding areas and a presence of some parts with dilated lumens.
Immunohistochemical analysis:
Estrogen receptor and PR: More obvious nuclear staining could be seen in endometrioid glands and stroma in both PEM and DIE specimens, which was interpreted as positive. According to the staining range and intensity of glandular epithelial and stromal cells, the hormone receptor expression was divided into ‘+’ (low), ‘++’ (moderate) or ‘+++’ (high) [Citation9].
Cell proliferation activity: Ki-67 staining was used to evaluate the proliferation activity of endometrioid glandular epithelium and stroma in PEM and DIE specimens. The regions with the highest proliferative activity as determined at low magnification (×40) were counted. The proliferative activity of endometrioid glandular epithelial cells was counted, and the mean number of Ki-67-positive nuclei in three regions of 100 adjacent epithelial cells was calculated. (‘Number of Ki-67-positive cells per 100 epithelial cells.’) Proliferative activity in endometrioid stromal cells was calculated as the average number of Ki-67-positive nuclei in three high power fields (×400) (hpf/FN 22) [Citation9].
Measurement of the invasiveness of the lesion to peritoneal structures and depth of invasion: Specimens around the PEM lesions and DIE with no adhesions and smooth peritoneum were selected for observation (to facilitate marking the peritoneal side of the specimen and measuring the subperitoneal depth of the lesion); included the degree of destruction of peritoneal mesothelial cells by the ectopic lesion, the integrity and continuity of the peritoneal mesothelial tissue, and the depth of infiltration of the ectopic lesion into the subperitoneum (mm).
Statistical analysis
SPSS27.0.1 statistical software (SPSS Inc., Chicago, IL) was used for statistical analysis, p < .05 was statistically significant. Fisher’s test was used to compare rates, Kruskal–Wallis test was used for ranked data, and ANOVA was used for measurement data. Welch’s test was used if the variance was not equal. Sidak test and Games-Howell test were used for post hoc analysis if the results were statistically significant.
Results
Distribution in the pelvis and morphological situation under laparoscopy of PEM and DIE
Distribution of PEM and DIE in the pelvic cavity
One to ten specimens of PEM and DIE were respectively collected from each case.
Distribution of PEM with different lesion morphological appearance: One hundred and thirty-one PEM specimens were collected, including 12 vesicular lesions (9.16%), 14 transparent lesions (10.69%), 15 red lesions (11.45%), 69 brown lesions (52.67%) and 21 white lesions (16.03%).
Distribution of PEM lesions: Twelve (9.16%) specimens were located in the anterior pelvis, 64 (48.85%) specimens were located in the posterior pelvis, 34 (25.95%) specimens were located in the left pelvis, 21 (16.03%) specimens were located in the right pelvis and 39 (29.78%) specimens were located in the uterosacral ligament in the posterior pelvis.
Distribution of PEM lesions with different morphological appearance: Transparent lesions were mainly distributed in the posterior and left pelvis; meanwhile, vesicular, red, brown and white lesions were distributed in the anterior, posterior, left and right pelvic cavities. A total of 41.98% (55/131) of the red, brown and white lesions were distributed in the posterior pelvic cavity.
Thirty-seven specimens of DIE were found among the cases. The distributions were as follows: a total of 2 (5.4%) in the peritoneal reflex in the bladder, 24 (64.86%) in the uterosacral ligament, six (16.21%) in the rectouterine pouch (RP), one (2.7%) in the surface of the left ureter, two (5.4%) in the right broad ligament and two (5.4%) in the surface of the right ureter.
The detailed distribution locations are shown in . PEM and DIE lesions were mainly located in the posterior pelvic cavity (p < .001).
Table 1. Distribution of PEM and deeper typical lesion in the pelvis (number of the specimen).
