Abstract
Introduction: Rheumatoid arthritis (RA) is a chronic, systemic autoimmune disease characterized by autoantibody production, joint inflammation and bone destruction. Nearly 1/3 of RA patients with the active disease also exhibit a normal range of ESR and CRP. Here we assessed the performance and clinical significance of soluble CD24 (sCD24) as a biomarker of disease activity in RA.
Methods: A total of 269 RA patients, 59 primary Sjogren’s syndrome (SS) patients, 81 systematic lupus erythematosus (SLE) patients, 76 osteoarthritis (OA) patients and 97 healthy individuals (HC) were included in this study. Soluble CD24 in sera were detected by ELISA. Therefore, the concentration of sCD24 was analyzed in RA patients with different disease activity statuses.
Results: The sCD24 was significantly increased in RA (2970 pg/mL), compared to other rheumatic diseases (380-520 pg/mL) and healthy individuals (320 pg/mL). Moreover, sCD24 was elevated in 66.67% of early RA and 61.11% of seronegative RA patients. In addition, sCD24 was significantly correlated with the disease duration and inflammatory indicators.
Conclusion: The sCD24 could be an inflammatory biomarker in RA patients, especially in early and seronegative patients.
Introduction
Rheumatoid arthritis (RA) is a common chronic inflammatory disease characterized by cartilage and bone destruction, leading to progressive disability [Citation1]. It affects 0.28-1% of the population worldwide [Citation2–4]. Nearly 50% of RA patients confront permanent work disability within 2 to 3 years after diagnosis, which highlights the importance of RA management strategies [Citation5,Citation6]. In detail, preserving articular function and preventing disability requires evaluation of disease activity. In the clinic, erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) have been used to evaluate disease ability [Citation7]. However, nearly 1/3 of RA patients with the active disease also exhibit a normal range of ESR and CRP. Furthermore, research has highlighted the timely diagnosis of early RA would improve the prognosis of RA [Citation8]. Therefore, current inflammatory markers are unsatisfying in warning of the presence and indicating disease activity of RA, exposing the urgent need for novel sensitive biomarkers to alert and evaluate RA disease activity.
CD24, known as heat-stable antigen (HSA), is a cell surface glycosyl -phosphatidylinositol-anchored protein [Citation9]. It plays a pivotal role in discriminating immune responses triggered by damage-associated molecular pattern (DAMP) from the pathogen-associated molecular pattern (PAMP) through binding to Siglec-10 in human or Siglec-G in murine [Citation10]. Moreover, CD24 was found highly abundant in multiple malignant tumors, even as a new prognostic marker for ovarian cancer. Furthermore, targeting CD24 signaling could be a new immunotherapy for cancer, especially triple-negative breast cancer [Citation11]. Meanwhile, in autoimmune diseases, previous studies have demonstrated that CD24 participate in the development of multiple autoimmune diseases, including rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), multiple sclerosis (MS) and experimental autoimmune encephalomyelitis (EAE) through in vitro and animal experiments [Citation12–16]. Beyond this point, it has been reported that dinucleotide deletion in CD24 protects against autoimmune disease [Citation17].Furthermore, the CD24V/V genotype was found a high prevalence in RA patients [Citation16]. However, the participation of CD24 in autoimmune diseases, especially rheumatoid arthritis, remains elusive. Here we investigated the clinical significance of soluble CD24 in early and seronegative RA patients through a retrospective study.
Methods
Patients and samples
Sera samples of 269 RA patients, 59 primary Sjogren’s syndrome (SS) patients, 81 systematic lupus erythematosus (SLE) patients, 76 osteoarthritis (OA) patients and 97 healthy individuals were collected between 2013 and 2018 as part of patient routine care at the Department of Rheumatology and Immunology, Peking University People’s Hospital. Corresponding medical records, demographic and clinical characteristics of patients were also collected and used for analysis. Sera from patients and healthy donors were aliquoted and stored at −80 °C until further analysis.
The strict inclusion criteria include the 2010 American College of Rheumatology (ACR) and European League Against Rheumatism (EULAR) classification criteria for RA, SLICC Revision of ACR 2009 criteria for SLE, ACR 2012 criteria for SS, and ACR 1995 criteria for OA. The study was approved by the Research Ethics Committee at Peking University People’s Hospital (2020PHB197-01), Beijing, China. Informed consent was provided from all patients and healthy donors.
