Abstract
Background
Bright light therapy (BLT) is widely used for treating Seasonal Affective Disorder (SAD). However, the neural mechanisms underlying the therapeutic effects of BLT remain largely unexplored. The present study used a diurnal rodent (Nile grass rats; Arvicanthis niloticus) to test the hypothesis that the therapeutic effects of BLT could be, in part, due to reduced neuroinflammation and/or enhanced neuroplasticity. Our previous research has demonstrated that compared to grass rats housed in a summer-like daytime bright light condition (1000 lux), those housed in a winter-like daytime dim light condition (50 lux) showed increased depression- and anxiety-like behaviours, as well as impaired sociosexual behaviours and spatial memory, similar to what is observed in patients suffering from SAD.
Materials and Methods
In the present study, male and female grass rats were housed under the winter-like dim daytime light condition (lights on 600–1800 hr, 50 lux). The experimental groups received daily 1-h early morning BLT from 0600-0700 using full-spectrum light (10,000 lux), while the control groups received narrowband red light (λmax, 780 nm). Following 4 weeks of treatment, the expression of several neuroinflammatory or plasticity markers was examined in the medial prefrontal cortex (mPFC), basolateral amygdala (BLA), and the CA1 of the dorsal hippocampus.
Results
For the neuroinflammatory markers, BLT reduced TNF-α in the BLA of females, and upregulated CD11b in the mPFC and IL6 in the BLA in males. For the neuroplasticity markers, BLT downregulated BDNF in the CA1 and TrkB in all three brain regions in females but upregulated BDNF in the BLA and CA1 in males.
Conclusions
These results indicate that the therapeutic effects of BLT on sleep, mood, and cognition may be attributed in part to mechanisms involving neuroinflammation and neuroplasticity in corticolimbic brain regions. Moreover, these effects appear to vary between sexes.
1. Introduction
Bright light therapy (BLT) has long been used to treat Seasonal Affective Disorder (SAD), a depressive disorder characterized by fatigue, anhedonia, and depressive symptoms in the fall and winter and alleviation of these symptoms in the spring and summer [Citation1,Citation2]. BLT is as effective as some antidepressant medications not only for treating SAD [Citation2], but also for treating nonseasonal depression. This is true when used as monotherapy or as an adjunct to antidepressant medications, with the latter producing a higher remission rate or faster remission when compared to medication alone [Citation3–7]. BLT has also been particularly valuable in treating depression in populations where noninvasive and nonpharmacological treatment options are preferred, such as pregnant and postpartum women, as well as adolescents [Citation8–10].
Beyond depressive disorders, BLT also alleviates symptoms of bipolar depression [Citation11,Citation12], adult ADHD [Citation13], and post-traumatic stress disorder [Citation14]. Besides psychiatric disorders, BLT can help counteract fatigue and sleep disturbances, particularly when they arise as side effects of cancer and immune disease medications [Citation15–19]. Additionally, in both dementia patients and general nursing home populations BLT improves sleep and slow cognitive decline, as well as lessens sundown syndrome, agitation, and depressive symptoms [Citation20–24].
Despite the widespread use of BLT, the neurobiological mechanisms driving its therapeutic effects are poorly understood. An emerging area of interest is how BLT affects neuroinflammation and neuroplasticity. Individuals with SAD exhibit notably elevated levels of plasma IL-6 and soluble IL-6 receptors compared to unaffected controls, indicating the presence of an increased pro-inflammatory state in these patients [Citation25]. It was recently reported that bright light therapy reduced peripheral pro-inflammatory cytokines TNF- α and IL6 in patients with traumatic brain injury [Citation26]. That study also found higher circulating pro-inflammatory cytokines associated with higher insomnia severity in the patients, and that BLT improved both sleep quality and inflammatory profile [Citation26]. Neuroinflammatory response, typically activating microglia and pro-inflammatory cytokines, is implicated in the pathophysiology of many neurological and psychiatric disorders, including dementia and depression [Citation27–32]. The neuroinflammatory response often exerts its cytotoxic effects through reductions in synaptic plasticity, by altering the signalling of neurotrophic and neuroplasticity markers such as BDNF and TrkB [Citation33–36]. BDNF and TrkB have been implicated in psychiatric disorders and offer potential therapeutic targets [Citation37–40]. Therefore, we hypothesized that neuroinflammatory responses and synaptic plasticity could be potential mechanisms underlying the therapeutic effects of BLT.
To begin testing this hypothesis, the current study utilized a diurnal rodent, the Nile grass rat (Arvicanthis niloticus). Chronically housing Nile grass rats in a winter-like light condition involving dim light during the day has been established as a model to study the effects of light on the brain and behaviour relevant to SAD [Citation41]. Our previous work found that, when compared to grass rats housed in a summer-like bright daytime light condition, grass rats housed in a winter-like dim daytime light condition showed more behavioural despair, anhedonia, and decreased exploratory behaviours [Citation42–45]; impaired spatial memory [Citation46,Citation47]; and disrupted sociosexual behaviours [Citation48]. The behavioural responses induced by winter-like dim light conditions are consistent with the symptoms reported by SAD patients. Using the grass rat model of SAD, the current study examined the impact of BLT on the expression of neuroinflammatory and synaptic plasticity markers in corticolimbic brain regions implicated in affective state and cognition - the medial prefrontal cortex (mPFC), basolateral amygdala (BLA), and the CA1 region of the dorsal hippocampus. Female and male grass rats were housed under winter-like dim light conditions as in previous studies and the experimental groups then received daily 1-h early morning BLT, while the control groups received narrowband red light (λmax 780 nm) over 4 weeks. We then examined the expression of neuroinflammation markers including the microglia activation marker CD11b and the proinflammatory cytokines TNF-α and IL6, as well as synaptic plasticity markers including the neurotrophic factor BDNF and its receptor TrkB. The results revealed brain region-specific effects that differed between males and females, suggesting that the therapeutic effects of BLT to improve sleep, affective state, and cognition are partly mediated by mechanisms involving neuroinflammation and neuroplasticity, and differently between the sexes.