Morphological appearance of PEM and DIE under laparoscopy
There were 10 cases of ipsilateral sacral ligament, two cases of ipsilateral ureteral surface peritoneum and three cases of utero-rectal depression in the same patient with PEM and DIE. Comparative analysis of the characteristics under laparoscopy showed that the peritoneum was flat on the surface of PEM lesions, with light pigmentation. The blood vessels around the lesions were sparse and scattered, and most of them were fine branches. While DIE showed peritoneal shrinkage and spasticity, with deep pigmentation, spider network distribution of blood vessels around the lesion, and thick branches. During the resection, it was found that the central part of DIE was often cystic cavity structure, coffee-like liquid and occasionally endometrial tissues seen. The lesion infiltrated deeply into the peritoneum, and the infiltration depth was ≥5 mm, which was confirmed as DIE by histopathology (see ).
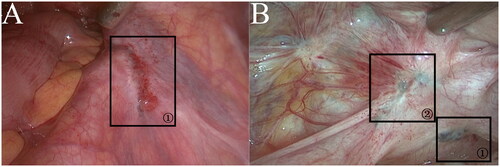
Figure 1. (a)DIE in the right anterior ureter. (b) PEM and DIE in the left sacral ligament.
Morphological features of PEM and DIE: (a) DIE in the right anterior ureter that morphologically behaved similarly to the brown lesions of PEM under laparoscopy; however, compared to PEM, the lesion was found to have infiltrated deeper into the subperitoneum during resection. The histopathology confirmed that it was DIE. (b)Marker ① indicated a brown lesion in the left sacral ligament, with visible pigmentation and no obvious vascular aggregation in the surrounding peritoneum. (b) marker ② indicated DIE in the left sacral ligament, with obvious vascular aggregation and a crinkled spasm in the surrounding peritoneum.
Histopathological and morphological features of PEM and DIE
Histopathological features of PEM and DIE: Statistical analysis of endometrioid glands, endometrioid stroma, smooth muscle, fibrous tissue and blood vessels in each lesion, p < .050, were statistically significant. Details are shown in .
Table 2. Histopathological features of PEM and DIE and relationship with peritoneum.
The predominant pattern of 22 eutopic endometrial tissues corresponded to proliferative phases, which was synchronous with the dating of those datings of menstrual cycle. In addition, we contrasted these 22 patients’ endometriotic lesions, which histopathological features accorded with proliferative phases.
Endometrioid glands and stroma
Endometrioid glands and endometrioid stroma were distributed in all lesions, ranging from ‘+’ to ‘+++’. In PEM, it increased first and then decreased from vesicular lesions, transparent lesions, red lesions, brown lesions to white lesions, especially in red lesions, and white lesions was low. DIE was ‘++’ in endometrioid glands and endometrioid stroma, and its content was similar to that of brown lesions.
In addition, we observed that the size of endometrioid glands varied in different lesions. The vesicular lesions were mostly small glands. The transparent lesions were mostly large glands with obvious dilatation of glandular cavity. The red lesions were mostly medium and large glands. The brown lesions were mainly medium glands. The white lesions were mostly microglands, and occasionally small glands were seen. DIE was mainly composed of small glands, but occasionally large glands were seen. These glands were surrounded by abundant smooth muscle tissue and fibrous tissue.
Smooth muscle tissue
The content of smooth muscle tissue varies in different lesions, ranging from G1 to G4. In PEM, the vesicular lesions and transparent lesions were mainly G1, but not G3 and G4. The red lesions were mainly G2, no G4. Brown and white lesions were distributed in all expressions, with G4 predominating in brown lesions and G3 predominating in white lesions. In the smooth muscle, DIE was mainly G3 and G4, but not G1 and G2. The content of DIE was similar to that of brown and white lesions.
Fibrous tissue
The amount of fibrous tissue varies from lesion to lesion, ranging from G1 to G4. vesicular lesions, transparent lesions; red lesions were mainly G1, no G3, G4; brown lesions and white lesions were distributed in all expression, among which the brown lesions were mainly G3; and the white lesions were mainly G4. DIE was mainly G3 on fibrous tissue without G1, and its content was similar to that of brown lesions.
Blood vessels
Blood vessels were distributed in all lesions, ranging from ‘+’ to ‘+++’. In PEM, red lesions was the most, mainly with the expression of ‘+++’, followed by the brown lesions with the expression of ‘++’, and there were more hemosiderin granules. Most DIE lesions showed ‘++’ expression, and the content was similar to that of brown lesions.