Enzyme-linked immunosorbent assay
The serum levels of soluble CD24 (sCD24) in patients and healthy individuals were detected and quantitated by enzyme-linked immunosorbent assay (ELISA). Commercially available human CD24 ELISA kits (Cloud-clone, Wuhan, China) were used according to the manufacturer’s instructions. The results were measured on a SyngeryTM 4 Multi-Mode Microplate Reader with software GEN5CH 2.0 (BioTek, Winooski, VT).
Diagnostic value analysis of the sCD24 for RA
The receiver-operating characteristic curve (ROC) analysis was performed with the statistical software SPSS 25 (SPSS Inc., Chicago, IL) to evaluate the diagnostic utility of the concentration of sCD24 for RA as estimated by the area under the ROC curve (AUC). The optimal positive cut-off value in the study was recommended by the ROC curve and Youden index analyses.
Correlation analysis
The Spearman’s rank correlation test was performed with the GraphPad Prism 8 (GraphPad Software Inc.) to evaluate the correlation between the concentration of sCD24 and the manifestations of RA patients, including age, disease duration, disease activity (DAS 28), tender joint count (TJC), swollen joint count (SJC), anti-citrullinated peptide antibody (ACPA), rheumatoid factor (RF), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) and serum IgA, IgM, IgG. Besides, Spearman’s rank correlation test was also performed to assess the correlation between the sCD24/RF ratio and manifestations of RA patients.
Statistical analysis
The serum concentration of CD24 was compared among RA patients and controls and the significant differences was evaluated by a non-parametric Kruskal-Wallis test followed by Dunn’s post-test for multiple comparisons. The Spearman’s rank correlation test was performed to evaluate the correlation between the concentration of sCD24 in serum and the manifestations of RA patients. 269 RA patients were recruited in this study for the detection of CD24. However, some clinical and laboratory features were missed for some patients. Therefore, there is a bit of inconsistency between the ELISA detection and the correlation or subgroup analysis. The threshold titers for ACPA, RF, ESR and CRP were 20 U/mL, 20 IU/mL, 20 mm/h and 20 mg/L, separately. All statistical analyses were conducted by GraphPad Prism 8 (GraphPad Software Inc.) and SPSS 25 (SPSS Inc.). All statistical analyses with p-value < 0.05 were considered statistically significant (*p < 0.05, **p < 0.01, ***p < 0.001, N.S., not significant).
Results
Characteristics of RA patients
A total of 269 RA patients were recruited for this study, with a median age of 53.3, and 85.5% female (230/269.) Detailed characteristics of RA patients are shown in . The RA patients had a mean disease duration of 8.77 years, ranging from 2 months to 40 years and the mean disease activity score (DAS 28) was 3.11, ranging from 1.04 to 8.12. Meanwhile, the control including 59 SS patients, 81 SLE patients, 76 OA patients and 97 healthy controls were recruited as well, corresponding clinical and laboratory characteristics were also shown in .
Table 1. Baseline characteristics of patients and healthy controls in this study.
Soluble CD24 (sCD24) was significantly increased in RA
We first evaluated sCD24 levels in patients with RA and other rheumatic diseases including SS, SLE and OA. As shown in , the serum sCD24 in RA patients (2970 ± 5780 pg/mL) was significantly higher than those from healthy individuals (320 ± 490 pg/mL) and patients with SS (380 ± 490 pg/mL), SLE (460 ± 590 pg/mL), and OA (520 ± 1090 pg/mL) (p < 0.001).
Figure 1. The prevalence and discrimination capacity of sCD24 for RA patients. A: The serum levels of sCD24 were detected in patients with RA than those of healthy individuals (n = 97, ***p < 0.001), and patients with sjogren’s syndrome (SS, n = 59, ***p < 0.001), systemic lupus erythematosus (SLE, n = 81, ***p < 0.001), and osteoarthritis (OA, n = 76, ***p < 0.001). B: ROC analysis was performed to evaluate the performance of sCD24 in the diagnosis of RA. The area under the ROC curve (AUC) was 0.8631 (95% CI extending from 0.834 to 0.892, std. Error = 0.014). kruskal–wallis test followed by dunn’s posttest for multiple comparisons, ***p < 0.001.