2. Materials and methods
2.1. Subjects and housing conditions
All procedures followed the ethical guidelines outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80-23) and received approval by the Institutional Animal Care and Use Committee of Michigan State University. Adult male and female adult grass rats (Arvicanthis niloticus) were sourced from our breeding colony at Michigan State University, aged between 4 and 6 months [Citation49]. The rats were housed in Plexiglass cages (43 × 23 × 20 cm) under a 12:12 hr light/dark cycle (lights on at 06:00 hr), with a metal hut provided for shelter and enrichment. They had unrestricted access to food (PMI Nutrition Prolab RMH 2000, Brentwood, MO, USA) and water. The colony room had a light intensity of ∼300 lux, generated by ceiling light fixtures. For this study, the animals were moved to a separate holding room and exposed to a dim light intensity of 50 lux during the 12-hr light phase, as described in previous studies [Citation42,Citation46]. Each cage was equipped with light fixtures (Multispectral Luminaire, Telelumen LLC, CA) positioned 12.7 cm (∼5 in) above, ensuring full illumination of the cage (excluding the interior of the metal hut) throughout the study. The animals were randomly divided into two groups (n = 6/group for males, n = 8/group for females), receiving either 1 hr (6:00-7:00) of full-spectrum white light BLT (∼10,000 lux, total irradiance: 7.92 mW/cm2, melanopic irradiance: 1.51 mW/cm2), or a control narrowband red light (λmax 780 nm, ∼180 lux, total irradiance: 1.51mW/cm2 with no melanopic irradiance). The red light served as the control, as rodents lack the necessary photoreceptor and spectral sensitivity to detect it [Citation50,Citation51]. The red light was overlaid onto the dim housing light provided in their home cages. This light exposure regimen was maintained daily for 4 weeks, as described previously [Citation52].
2.2. Brain RNA extraction and real time-qPCR
At the conclusion of the four weeks of BLT or control red light exposure, animals were euthanized at midday, specifically between 1100–1300 hr. Euthanasia was performed by administering a peritoneal injection of sodium pentobarbital at a dose of 150 mg/kg, followed by rapid decapitation. The entire brains were promptly flash-frozen and stored at a temperature of −80 °C. Subsequent cryostat sectioning was conducted at a thickness of 200 µm, and the sections were then thaw-mounted onto glass slides. These brain samples had been previously utilized in an earlier study examining the expression of hypothalamic orexin and its receptors OX1R and OX2R, following BLT [Citation52]. To analyze specific regions of interest, namely the medial prefrontal cortex (mPFC), basolateral amygdala (BLA), and dorsal hippocampal subregion CA1, 1-mm micropuncture (Harris Micropunch, Hatfield, PA) was used to punch these sections. RNA extraction was performed using the Qiagen RNeasy Plus Mini kits (Qiagen, Valencia, CA, Cat# 74134), and the resulting RNA was quantified using a Qubit Flex Fluorometer (Thermo Fisher Scientific). To facilitate further analysis, the RNAs were converted to cDNA using a high-capacity cDNA Reverse Transcription Kit (Applied Biosystems, CA, USA, Cat# 4368814).
The RT-qPCR analysis was carried out using an SYBR green master mix consisting of 5 ng cDNA and 0.25 uM of each primer set. The reactions were performed in triplicate to ensure accuracy. The primer sequences for CD11b, TNF-α, IL-6, BDNF, TrkB, and the reference gene Hprt-1 were designed based on corresponding sequences in Nile grass rats [Citation53]. To ensure specificity and efficiency, the primer sets underwent validation through melt curve analysis and assessment of CT value consistency and precision. The reference genet Hprt-1 was utilized as a “housekeeping” gene, which was confirmed to remain constant across both sexes and treatment groups in the current study. Forward and reverse primer sequences used were 5′-GCA GAC TTG CAA GGG TTC AG-3′ and 5′-GGA GGT ATC TTA CTC TTC GCT G-3′, respectively for CD11b; 5′- GGT TTT CTC CAC CAA GGA AGT TTT C −3′ and 5′-TCT GCT TGC TGC CTG TGC-3′, respectively for TNF- α; 5′-TCT ACT AGA GCC TAG TGA GCT CTG C-3′ and 5′-ACA TGA GTC AGA TAC CCG ACG A-3′, respectively for IL-6; 5′-GTC CCG CTA TCA AAA CCG CA-3′ and 5′-GCC TTC CTT CGT GTA ACC CCA-3′, respectively for BDNF; 5′-TGC ACA TCG CTC AGC AAA TCG-3’and 5′- ATC GGA TGG GCA ACA TTG TGT G-3′, respectively for TrkB; 5′-CTC ATG GAC TGA TTA TGG ACA GGA C-3′ and 5′- GCA GGT CAG CAA AGA ACT TAT AGC C-3′, respectively for Hprt-1. The CT values were automatically determined using the QuantStudio-5 Real-Time PCR System analysis software (Applied Biosystems, 272511214). ΔCT was used to calculate the relative expression of the targeted gene over the housekeeping gene (HPRT). ΔΔCT was then used to calculate the relative gene expression level in the BLT group relative to the control red light group. The fold change of the BLT group over the control group was then calculated using 2−ΔΔCT.