The histopathological features of PEM and DIE are shown in .
Figure 2. (a)Vesicular lesion(HE × 40). (b)Vesicular lesion(HE × 100).
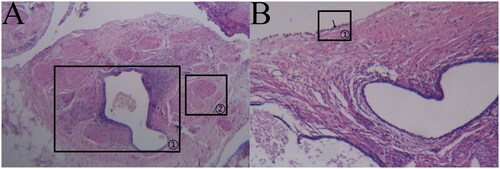
Vesicular lesion: the ectopic lesions were visible both supraperitoneally and subperitoneally; they were distributed close to the peritoneum, with a regular glandular structure, flattened cavity, and sparse occurrence of peri-glandular stromal cells, as indicated in (a) marker ①; scattered hemorrhages were visible subperitoneally, as indicated in (a) marker ②; and the peritoneal mesothelial tissue was structurally intact and continuous, as indicated in (b) marker ①.
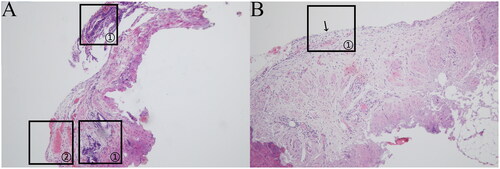
Figure 3. (a)Transparent lesion (HE × 40). (b)Transparent lesion (HE × 400).
Transparent lesion: the ectopic lesion was located in the subperitoneum and close to the peritoneum, with an irregular glandular structure, obvious dilated glandular cavity and sparse occurrence of peri-glandular stromal cells, as indicated in (a) marker ①; the peritoneal mesothelial tissue was intact and continuous, as indicated in (b) marker ①; and scattered hemorrhages were visible in the subperitoneum, as indicated in (b) marker ②.
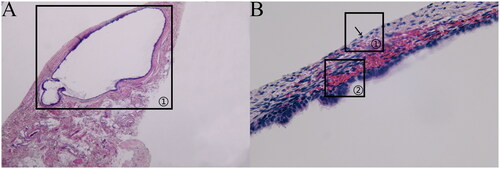
Figure 4. (a)Red lesion (HE × 100). (b)Red lesion (HE × 100).
Red lesion: The ectopic lesion was located in the subperitoneum, and the glands were mainly large and medium in size, with a dilated glandular cavity and accumulation of peri-glandular stromal cells, as indicated in (a) marker ①; the peritoneal mesothelial tissue structure was destroyed, as indicated in (a) marker ②; interrupted peritoneal mesothelial tissue structure was visible, as indicated in (a) marker ③; and scattered hemorrhage was visible in the subperitoneum, as indicated in (b) marker ①, with abundant microvascular dilatation, as indicated in (b) marker ②.
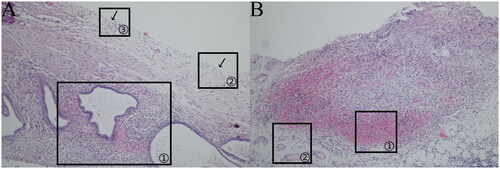
Figure 5. (a)Brown lesion (HE × 400). (b)Brown lesion (HE × 100).
Brown lesion: The ectopic lesion was located in the subperitoneum, and most of the glandular structures were medium in size, with dilated cavities and abundant stromal cell aggregation around the gland, as indicated in (a) marker ①; microvessels were occasionally visible around the lesion, as indicated in (a) marker ②, with abundant smooth muscle tissue distributed around the lesion, as indicated in (a) marker ③, and destroyed peritoneal mesothelial tissue structure, as indicated in (b) marker ①; and interrupted peritoneal mesothelial tissue structure was visible, as indicated in (b) marker ②.
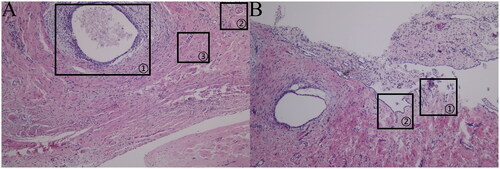
Figure 6. (a)White lesion (HE × 100). (b) White lesion (HE × 400).