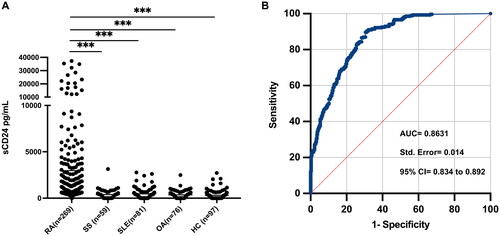
Next, receiver-operating characteristic curve (ROC) analysis was performed to assess the discriminating power of sCD24 for RA. The area under the ROC curve (AUC) was 0.8631 (95% CI extending from 0.834 to 0.892) (). The optimal cut-off value (802.8 pg/mL) revealed a sensitivity of 61.71% and specificity of 85.35% for sCD24 in RA diagnosis.
According to the cut-off value, all the RA patients were divided into sCD24-positive and negative groups. Consequent analysis showed that the therapy of DMARDs and NSAIDs medication was significantly higher in the sCD24-negative group, shown in Supplementary Table 1.
sCD24 is associated with disease activity
Successively, the correlation between sCD24 and multiple clinical and laboratory manifestations of RA patients was revealed by Spearman’s correlation analysis. The sCD24 positively correlates with ESR (r = 0.3184, *p = 0.0292), CRP (r = 0.3061, *p = 0.0364), and DAS28 (r = 0.2892, p = 0.0513) ( and ). These results suggest sCD24 was a disease activity marker in RA patients.
Figure 2. Correlation between serum level of soluble CD24 with RA patient clinical manifestation and laboratory features. The plots demonstrate laboratory features of RA including ESR (A), CRP (B), and RF-IgG isotype (C). and disease activity score of 28 for RA (D). Spearman’s correlation analysis was performed between sCD24 concentration and each of these parameters. ESR, CRP and RF-IgG showed a strong positive correlation with soluble CD24 in RA serum. *p < 0.05, **p < 0.01 (two-tailed spearman’s rank correlation test). n: the number of sCD24-positive patients; N: the number of total patients. −: normal; +: increased. ***p < 0.001 (kruskal–wallis test followed by dunn’s posttest for multiple comparisons).
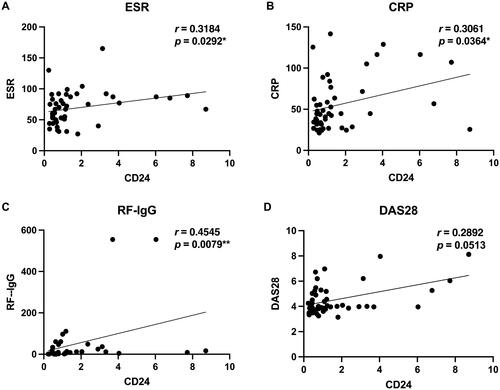
Table 2. Association between sCD24 and RA patient clinical and immunological features.
Interestingly, as shown in , the sCD24 concentration in low disease activity is comparable with that in high disease activity RA patients. Consequently, we divided RA patients according to ESR and CRP levels, it appears the positive rate of sCD24 in RA patients with normal ESR and CRP reached 64.23% (88/137), compared to the group with increased ESR and CRP of 50.98% (26/51) and the group with increased ESR or CRP of 62.90% (39/62) (). Furthermore, the laboratory features among three groups were significantly different, shown in .
Figure 3. Significance of sCD24 for disease activity evaluation in ESR/CRP normal RA patients. A: the distribution of sCD24 among the different disease Activities of RA patients. High: high disease activity (DAS28 > 5.1, n = 31); moderate: moderate activity group (5.1 ≥ DAS28 > 3.2, n = 87); low: low activity group (DAS28 ≤ 3.2, n = 140). the positive rates of sCD24 were also analyzed. B: according to ESR and CRP, divide the RA patients into three groups, the positivity of sCD24 was shown in each group. C: the distribution of DAS28 in negative ESR and/or CRP patients. D: the concentration of sCD24 in ESR/CRP double-negative patients. n: the number of sCD24-positive patients, N: total number of RA patients of the category; ***p < 0.001 (kruskal–wallis test followed by dunn’s posttest for multiple comparisons).