2.3. Statistical analysis
Normality was assessed using Shapiro–Wilk’s tests, homogeneity of variance was evaluated using Levene’s tests, and outliers were identified using Grubb’s Test for a Single Outlier. Unless stated otherwise below, the data exhibited normal distribution and equal variances. One-way ANOVAs conducted in SPSS IBM Version 27 were employed to analyze the impact of early morning BLT on the expression of neuroinflammation and neuroplasticity markers in the brain regions of interest. Sex was not included as a factor in the analysis, since the male and female cohorts did not receive treatment simultaneously. Statistical significance for all tests is indicated by a p-value less than 0.05.
3. Results
3.1. Effects of BLT on neuroinflammatory markers
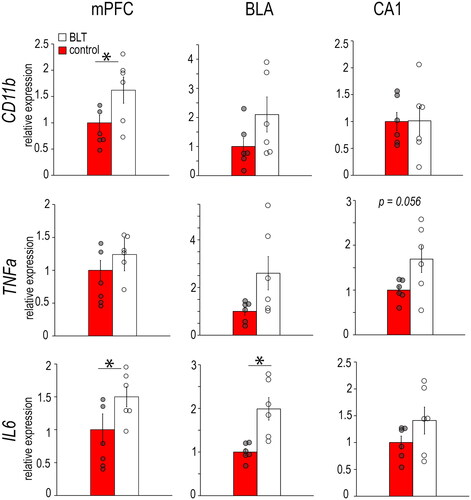
In the female mPFC (), there were no significant differences between the BLT and control groups in the expression of the microglia marker CD11b (F1,14 = 1.937, p = 0.186), or the pro-inflammatory cytokine TNF-α (F1,12 = 0.603, p = 0.452) and IL6 (F1,12 = 4.583, p = 0.053). In the female BLA, the BLT group had significantly lower expression of TNF-α compared to controls (F1,14 = 4.797, p = 0.04), but the groups did not differ in expression of CD11b (F1,14 = 1.189, p = 0.294) or IL6 (F1,14 = 3.353, p = 0.08). In the female CA1, the groups did not differ in any of the inflammatory markers (CD11b, F1,13 = 0.022, p = 0.884; TNF-α, F1,13 = 1.508, p = 0.241; IL6, F1,14 = 0.123, p = 0.731).
Figure 1. Levels of mRNAs for the microglia marker CD11b and pro-inflammatory cytokines TNF-α and IL6 in the mPFC, BLA, and CA1 of female grass rats exposed to BLT or control red light. Data are shown as Means ± SEMs, n = 8/group. *p < 0.05. Controls are set to 1.0.
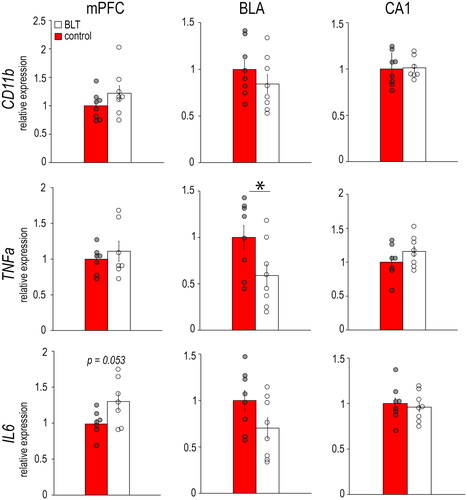
In the male mPFC (), BLT significantly increased CD11b (F1,10 = 7.293, p = 0.022) and IL6 expression (F1,9 = 7.488, p = 0.021), but not TNF-α (F1,10 = 3.677, p = 0.084), when compared to controls (). Within the male BLA, BLT also significantly upregulated IL6 (F1,10 = 13.231, p = 0.005), but not the other inflammatory markers (CD11b, F1,10 = 2.371, p = 0.155; TNF-α, F1,10 = 4.465, p = 0.061). In the male CA1, there were no significant differences between BLT and control groups in inflammatory marker expression (CD11b: F1,10 = 0.001, p = 0.975; TNF-α: F1,10 = 4.629, p = 0.056; IL6: F1,10 = 2.143, p = 0.174).