White lesion: The ectopic lesions were located in the subperitoneum, and most of the glands were micro glands with narrow cavities and few stromal cells surrounding the glands; however, small glands were occasionally visible, as indicated in (a) marker ①; the lesions were mainly composed of fibrous tissue, as indicated in (a) marker ②; and the peritoneal mesothelial tissue structure was destroyed and almost invisible, as indicated in (b) marker ①.
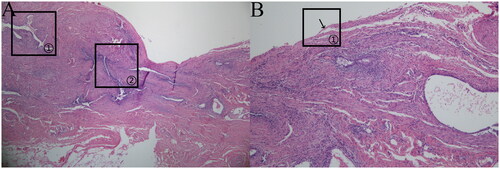
Figure 7. (a)DIE (HE × 40). (b) DIE (HE × 100).
DIE: The ectopic lesion was located in the subperitoneum, and the glands were mainly small in size; there were occasionally large glands as well as glands surrounding stromal cell aggregates, as indicated in (a) marker ①; the lesion was mainly composed of smooth muscle tissue, as indicated in (a) marker ②; and the peritoneal mesothelial tissue structure was destroyed, with a small amount of peritoneal mesothelial tissue visible, as indicated in (b) marker ①.
Immunohistochemical study of PEM and DIE
ER and PR: immunohistochemical analysis of ER and PR was performed in PEM and DIE
ER was expressed in the glandular epithelium and stroma of all lesions, and the expression was mostly ‘+++’. Statistical analysis showed that there was no significant difference in the expression of ER in the endometrioid glandular epithelium of different lesions (p = .330), and there was no significant difference in the expression of ER in the endometrioid stroma of different lesions (p = .113).
PR was also expressed in the glandular epithelium and stroma of all lesions, and the expression was mostly ‘+++’. Statistical analysis showed that there was no significant difference in the expression of PR in the endometrioid glandular epithelium of different lesions (p = .757), and no significant difference in the expression of PR in the endometrioid stroma of different lesions (p = .798). None of the endometriotic lesions were completely negative for ER or PR.
Proliferative activity
The expression of Ki-67 was ranked from low to high in PEM and DIE lesions: white lesions, brown lesions, transparent lesions, vesicular lesions, red lesions and DIE. The expression of Ki-67 in DIE was significantly higher than that in brown and white lesions (p < .001), but not significantly different from that in other PEM lesions (all p > .05). The expression of proliferative activity Ki-67 in endometrioid stromal cells ranked from low to high in PEM and DIE lesions: white lesions, transparent lesions, vesicular lesions, brown lesions, red lesions and DIE. Among the different PEM lesions, the expression of Ki-67 in brown lesions was significantly higher than that in white lesions (p < .001). There was no significant difference between the expression of Ki-67 in DIE and red lesions (p = .070), but the expression of Ki-67 in DIE was significantly higher than that in other PEM lesions (p < .001).
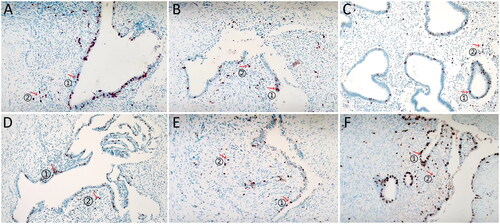
The expression of Ki-67 in endometrioid glandular epithelium and endometrioid stroma of different lesions is shown in . The brown particles indicate the expression of Ki-67 in the lesions, ‘↑’ ① represents the expression of Ki-67 in endometrioid glandular epithelium, and ‘↑’ ② represents the expression of Ki-67 in endometrioid stroma.
Relationship of PEM and DIE with the peritoneum
The relationships of different lesions of PEM and DIE with the peritoneum are given in .
Vesicular lesions
The peritoneal mesothelial tissue was structurally intact and continuous, with ectopic lesions visible above and below the peritoneum and subperitoneal lesions close to the peritoneum (depth of ≤3 mm).
Transparent lesions
The peritoneal mesothelial tissue was structurally intact and continuous, and the lesion was located below the peritoneum, immediately adjacent to the peritoneum (depth of ≤3 mm).