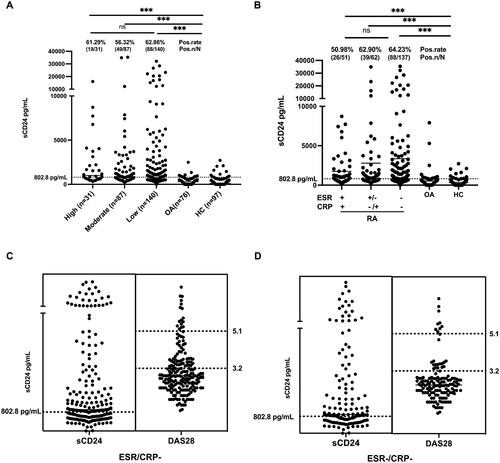
Table 3. Clinical and immunological features of RA patients among ESR/CRP groups.
sCD24 is an inflammatory marker in ESR/CRP normal patients
Within the RA patients, 72.5% (195/269) of them with normal ESR and/or CRP. While 64.62% (126/195) of these patients exhibited an increased sCD24, most of which were with high and moderate disease activity according to DAS28 ().
Furthermore, there were 50.93% (137/269) of RA patients with double-negative ESR and CRP levels, 64.23% (88/137) of them were sCD24-positive whereas 17.52% (24/137) were in high and moderate disease activity, shown in . Among these ESR and CRP double-negative RA patients, the sCD24/RF ratio exhibits a significantly positive correlation with ESR, ACPA, IgM, IgA and DAS28, as shown in Supplementary Table 2. Therefore, sCD24 could be an option to evaluate RA disease activity in ESR and CRP double-negative patients.
sCD24 is a sensitive disease marker in low disease activity RA
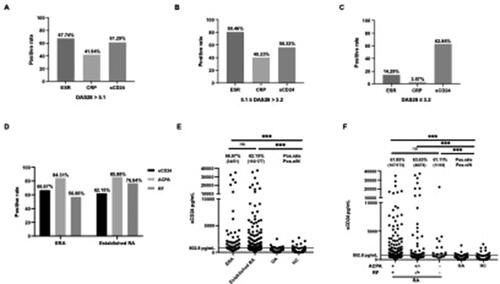
According to DAS28, the RA patients were grouped into 3 groups: the high activity group (DAS28 > 5.1, 31 patients), the moderate activity group (3.2 < DAS28 ≤ 5.1, 87 patients) and the low activity group (DAS28 ≤ 3.2, 140 patients). We further compare the positive rate of sCD24 with ESR and CRP in all three groups. In the high disease activity RA group, the positivity of ESR, CRP and sCD24 was observed in 67.74%, 41.94% and 61.29% of RA (). A same situation was also found in the moderate activity group with 80.46% for ESR, 40.23% for CRP and 56.32% for sCD24 (). However, in the low disease activity group, only sCD24 was still significantly increased in a large proportion of RA patients with 14.29% for ESR, 3.57% for CRP and 62.86% for sCD24, as shown in , suggesting that sCD24 could be a more sensitive disease activity marker in RA patients with low disease activity.
Figure 4. Significance of sCD24 for disease activity evaluation in ACPA/RF negative RA patients. A: High: high activity group; B: Moderate: moderate activity group; C: Low: low activity group. D: the distribution of sCD24, ACPA and RF in early and established RA patients. E: according to disease duration, divide the RA patients into early (ERA) and established RA two groups, the positivity of sCD24 was shown in each group. F: the distribution of sCD24 in seropositive (ACPA +, RF +), semi-seronegative (ACPA ±, RF −/+) and seronegative (ACPA -, RF -) RA patients. n: the number of sCD24-positive patients, N: total number of RA patients of the category; ***p < 0.001 (kruskal–wallis test followed by dunn’s posttest for multiple comparisons).
sCD24 is an inflammatory indicator in early and seronegative RA
The diagnosis of early RA and seronegative RA has long been challenged in clinical. According to the disease duration, the RA patients were divided into two groups: early RA (ERA, disease duration < 2 years, 51 patients) and established RA (disease duration ≥ 2 years, 177 patients). The laboratory features between early and established RA were similar (Supplementary Table 3). We further compare the positive rate of sCD24, ACPA and RF in two groups. In ERA groups, positive sCD24, ACPA and RF were found in 66.67%, 84.31% and 56.86% of RA patients, respectively. While in established RA groups, the positivity of sCD24, ACPA and RF was 62.15%, 85.88% and 76.84%, respectively (). Compared with the positivity of ACPA and RF in ERA, sCD24 could be more sensitive in ERA diagnosis.