3.2. Effects of BLT on synaptic plasticity markers
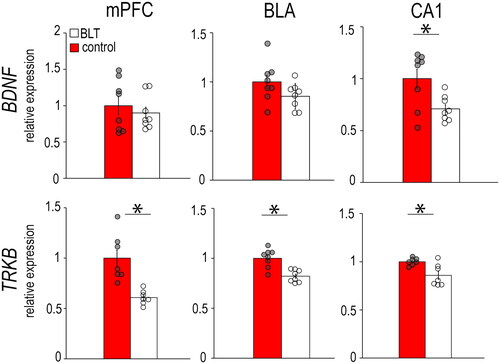
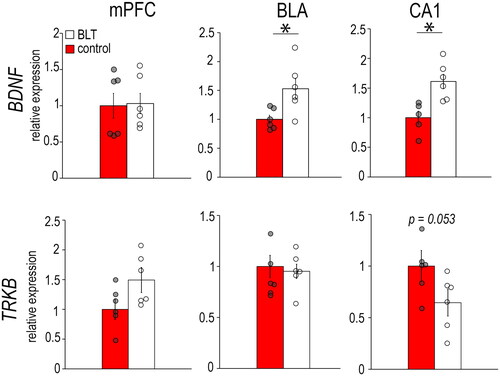
In females (), the BLT group had significantly lower expression of BDNF in the CA1 (F1,14 = 7.638, p = 0.015) and lower expression of TrkB in the mPFC (F1,12 = 17.878, p = 0.001), BLA (F1,14 = 20.636, p < 0.001) and CA1 (F1,12 = 9.907, p = 0.008) compared to the controls. In males (), the BLT group had higher BDNF expression in the BLA (F1,10 = 7.937 p = 0.018) and CA1 (F1,10 = 12.456, p = 0.006), but no significant differences between groups were found in their expression of TrkB (mPFC: F1,10 = 4.248, p = 0.066; BLA: F1,12 = 0.130, p = 0.726; CA1: F1,10 = 4.780, p = 0.053).
4. Discussion
Following 4 weeks of early morning BLT, numerous changes were found in the expression of neuroinflammatory or neuroplasticity markers in corticolimbic brain regions of diurnal grass rats when compared to animals in the red-light control group (). The results support the hypothesis that neuroinflammation and neuroplasticity are potential mechanisms underlying the therapeutic effects of BLT.
Table 1. Summary of mRNA expression of neuroinflammatory markers CD11b, TNF-α, and IL6, and neuroplasticity markers BDNF and TrkB in the medial prefrontal cortex (mPFC), basolateral amygdala (BLA), and CA1 subregion of the hippocampus (CA1) of female and male grass rats exposed to BLT.
Brain region- and sex-specific responses in neuroinflammatory markers were found in our previous study comparing grass rats chronically housed in either a winter-like dim daylight condition or a summer-like bright daylight condition (∼1000 lux) [Citation54]. It was reasonable to expect that animals housed in the winter-like dim light but exposed to daily early morning BLT would show a restoration of the neuroinflammatory status seen in animals housed in summer-like bright light. That was not always the case. For example, our prior study found lower IL-6 expression in the BLA of males housed in summer-like bright light than those housed in winter-like dim light [Citation54], but here we found IL-6 upregulated in males by BLT. Furthermore, while we previously found that females housed in bright light during the day had lower TNF-α expression in the CA1, the present study found that BLT had no significant effect thereon TNF-α. These few inconsistencies are likely due to differences between our studies in the lighting paradigms used to address the specific questions asked, including the light intensity (1000 vs. 10,000 lux) and the timing/duration of light exposure (all day vs only 1 h in the early morning). It has been shown that combined BLT and antidepressant treatment decreases the proinflammatory profile (as defined by peripheral circulating neutrophil count) in SAD patients [Citation55]; BLT alone reduces peripheral proinflammatory cytokines including IL-6 and TNF- α [Citation26]. Our findings of region-specific changes in IL-6 and TNF-α expression within the brain of diurnal grass rats suggest that neuroinflammation is a potential mechanism underlying the effects of BLT, which could be further tested in clinical studies.
We previously found significantly lower CA1 apical dendritic spine density in both male and female grass rats housed in winter-like dim daylight when compared to grass rats housed in summer-like bright daylight [Citation46,Citation47]. These findings suggest that synaptic plasticity could be a potential mediator through which seasonal light conditions influence the affective state. The present study examined the expression of two synaptic plasticity markers, BDNF and TrkB, and revealed that BLT effects were generally consistent with those from summer-like bright light housing. In females, the BLT group had lower BDNF mRNA expression in the CA1 and lower TrkB mRNA expression in mPFC, BLA, and CA1 (). When BDNF and TrkB protein levels were previously compared between grass rats housed in bright or dim daytime light conditions, the differences did not reach statistical significance despite mature BDNF and the phospho- over total-TrkB ratio in the CA1 being ∼30% lower in the bright light group [Citation47]. In males, we here found that BLT increased BDNF expression in the BLA and CA1 (). Consistently, we previously found higher levels of BDNF immunoreactivity in the CA1 of males housed in bright light [Citation46,Citation47]. Although we were unable to directly test for sex differences in the present study, they are suggested by our results and consistent with the sex-specific responses in BDNF and TrkB reported by others. Social isolation and maternal deprivation significantly reduce BDNF in male mice and rats, but not in females [Citation56–58]. Environmental enrichment, however, increases BDNF in females, but not in males [Citation59,Citation60]. Lastly, reducing forebrain BDNF leads to a higher phospho- over total-TrkB ratio in male but not in female mice [Citation61]. Gonadal hormones, particularly estrogens, likely play a role in these sex differences [Citation62–65], which could contribute to sex differences in the pathophysiology of depression. In human postmortem tissue, depressed men have less hippocampal BDNF compared to non-depressed men, while no difference was found between depressed and non-depressed women [Citation66]; this is consistent with the light (BLT or summer-like bright light)-induced changes of BDNF in our male and female grass rats.