Red lesions
The peritoneal mesothelial cells were destroyed, with discontinuous peritoneal mesothelial tissue visible around the lesions, and ectopic lesions were visible in the superficial area below the peritoneum (infiltration depth of ≤3 mm).
Brown lesions
The peritoneal mesothelial cells were destroyed, with discontinuous peritoneal mesothelial tissue visible, and the ectopic lesions infiltrated the subperitoneum (depth <5 mm).
White lesions
The peritoneal mesothelial cells were destroyed, with the peritoneal mesothelial tissue barely visible, and the ectopic lesions infiltrated the subperitoneum (depth <5 mm).
DIE
The peritoneal mesothelial cells were destroyed, with the peritoneal mesothelial tissue barely visible, and the ectopic lesions infiltrated into the subperitoneum (depth of ≥5 mm).
Discussion
The present study analysed 100 patients with PEM and DIE, attempted to explore the association between them in terms of the lesion distribution characteristics, histopathological features and invasiveness towards the peritoneum.
The distribution of PEM and DIE in the pelvis
In our study, 48.85% of the PEM lesions were located in the posterior pelvis; of these, 60.94% were in the uterosacral ligament. A possible reason for this is that the posterior pelvic cavity is the lowest point of the abdominal cavity, where retrograde menstrual blood tends to aggregate; furthermore, abdominal pressure together with intestinal peristalsis also promotes the flow of peritoneal fluid containing endometrial cells to the posterior pelvic cavity, causing active endometrial cells to implant into the peritoneum and grow, shed and bleed cyclically with hormonal levels, thus resulting in different morphological appearance of PEM [Citation11].
The present study also found that the vesicular, transparent and red lesions in early PEM had smaller proportions than those reported in our present study [Citation12]. One of the possible reasons is that early lesions might be very easily misdiagnosed under the plain white light of laparoscopy [Citation3]; another potential reason is that early lesions exist for a shorter period during the development of PEM, while brown lesions last for a relatively longer duration. Therefore, brown lesions might be more commonly detected under laparoscopy.
Researchers have investigated the distribution of DIE in the pelvis by adopting the traditional DIE definition; the results show the posterior pelvis as the most frequent DIE site [Citation13,Citation14].
The study results showed that the uterosacral ligament in the posterior pelvis was also a site with a high prevalence of DIE (64.86%); which is consistent with the predisposing site of PEM. Therefore, it was further hypothesized that PEM might be a pre-existing state of DIE. The possibility that the DIE was formed by the continuous infiltration of PEM into the deep peritoneum could not be excluded; it is possible that PEM and DIE are different development stages of the same disease.
Histopathological and morphological features of PEM and DIE
Through a histopathological analysis of different PEM lesions, red lesions, brown lesions and white lesions were consistent with the study of Johanna et al. [Citation9]. We further found that the advent of vesicular and transparent lesions was before red lesions, which belonged to the budding state of the disease, should be the first stage in the development of PEM. Red lesions indicated that the disease was in an active stage. With an emergence of iron-containing heme granules and smooth muscle fibres, brown lesions indicated that the disease was changing from an active to a resting state. While in the white lesions, there were predominantly dense fibrous tissue, suggested that the lesions eventually returned to a resting state.
These findings further confirmed that PEM is an aging disease; it is a process from lesion budding to tissue destruction, resorption and ultimately fibrosis.
In our study, PEM and DIE at the same site were compared. The results showed that the morphological presentations of DIE under laparoscopy were similar to those of brown and white lesions in PEM; however, the DIE lesion lasted for a long time, caused peritoneal inflammation, and further infiltrated more deeply and extensively into the subperitoneum. The results also showed that the DIE had similar histopathological contents as the brown lesions; meanwhile, the white lesions were mainly fibrous tissue.
Therefore, we speculate that red lesions are the most active phase, and after it is continued into the brown lesion phase, we can assume that there are two development trends: on the one hand, the lesions were inactivated and changed to white lesions, which eventually become resting; on the other hand, they were still invasive, infiltrating deeper into the peritoneum to form DIE.