According to the serum level of ACPA and RF, the RA patients were divided into three groups: seropositive RA (ACPA + and RF+, 175 patients), semi-seronegative RA (ACPA + or RF+, 55 patients) and seronegative RA (ACPA- and RF-, 18 patients). We further compare the positive rate of sCD24, ACPA and RF in three groups. In seropositive RA, positive sCD24 was found in 61.85% of RA patients. While in semi-seropositive RA groups, the positivity of sCD24, ACPA and RF was 63.64%, 12.73% and 87.27%, respectively. And in seronegative RA, positive sCD24 was found in 61.11% of patients (). And the laboratory features among the three groups were significantly different, shown in . These results suggest sCD24 showed a considerable value for identifying seronegative RA.
Table 4. Clinical and immunological features of RA patients among ACPA/RF groups.
Discussion
This is the first study to assess the serum concentration of CD24 in rheumatic disease. Our study demonstrated that elevated sCD24 levels are associated with the disease activity of RA patients. In addition, there was a significant difference between the serum CD24 levels in participants with RA as compared to disease controls and healthy subjects. We also identified sCD24 as an inflammatory biomarker for RA by consequent analysis. In clinical practice, sCD24 levels may serve as a supplement marker in the initiation and partial seronegative RA. Meanwhile, the significant elevation of sCD24 levels in RA may expose a new therapeutic target for RA.
CD24 is known to interact with Siglec-10 on innate immune cells to dampen damaging inflammatory responses to infection, sepsis, and graft versus host disease [Citation10, Citation18–20]. While the host can discriminate DAMPs from PAMPs via CD24-Siglec-10 signaling. Apart from these findings, CD24-Siglec-10 signaling also regulates the macrophage-mediated immune response to cancer and tumour-expressed CD24 promoting immune evasion of cancer cells [Citation21–23]. Intriguingly, the blockade of CD24-Siglec-10 signaling seems promising immunotherapy in several cancers [Citation10].
CD24 was first discovered in 1978, initially named heat-stable antigen for its stability upon heat inactivation [Citation9]. And CD24 was found expressed in multiple cells, especially immature cells. Subsequent research has demonstrated that CD24 is an important co-stimulatory factor in promoting T cell proliferation and CD24 is also critical for the survival of autoreactive T cells in negative selection occurred in the thymus [Citation24]. For B cells, CD24 expression is pivotal for B cell maturation and deletion of specific precursor B cells in the bone marrow [Citation25]. It’s well-established that both T and B cells cooperatively and separately participate in the progression of autoimmune disease [Citation26].
Accumulative evidence has found a tight association between CD24 and autoimmune disease. CD24 gene polymorphisms have been linked to the disease risks for developing autoimmune diseases, including RA, SLE, and MS [Citation17]. Typically, the CD24 polymorphisms result in increased incidence or severity of the disease. And CD24V/V genotype and CD24 V allele were found to increase the susceptibility to RA [Citation16, Citation27].
Moreover, CD24 expression was found among a diversity of cells, such as B cells, immature T cells, granulocytes and monocytes. And the altered cellular function of CD24-expressing regulatory B cells was also demonstrated in RA patients [Citation28]. In RA patients, CD19+CD24hiCD38hi B cells lose the ability to inhibit the differentiation of Th17 cells and the transformation of Treg cells [Citation29,Citation30]. The Th17 and IL17 pathways have been implicated in augmenting RA progression [Citation31]. Furthermore, active RA patients have less circulating CD19+CD24hiCD38hi Breg cells, and further investigations suggest that Breg cells have compromised regulatory function in suppressing autoreactive responses and aberrant inflammation [Citation32].
However, the origin of the elevated sCD24 in RA serum is less known. Considering the wide-expressed CD24 among the cells, we assumed there is an overactivated enzyme that released the CD24 from the cell membrane and consequently altered the corresponding signaling pathway which may participate in autoimmunity [Citation33]. This hypothesis needs further research.
There are several limitations to this study. Firstly, this is a retrospective study and the majority of the recruited RA patients are outpatients, implying that the disease has been treated and plateaued. Thus, our results were weakened when comparing the clinical and laboratory features among the RA patients. The other weakness is the variation of CD24 concentration before and after treatment is less known and requires further research.