Behavioural sleep in the BLT and control grass rats from the current study was recently reported by our group [Citation52]. Although the total amounts of sleep during the day or night were not significantly affected by BLT, it did lead to longer nighttime sleep bout length in both sexes, indicating better sleep quality or less sleep fragmentation in these animals. Sleep is known to affect neuroinflammation. In male C57BL/6J mice, an acute sleep disturbance (1 day) was sufficient to induce memory impairments and neuroinflammation as indicated by higher IL-6 protein levels and the number of microglia in the CA1 [Citation67]. In male Wistar rats, chronic sleep restriction (only 6 hrs permitted per day over 21 days) also caused memory impairments and increased hippocampal IL-6 and TNF-α [Citation68]. In humans, sleep duration and sleep disturbance affect inflammatory cytokine levels [Citation69–71]. For instance, a recent study reported that sleep restriction from 8 to 6 hrs per night in a small sample of both men and women led to enhanced monocytosis; this effect was attributed to alterations in the epigenome of hematopoietic stem and progenitor cells, which in turn primed these cells for heightened inflammatory responses [Citation72]. There is also a link between poor sleep quality and neuroinflammation, suggested by their comorbidity in dementia, and neuroinflammation has even been proposed as a mediator of the relationship between sleep disturbance and neuropathology in dementia [Citation73].
Relationships between sleep, synaptic plasticity, and depression have also been well-established [Citation74–76]. Numerous studies have examined the impact of sleep deprivation on hippocampal BDNF expression, but results are equivocal probably due to the differences in species studied (mice vs rats), age of the animals, and methodological details including the procedures inducing sleep deprivation and their durations (reviewed in [Citation77]). It is also noteworthy that those studies almost exclusively involved male subjects. Our present and recent findings [Citation52] help fill this important gap in knowledge, by showing a potential association between improved sleep quality and the central expression of BDNF and TrkB in both male and female grass rats following BLT.
In addition to regulating sleep/wakefulness, we recently found that BLT alters the expression of hypothalamic orexin/hypocretin and its receptors [Citation52]. The central orexin system is another potential pathway through which BLT modulates neuroinflammation and plasticity [Citation78–83]. Orexin administration attenuates the production of TNF-a and IL-6 [Citation84,Citation85], whereas orexin-knockout mice show increased TNF-a, IL-6, and activated microglia [Citation84,Citation85]. In humans, preliminary trials suggest that intranasal orexin dampens pro-inflammatory responses in cardiac patients [Citation86]. Orexin also upregulates the expression of neurotrophic factors, such as BDNF [Citation83,Citation87–89]. In the current study, we found that BLT increased BDNF in the male CA1, but downregulated it in females ( and ). This pattern of changes in BDNF is consistent with BLT-induced changes in OX1R in the grass rat CA1 [Citation52]. Further analyses on our datasets arising from these same grass rats exposed to BLT or not confirmed that there is a significant positive correlation between OX1R and BDNF mRNAs in the CA1 of both males (r = 0.770, p = 0.006) and females (r = 0.579, p = 0.019). This intriguing, and the possible causal role of orexin in light-dependent neuroinflammatory and neuroplastic responses will be tested in the grass rat SAD model in our future studies.
5. Conclusions
Using the diurnal grass rat model of SAD we demonstrated that early morning BLT given daily for 4 weeks leads to changes in the expression of neuroinflammatory and neuroplasticity markers in corticolimbic brain regions involved in regulating affective and cognitive functions. Consistent with our previous findings in grass rats housed in winter- or summer-like lighting conditions [Citation54,Citation90] or following a BLT paradigm [Citation52], the effects of BLT are brain region-specific and differ between males and females. The results from our present and previous studies provide novel insights into how seasonal light conditions and light therapy influence a variety of neural functions including neuropeptides and neurotransmitter systems, neuroinflammation, and neuroplasticity with concomitant effects on numerous behavioural outcomes highly relevant to SAD and other depressive disorders in humans [Citation41].
Author contributions
AC, JSL, and LY designed the study and wrote the manuscript. AC, KL-D, and CV conducted the experiments and analyzed data. All authors have approved the final version of the paper for submission.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data supporting the findings of this study are viewable within the article’s figures and will be made available in spreadsheet form upon request made to the corresponding author.
Additional information
Funding
References
- Rosenthal NE, Sack DA, Gillin JC, et al. Seasonal affective disorder. A description of the syndrome and preliminary findings with light therapy. Arch Gen Psychiatry. 1984;41(1):1–11. doi: 10.1001/archpsyc.1984.01790120076010.
- Terman M, Amira L, Terman JS, et al. Predictors of response and nonresponse to light treatment for winter depression. Am J Psychiatry. 1996;153(11):1423–1429. doi: 10.1176/ajp.153.11.1423.
- Ruhrmann S, Kasper S, Hawellek B, et al. Effects of fluoxetine versus bright light in the treatment of seasonal affective disorder. Psychol Med. 1998;28(4):923–933. doi: 10.1017/s0033291798006813.