Immunohistochemical features of PEM and DIE
ER and PR were highly expressed and not negatively expressed in all lesions, suggesting that the endometriotic lesion is a hormone-dependent disease. ER promoted the adhesion and proliferation of ectopic endometrial cells and neovascularization in lesions through a variety of ways, while inhibiting PR and increasing progesterone resistance to promote lesions [Citation15,Citation16]. Johanna et al. [Citation9] found that the expression of ER and PR in the proliferative phase of PEM is higher than that in the secretory phase. The reason is that estrogen in the proliferative phase promotes the expression of ER and PR, while progesterone in the secretory phase reduces the expression of ER and PR [Citation17]. While Lenz at al. [Citation18] found that ER was highly expressed during the proliferative phase, which was slightly decreased during the secretory phase and PR was significantly decreased during the secretory phase in DIE. In this study, because all cases were in proliferative phase, the high expression of ER and PR may be related to the proliferative phase. Although PR was highly expressed in most different lesions, the expression of PR is relatively scattered than that of ER in the same lesions, suggesting that the difference in PR expression in the same lesion may play a different role in subsequent drug treatment.
Ki-67 is often used as an antibody to show the proliferation of tumour cells. The higher the expression of Ki-67, the more aggressive the tumour is, and the vascular invasion and cell metastasis of tumour cells are prone to occur [Citation19]. In this study, the expression of Ki-67 in endometrioid glandular epithelium was higher in DIE than that in brown lesions and white lesions, indicating that DIE has the ability to invade surrounding tissues, which is the same as early and middle lesions of PEM [Citation3], and the invasive ability of late lesions of PEM is weakened and enter the aging stage. However, the Ki-67 expression in DIE (36.47 ± 22.09) was significantly higher than that of red lesions (19.88 ± 17.33), which was further higher than that of other lesions, indicating that the invasive ability of DIE was the strongest. The study found that there were still a few lesions with high Ki-67 expression in the brown lesions, so it cannot be excluded that they have the ability to continue to invade the surrounding tissues and form DIE when the infiltrating depth is ≥5 mm. Similarly, we can assume that the brown lesion continues to develop in two ways, one is the white lesion, and the other is to develop into DIE.
Konrad et al. [Citation20] pointed out that endometrioid glands rarely exist alone in endometriosis, but are often accompanied by endometrioid stroma, and found that the expression of platelet growth factor β in endometrioid stromal cells was significantly higher than that in endometrioid glandular epithelial cells, indicating that endometrioid stromal cells have high proliferative activity. Stromal endometriosis also occurs often in the form of small and well-defined nodules or plaques [Citation21]. These findings suggest that the invasive capacity of endometrioid stroma plays a major role in the endometriosis.
Invasion of the peritoneum by PEM and DIE
The etiology and mechanism of endometriosis are still controversial; however, the ‘implantation theory’, which is the most likely theory of PEM development, is widely accepted [Citation22].
Endometrial cells adhere to the peritoneal surface and invade the peritoneal mesothelium, causing the destruction and subsequent recruitment of inflammatory cells to the endometrial cell implantation site. At the same time, neovascularization allowed the endothelial cells to proliferate and form ectopic lesions [Citation23]. It has been suggested that ectopic endometrium adheres to the peritoneal surface and induces a local inflammatory response in the peritoneum. If the ectopic endometrium is persisted, it will disrupt the peritoneal barrier, invade the subperitoneal mesothelial tissue, and induce neovascularization; this is necessary for the infiltration of the lesion deeper into the peritoneum [Citation24].
In the vesicular and transparent lesions, ectopic endometrial cells implanted on the peritoneal surface, did not cause the destruction of the intact peritoneal structure. With the process of PEM, peritoneal mesothelial cells began to be destroyed, indicating that the ectopic lesion was infiltrating to subperitoneum. The endometrial cells with growing activity further destroyed the peritoneal mesothelial cells under the action of the peritoneal microenvironment. With an infiltration depth exceeding 5 mm, the lesion developed into DIE.