In conclusion, soluble CD24 serves as a sensitive inflammatory marker to assess disease activity in RA, especially in RA with normal ESR and CRP. And it could supplement the diagnosis of early and seronegative RA. The accumulation of circulating CD24 in RA serum and its correlation with disease activity implicates its participation in RA development. Further investigation of the pathologic role of CD24 in RA progression is necessary.
Contributions
X.Z., F.L.H. and Z.G.L conceived the study design, X.Z., P.W., and J.S performed the experiments. X.Z, Y.D.T, Y.X., X.J., and D.X.Z analyzed the data. X.Y.F., C.N.W and R.L. contributed with reagents/materials/analysis tools. X.Z. wrote the manuscript. F.L.H. and Z.G.L. interpreted the data, and revised and edited the manuscript. All the authors approved the final manuscript.
Ethical approval
This study was approved by the ethics committees of Peking University People’s Hospital. All patients fulfilled the consent forms.
Supplementary functioning in a supporting capacity More (Definitions, Synonyms, Translation)
Supplemental Material
Download MS Word (16.9 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Smolen JS, Aletaha D, Barton A, et al. Rheumatoid arthritis. Nat Rev Dis Primers. 2018. doi:10.1038/nrdp.2018.1.
- Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):1–10. doi:10.1016/S0140-6736(16)30173-8.
- Sparks JA. Rheumatoid arthritis. Ann Intern Med. 2019;170(1):ITC1–ITC16. doi:10.7326/AITC201901010.
- Li R, Sun J, Ren LM, et al. Epidemiology of eight common rheumatic diseases in China: a large-scale cross-sectional survey in Beijing. Rheumatology (Oxford). 2012;51(4):721–729. doi:10.1093/rheumatology/ker370.
- Verstappen SMM, Bijlsma JWJ, Verkleij H, et al. Overview of work disability in rheumatoid arthritis patients as observed in cross-sectional and longitudinal surveys. Arthritis Rheum. 2004;51(3):488–497. doi:10.1002/art.20419.
- Aletaha D, Smolen JS. Diagnosis and management of rheumatoid arthritis: a review. JAMA. 2018;320(13):1360–1372. doi:10.1001/jama.2018.13103.
- Van der Heijde DMFM, Van’t Hof MA, Van Riel PLCM, et al. Validity of single variables and composite indices for measuring disease activity in rheumatoid arthritis. Ann Rheum Dis. 1992;51(2):177–181. doi:10.1136/ard.51.2.177.
- Bandinelli F, Benucci M, Salaffi F, et al. Do new and old biomarkers of early undifferentiated arthritis correlate with arthritis impact measurement scales? Clin Exp Rheumatol. 2021;39(1):79–83. doi:10.55563/clinexprheumatol/nqqx5k.
- Springer T, Galfrè G, Secher DS, et al. Monoclonal xenogeneic antibodies to murine cell surface antigens: identification of novel leukocyte differentiation antigens. Eur J Immunol. 1978;8(8):539–551. doi:10.1002/eji.1830080802.
- Chen G-Y, Tang J, Zheng P, et al. CD24 and siglec-10 selectively repress tissue damage–induced immune responses. Science. 2009;323(5922):1722–1725. doi:10.1126/science.1168988.
- Barkal AA, Brewer RE, Markovic M, et al. CD24 signalling through macrophage siglec-10 is a target for cancer immunotherapy. Nature. 2019;572(7769):392–396. doi:10.1038/s41586-019-1456-0.
- Bai X-F, Liu J-Q, Liu X, et al. The heat-stable antigen determines pathogenicity of self-reactive T cells in experimental autoimmune encephalomyelitis. J Clin Invest. 2000;105(9):1227–1232. doi:10.1172/JCI9012.
- Sánchez E, Abelson A-K, Sabio JM, et al. Association of a CD24 gene polymorphism with susceptibility to systemic lupus erythematosus. Arthritis Rheum. 2007;56(9):3080–3086. doi:10.1002/art.22871.
- Zhou Q, Rammohan K, Lin S, et al. CD24 is a genetic modifier for risk and progression of multiple sclerosis. Proc Natl Acad Sci USA. 2003;100(25):15041–15046. doi:10.1073/pnas.2533866100.
- Liu J-Q, Carl JW, Joshi PS, et al. CD24 on the resident cells of the central nervous system enhances experimental autoimmune encephalomyelitis. J Immunol. 2007;178(10):6227–6235. doi:10.4049/jimmunol.178.10.6227.