- Golden RN, Gaynes BN, Ekstrom RD, et al. The efficacy of light therapy in the treatment of mood disorders: a review and meta-analysis of the evidence. Am J Psychiatry. 2005;162(4):656–662. doi: 10.1176/appi.ajp.162.4.656.
- Özdemir PG, Boysan M, Smolensky MH, et al. Comparison of venlafaxine alone versus venlafaxine plus bright light therapy combination for severe major depressive disorder. J Clin Psychiatry. 2015;76(05):e645-54–e654. doi: 10.4088/JCP.14m09376.
- Even C, Schröder CM, Friedman S, et al. Efficacy of light therapy in nonseasonal depression: a systematic review. J Affect Disord. 2008;108(1-2):11–23. doi: 10.1016/j.jad.2007.09.008.
- Al-Karawi D, Jubair L. Bright light therapy for nonseasonal depression: meta-analysis of clinical trials. J Affect Disord. 2016;198:64–71. doi: 10.1016/j.jad.2016.03.016.
- Niederhofer H, von Klitzing K. Bright light treatment as mono-therapy of non-seasonal depression for 28 adolescents. Int J Psychiatry Clin Pract. 2012;16(3):233–237. doi: 10.3109/13651501.2011.625123.
- Wirz-Justice A, Bader A, Frisch U, et al. A randomized, double-blind, placebo-controlled study of light therapy for antepartum depression. J Clin Psychiatry. 2011;72(7):986–993. doi: 10.4088/JCP.10m06188blu.
- Corral M, Wardrop AA, Zhang H, et al. Morning light therapy for postpartum depression. Arch Womens Ment Health. 2007;10(5):221–224. doi: 10.1007/s00737-007-0200-1.
- Colombo C, Lucca A, Benedetti F, et al. Total sleep deprivation combined with lithium and light therapy in the treatment of bipolar depression: replication of main effects and interaction. Psychiatry Res. 2000;95(1):43–53. doi: 10.1016/s0165-1781(00)00164-5.
- Tseng P-T, Chen Y-W, Tu K-Y, et al. Light therapy in the treatment of patients with bipolar depression: a meta-analytic study. Eur Neuropsychopharmacol. 2016;26(6):1037–1047. doi: 10.1016/j.euroneuro.2016.03.001.
- Rybak YE, McNeely HE, Mackenzie BE, et al. An open trial of light therapy in adult attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2006;67(10):1527–1535. doi: 10.4088/jcp.v67n1006.
- Zalta AK, Bravo K, Valdespino-Hayden Z, et al. A placebo-controlled pilot study of a wearable morning bright light treatment for probable PTSD. Depress Anxiety. 2019;36(7):617–624. doi: 10.1002/da.22897.
- Phipps-Nelson J, Redman JR, Dijk D-J, et al. Daytime exposure to bright light, as compared to dim light, decreases sleepiness and improves psychomotor vigilance performance. Sleep. 2003;26(6):695–700. doi: 10.1093/sleep/26.6.695.
- Kohyama J. Sleep health and asynchronization. Brain Dev. 2011;33(3):252–259. doi: 10.1016/j.braindev.2010.09.006.
- Johnson JA, Garland SN, Carlson LE, et al. Bright light therapy improves cancer-related fatigue in cancer survivors: a randomized controlled trial. J Cancer Surviv. 2018;12(2):206–215. doi: 10.1007/s11764-017-0659-3.
- Starreveld DEJ, et al. Light therapy for cancer-related fatigue in (non-)Hodgkin lymphoma survivors: results of a randomized controlled trial. Cancers (Basel.). 2021;13(19):4948 doi: 10.3390/cancers13194948.
- Wu H-S, Gao F, Yan L, et al. Evaluating chronotypically tailored light therapy for breast cancer survivors: preliminary findings on fatigue and disrupted sleep. Chronobiol Int. 2022;39(2):221–232. doi: 10.1080/07420528.2021.1992419.
- Haffmans PMJ, Sival RC, Lucius SAP, et al. Bright light therapy and melatonin in motor restless behaviour in dementia: a placebo-controlled study. Int J Geriat Psychiatry. 2001;16(1):106–110. doi: 10.1002/1099-1166(200101)16:1<106::AID-GPS288>3.0.CO;2-9.
- Ancoli-Israel S, Gehrman P, Martin JL, et al. Increased light exposure consolidates sleep and strengthens circadian rhythms in severe Alzheimer’s disease patients. Behav Sleep Med. 2003;1(1):22–36. doi: 10.1207/S15402010BSM0101_4.
- Sloane PD, Figueiro M, Garg S, et al. Effect of home-based light treatment on persons with dementia and their caregivers. Light Res Technol. 2015;47(2):161–176. doi: 10.1177/1477153513517255.
- Yamadera H, Ito T, Suzuki H, et al. Effects of bright light on cognitive and sleep-wake (circadian) rhythm disturbances in Alzheimer-type dementia. Psychiatry Clin Neurosci. 2000;54(3):352–353. doi: 10.1046/j.1440-1819.2000.00711.x.
- Riemersma-van der Lek RF, Swaab DF, Twisk J, et al. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: a randomized controlled trial. JAMA. 2008;299(22):2642–2655. doi: 10.1001/jama.299.22.2642.