AAGL, ESGE, ESHRE, WES and other international working groups [Citation4] believe that the depth of the lesion cannot be accurately measured, and generally define DIE as a lesion of endometrioid tissue in the abdominal cavity that is convex above or below the peritoneal surface, nodular, invading adjacent structures, accompanied by fibrosis and destruction of normal anatomical structures. However, according to the results of our study, no matter the morphological changes, histological and immunohistochemical manifestations of the lesions seen under laparoscopy, the white lesions of PEM are completely different from those of DIE. The results of our study come from the definition of PEM and DIE by taking 5 mm below the peritoneum as the boundary [Citation4].
Clinical management of PEM and DIE
According to the present study, it was recommended that different treatment modalities should be adopted according to PEM staging. Vesicular, transparent and red lesions were the early and middle stages of PEM; these only infiltrate the peritoneum shallowly. They could thus be eliminated by ablation with energy devices.
Brown and white lesions had a great depth of invasion than other lesions. Ablation could only eliminate lesions located on the surface and the superficial parts of the peritoneum; it failed to completely remove the active lesions that still existed in the deeper part of the peritoneum. Therefore, excision should be recommended [Citation25]. Complete excision of the lesion should also be recommended for DIE, and attention should be paid to avoiding damage to surrounding organs, such as the ureter.
In summary, the development of PEM has two outcomes: these lesions remain in the peritoneum, which are inactive, in another way, they can invade peritoneum and subperitoneal tissues, progress into DIE. Thus, they become clinically significant. PEM and DIE are not always different entities, they may represent different developmental stages of the same diseases, but the homology between them needs to be explored from the perspective of pathogenesis.
Author contributions
Conception and design of the research: MYQ and LH; acquisition of data: MYQ, RR, YRS, SQX and LH; analysis and interpretation of the data: YPW, MYQ and LH; statistical analysis: MYQ and LH; writing of the manuscript: MYQ; critical revision of the manuscript for intellectual content: LH; all authors read and approved the final draft.
Ethical approval
This study was conducted with approval from the Ethics Committee of Dalian Municipal Women and Children’s Medical Center (no. 2018040). This study was conducted in accordance with the Declaration of Helsinki.
Consent form
Written informed consent was obtained from all participants.
Acknowledgements
We would like to acknowledge the hard and dedicated work of all the staff that implemented the intervention and evaluation components of the study.
Disclosure statement
The authors declare that they have no competing interests.
Data availability statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request. We declared that materials described in the manuscript, including all relevant raw data, will be freely available to any scientist wishing to use them for non-commercial purposes, without breaching participant confidentiality.
Additional information
Funding
References
- Zondervan KT, Becker CM, Missmer SA. Endometriosis. N Engl J Med. 2020;382(13):1–12. doi: 10.1056/NEJMra1810764.
- Gynecologists and Obstetricians Branch of Chinese Medical Association, Endometriosis Cooperation Group of Obstetrics and Gynecology Branch of Chinese Medical Association. Guideline for the diagnosis and treatment of endometriosis (third edition). Chin J Obstet Gynecol. 2021;56(12):812–824.
- Sun YR, Han L, Yu XH, et al. Macroscopic appearance analysis of peritoneal endometriosis with laparoscopic narrow-band imaging. Prog Obstet Gynecol. 2017;26(4):262–265.
- International Working Group of AAGL, ESGE, ESHRE and WES, Tomassetti C, Johnson NP, et al. An international terminology for endometriosis, 2021. Hum Reprod Open. 2021;28(11):1849–1859.
- Guerriero S, Saba L, Pascual MA, et al. Transvaginal ultrasound vs magnetic resonance imaging for diagnosing deep infiltrating endometriosis: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2018;51(5):586–595. doi: 10.1002/uog.18961.
- Chen SQ, Fan L, Jin WY, et al. Distribution characteristics of deep infiltrating endometriosis and evaluation of accuracy of laparoscopic diagnosis. Chin J Pract Gynecol Obstet. 2014;30(8):603–607.
- D’Alterio MN, Saponara S, D’Ancona G, et al. Role of surgical treatment in endometriosis. Minerva Obstet Gynecol. 2021;73(3):317–332. doi: 10.23736/S2724-606X.21.04737-7.
- D’Alterio MN, D’Ancona G, Raslan M, et al. Management challenges of deep infiltrating endometriosis. Int J Fertil Steril. 2021;15(2):88–94.