- Sánchez E, Fernández-Gutierrez B, González-Gay MÁ, et al. Investigating the role of CD24 gene polymorphisms in rheumatoid arthritis. Ann Rheum Dis. 2008;67(8):1197–1198. doi:10.1136/ard.2007.084475.
- Wang L, Lin S, Rammohan KW, et al. A dinucleotide deletion in CD24 confers protection against autoimmune diseases. PLoS Genet. 2007;3(4):e49. doi:10.1371/journal.pgen.0030049.
- Paulson JC, Kawasaki N. Sialidase inhibitors DAMPen sepsis. Nat Biotechnol. 2011;29(5):406–407. doi:10.1038/nbt.1859.
- Liu Y, Chen G-Y, Zheng P. CD24-Siglec G/10 discriminates danger- from pathogen-associated molecular patterns. Trends Immunol. 2009;30(12):557–561. doi:10.1016/j.it.2009.09.006.
- Toubai T, Hou G, Mathewson N, et al. Siglec-G–CD24 axis controls the severity of graft-versus-host disease in mice. Blood. 2014;123(22):3512–3523. doi:10.1182/blood-2013-12-545335.
- Lim SC. CD24 and human carcinoma: tumor biological aspects. Biomed Pharmacother. 2005;59 Suppl 2: S351–S354. doi:10.1016/S0753-3322(05)80076-9.
- Kristiansen G, Denkert C, Schlüns K, et al. CD24 is expressed in ovarian cancer and is a new independent prognostic marker of patient survival. Am J Pathol. 2002;161(4):1215–1221. doi:10.1016/S0002-9440(10)64398-2.
- Sano A, Kato H, Sakurai S, et al. CD24 expression is a novel prognostic factor in esophageal squamous cell carcinoma. Ann Surg Oncol. 2009;16(2):506–514. doi:10.1245/s10434-008-0252-0.
- Li O, Zheng P, Liu Y. CD24 expression on T cells is required for optimal T cell proliferation in lymphopenic host. J Exp Med. 2004;200(8):1083–1089. doi:10.1084/jem.20040779.
- Suzuki T, Kiyokawa N, Taguchi T, et al. CD24 induces apoptosis in human B cells via the glycolipid-enriched membrane domains/rafts-mediated signaling system. J Immunol. 2001;166(9):5567–5577. doi:10.4049/jimmunol.166.9.5567.
- Weyand CM, Goronzy JJ. The immunology of rheumatoid arthritis. Nat Immunol. 2021;22(1):10–18. doi:10.1038/s41590-020-00816-x.
- Liu Y, Zheng P. CD24: a genetic checkpoint in T cell homeostasis and autoimmune diseases. Trends Immunol. 2007;28(7):315–320. doi:10.1016/j.it.2007.05.001.
- Hu F, Liu H, Liu X, et al. Pathogenic conversion of regulatory B10 cells into osteoclast-priming cells in rheumatoid arthritis. J Autoimmun. 2017;76:53–62. doi:10.1016/j.jaut.2016.09.002.
- Mielle J, Audo R, Hahne M, et al. IL-10 producing B cells ability to induce regulatory T cells is maintained in rheumatoid arthritis. Front Immunol. 2018;9:961. doi:10.3389/fimmu.2018.00961.
- Flores-Borja F, Bosma A, Ng D, et al. CD19 + CD24hiCD38hi B cells maintain regulatory T cells while limiting TH1 and TH17 differentiation. Sci Transl Med. 2013;5(173):173ra23. doi:10.1126/scitranslmed.3005407.
- Niu Q, Cai B, Huang ZC, et al. Disturbed Th17/treg balance in patients with rheumatoid arthritis. Rheumatol Int. 2012;32(9):2731–2736. doi:10.1007/s00296-011-1984-x.
- Daien CI, Gailhac S, Mura T, et al. Regulatory B10 cells are decreased in patients with rheumatoid arthritis and are inversely correlated with disease activity. Arthritis Rheumatol. 2014;66(8):2037–2046. doi:10.1002/art.38666.
- Kay R, Rosten PM, Humphries RK. CD24, a signal transducer modulating B cell activation responses, is a very short peptide with a glycosyl phosphatidylinositol membrane anchor. J Immunol. 1991;147(4):1412–1416. doi:10.4049/jimmunol.147.4.1412.