- Leu SJ, Shiah IS, Yatham LN, et al. Immune-inflammatory markers in patients with seasonal affective disorder: effects of light therapy. J Affect Disord. 2001;63(1-3):27–34. doi: 10.1016/s0165-0327(00)00165-8.
- Elliott JE, McBride AA, Balba NM, et al. Feasibility and preliminary efficacy for morning bright light therapy to improve sleep and plasma biomarkers in US veterans with TBI. A prospective, open-label, single-arm trial. PLoS One. 2022;17(4):e0262955. doi: 10.1371/journal.pone.0262955.
- Bloomfield PS, Selvaraj S, Veronese M, et al. Microglial activity in people at ultra-high risk of psychosis and in schizophrenia: an [(11)C]PBR28 PET brain imaging study. Am J Psychiatry. 2016;173(1):44–52. doi: 10.1176/appi.ajp.2015.14101358.
- Gilhus NE, Deuschl G. Neuroinflammation – a common thread in neurological disorders. Nat Rev Neurol. 2019;15(8):429–430. doi: 10.1038/s41582-019-0227-8.
- van Berckel BN, Bossong MG, Boellaard R, et al. Microglia activation in recent-onset schizophrenia: a quantitative (R)-[11C]PK11195 positron emission tomography study. Biol Psychiatry. 2008;64(9):820–822. doi: 10.1016/j.biopsych.2008.04.025.
- Hinwood M, Morandini J, Day TA, et al. Evidence that microglia mediate the neurobiological effects of chronic psychological stress on the medial prefrontal cortex. Cereb Cortex. 2012;22(6):1442–1454. doi: 10.1093/cercor/bhr229.
- Muller N. The role of anti-inflammatory treatment in psychiatric disorders. Psychiatr Danub. 2013;25(3):292–298.
- Torres-Platas SG, Cruceanu C, Chen GG, et al. Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain Behav Immun. 2014;42:50–59. doi: 10.1016/j.bbi.2014.05.007.
- Fragale JEC, Khariv V, Gregor DM, et al. Dysfunction in amygdala-prefrontal plasticity and extinction-resistant avoidance: a model for anxiety disorder vulnerability. Exp Neurol. 2016;275(Pt 1):59–68. doi: 10.1016/j.expneurol.2015.11.002.
- Zhang JC, Yao W, Hashimoto K. Brain-derived neurotrophic factor (BDNF)-TrkB signaling in inflammation-related depression and potential therapeutic targets. Curr Neuropharmacol. 2016;14(7):721–731. doi: 10.2174/1570159x14666160119094646.
- Guan Z, Fang J. Peripheral immune activation by lipopolysaccharide decreases neurotrophins in the cortex and hippocampus in rats. Brain Behav Immun. 2006;20(1):64–71. doi: 10.1016/j.bbi.2005.04.005.
- Lapchak PA, Araujo DM, Hefti F. Systemic interleukin-1 beta decreases brain-derived neurotrophic factor messenger RNA expression in the rat hippocampal formation. Neuroscience. 1993;53(2):297–301. doi: 10.1016/0306-4522(93)90196-m.
- Autry AE, Monteggia LM. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev. 2012;64(2):238–258. doi: 10.1124/pr.111.005108.
- Lin CC, Huang TL. Brain-derived neurotrophic factor and mental disorders. Biomed J. 2020;43(2):134–142. doi: 10.1016/j.bj.2020.01.001.
- Castren E, Monteggia LM. Brain-derived neurotrophic factor signaling in depression and antidepressant action. Biol Psychiatry. 2021;90(2):128–136. doi: 10.1016/j.biopsych.2021.05.008.
- Bazzari AH, Bazzari FH. BDNF therapeutic mechanisms in neuropsychiatric disorders. Int J Mol Sci. 2022;23(15):8417. doi: 10.3390/ijms23158417.
- Yan L, Lonstein JS, Nunez AA. Light as a modulator of emotion and cognition: lessons learned from studying a diurnal rodent. Horm Behav. 2019;111:78–86. doi: 10.1016/j.yhbeh.2018.09.003.
- Leach G, Adidharma W, Yan L. Depression-like responses induced by daytime light deficiency in the diurnal grass rat (Arvicanthis niloticus). PLoS One. 2013;8(2):e57115. doi: 10.1371/journal.pone.0057115.
- Leach G, Ramanathan C, Langel J, et al. Responses of brain and behavior to changing day-length in the diurnal grass rat (Arvicanthis niloticus). Neuroscience. 2013;234:31–39. doi: 10.1016/j.neuroscience.2013.01.002.
- Ikeno T, Deats SP, Soler J, et al. Decreased daytime illumination leads to anxiety-like behaviors and HPA axis dysregulation in the diurnal grass rat (Arvicanthis niloticus). Behav Brain Res. 2016;300:77–84. doi: 10.1016/j.bbr.2015.12.004.
- Deats SP, Adidharma W, Lonstein JS, et al. Attenuated orexinergic signaling underlies depression-like responses induced by daytime light deficiency. Neuroscience. 2014;272:252–260. doi: 10.1016/j.neuroscience.2014.04.069.
- Soler JE, Robison AJ, Núñez AA, et al. Light modulates hippocampal function and spatial learning in a diurnal rodent species: a study using male nile grass rat (Arvicanthis niloticus). Hippocampus. 2018;28(3):189–200. doi: 10.1002/hipo.22822.