- Johanna DS, Hackl J, Wachter DL, et al. Correlation of histological and macroscopic findings in peritoneal endometriosis. Int J Clin Exp Pathol. 2014;7(1):152–162.
- Itoga T, Matsumoto T, Takeuchi H, et al. Fibrosis and smooth muscle metaplasia in rectovaginal endometriosis. Pathol Int. 2003;53(6):371–375. doi: 10.1046/j.1440-1827.2003.01483.x.
- Morgan-Ortiz F, López-de la T, López-Zepeda MA, et al. Clinical characteristics and location of lesions in patients with deep infiltrating endometriosis using the revised Enzian classification. J Turk Ger Gynecol Assoc. 2019;20(3):133–137. doi: 10.4274/jtgga.galenos.2018.2018.0120.
- Sun YR, Han L, Yu XH, et al. Diagnostic value of narrow band imaging for detecting peritoneal endometriosis. Chin J Minim Invas Surg. 2017;17(12):1087–1090.
- Audebert A, Petousis S, Margioula-Siarkou C, et al. Anatomic distribution of endometriosis: a reappraisal based on series of 1101 patients. Eur J Obstet Gynecol Reprod Biol. 2018;230:36–40. doi: 10.1016/j.ejogrb.2018.09.001.
- Kwok H, Jiang H, Li T, et al. Lesion distribution characteristics of deep infiltrating endometriosis with ovarian endometrioma: an observational clinical study. BMC Womens Health. 2020;20(1):111.
- Massarotti C, Mirabelli BI, Paudice M, et al. Steroids receptors immunohistochemical expression in different sites of endometriosis. J Gynecol Obstet Hum Reprod. 2021;50(3):101861. doi: 10.1016/j.jogoh.2020.101861.
- Flores V, Vanhie A, Dang T, et al. Progesterone receptor status predicts response to progestin therapy in endometriosis. J Clin Endocrinol Metab. 2018;103(12):4561–4568. doi: 10.1210/jc.2018-01227.
- Nisolle M, Casanas-Roux F, Donnez J. Immunohistochemical analysis of proliferative activity and steroid receptor expression in peritoneal and ovarian endometriosis. Fertil Steril. 1997;68(5):912–919. doi: 10.1016/s0015-0282(97)00341-5.
- Lenz J, Chvatal R, Fiala L, et al. Comparative immunohistochemical study of deep infiltrating endometriosis, lymph node endometriosis and atypical ovarian endometriosis including description of a perineural invasion. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2021;165(1):69–79. doi: 10.5507/bp.2020.006.
- Yalcin SE, Ocal I, Yalcin Y, et al. Evaluation of the Ki-67 proliferation index and urocortin expression in women with ovarian endometriomas. Eurasian J Med. 2017;49(2):107–112. doi: 10.5152/eurasianjmed.2017.17070.
- Konrad L, Kortum J, Nabham R, et al. Composition of the stroma in the human endometrium and endometriosis. Reprod Sci. 2018;25(7):1106–1115. doi: 10.1177/1933719117734319.
- Mecha E, Makunja R, Maoga J, et al. The importance of stromal endometriosis in thoracic endometriosis. Cells. 2021;10(1):180. doi: 10.3390/cells10010180.
- Stephens VR, Rumph JT, Ameli S, et al. The potential relationship between environmental endocrine disruptor Exposure and the development of endometriosis and adenomyosis. Front Physiol. 2021;12:807685. DOI: 10.3389/fphys.2021.807685
- Miller JE, Ahn Soo H, Marks RM, et al. IL-17A modulates peritoneal macrophage recruitment and M2 polarization in endometriosis. Front Immunol. 2020;11:108. doi: 10.3389/fimmu.2020.00108.
- Capobianco A, Cottone L, Monno A, et al. The peritoneum: healing, immunity, and diseases. J Pathol. 2017;243(2):137–147. doi: 10.1002/path.4942.
- Lang JH, Cui H, Dai Y, et al. Expert interpretation of 2015 endometriosis diagnosis and treatment guidelines. Chin J Obstet Gynecol. 2017;52(12):857–861.