- Soler JE, Stumpfig M, Tang Y-P, et al. Daytime light intensity modulates spatial learning and hippocampal plasticity in female nile grass rats (Arvicanthis niloticus). Neuroscience. 2019;404:175–183. doi: 10.1016/j.neuroscience.2019.01.031.
- Lonstein JS, Linning-Duffy K, Yan L. Low daytime light intensity disrupts male copulatory behavior, and upregulates medial preoptic area steroid hormone and dopamine receptor expression, in a diurnal rodent model of seasonal affective disorder. Front Behav Neurosci. 2019;13:72. doi: 10.3389/fnbeh.2019.00072.
- McElhinny TL, Smale L, Holekamp KE. Patterns of body temperature, activity, and reproductive behavior in a tropical murid rodent, Arvicanthis niloticus. Physiol Behav. 1997;62(1):91–96. doi: 10.1016/s0031-9384(97)00146-7.
- Rocha FAdF, Gomes BD, Silveira LCdL, et al. Spectral sensitivity measured with electroretinogram using a constant response method. PLoS One. 2016;11(1):e0147318. doi: 10.1371/journal.pone.0147318.
- Jacobs GH, Fenwick JA, Williams GA. Cone-based vision of rats for ultraviolet and visible lights. J Exp Biol. 2001;204(Pt 14):2439–2446. doi: 10.1242/jeb.204.14.2439.
- Costello A, Linning-Duffy K, Vandenbrook C, et al. Effects of light therapy on sleep/wakefulness, daily rhythms, and the Central orexin system in a diurnal rodent model of seasonal affective disorder. J Affect Disord. 2023;332:299–308. doi: 10.1016/j.jad.2023.04.012.
- Toh H, Yang C, Formenti G, et al. A haplotype-resolved genome assembly of the nile rat facilitates exploration of the genetic basis of diabetes. BMC Biol. 2022;20(1):245. doi: 10.1186/s12915-022-01427-8.
- Costello A, Linning-Duffy K, Vandenbrook C, et al. Daytime light deficiency leads to sex- and brain region-specific neuroinflammatory responses in a diurnal rodent. Cell Mol Neurobiol. 2023;43(3):1369–1384. doi: 10.1007/s10571-022-01256-x.
- Demirkol ME, Namlı Z, Tamam L. Efficacy of light therapy on non-seasonal depression and inflammatory markers. European J Psych. 2019;33(3):104–111. doi: 10.1016/j.ejpsy.2019.03.002.
- Kikusui T, Ichikawa S, Mori Y. Maternal deprivation by early weaning increases corticosterone and decreases hippocampal BDNF and neurogenesis in mice. Psychoneuroendocrinology. 2009;34(5):762–772. doi: 10.1016/j.psyneuen.2008.12.009.
- Viveros M-P, Díaz F, Mateos B, et al. Maternal deprivation induces a rapid decline in circulating leptin levels and sexually dimorphic modifications in hypothalamic trophic factors and cell turnover. Horm Behav. 2010;57(4-5):405–414. doi: 10.1016/j.yhbeh.2010.01.009.
- Weintraub A, Singaravelu J, Bhatnagar S. Enduring and sex-specific effects of adolescent social isolation in rats on adult stress reactivity. Brain Res. 2010;1343:83–92. doi: 10.1016/j.brainres.2010.04.068.
- Bakos J, Hlavacova N, Rajman M, et al. Enriched environment influences hormonal status and hippocampal brain derived neurotrophic factor in a sex dependent manner. Neuroscience. 2009;164(2):788–797. doi: 10.1016/j.neuroscience.2009.08.054.
- Zhu S-W, Yee BK, Nyffeler M, et al. Influence of differential housing on emotional behaviour and neurotrophin levels in mice. Behav Brain Res. 2006;169(1):10–20. doi: 10.1016/j.bbr.2005.11.024.
- Hill RA, van den Buuse M. Sex-dependent and region-specific changes in TrkB signaling in BDNF heterozygous mice. Brain Res. 2011;1384:51–60. doi: 10.1016/j.brainres.2011.01.060.
- Sohrabji F, Miranda RC, Toran-Allerand CD. Identification of a putative estrogen response element in the gene encoding brain-derived neurotrophic factor. Proc Natl Acad Sci USA. 1995;92(24):11110–11114. doi: 10.1073/pnas.92.24.11110.
- Harte-Hargrove LC, Maclusky NJ, Scharfman HE. Brain-derived neurotrophic factor-estrogen interactions in the hippocampal mossy fiber pathway: implications for normal brain function and disease. Neuroscience. 2013;239:46–66. doi: 10.1016/j.neuroscience.2012.12.029.
- Scharfman HE, MacLusky NJ. Estrogen and brain-derived neurotrophic factor (BDNF) in hippocampus: complexity of steroid hormone-growth factor interactions in the adult CNS. Front Neuroendocrinol. 2006;27(4):415–435. doi: 10.1016/j.yfrne.2006.09.004.
- Tang YP, Wade J. 17beta-estradiol regulates the sexually dimorphic expression of BDNF and TrkB proteins in the song system of juvenile zebra finches. PLoS One. 2012;7(8):e43687. doi: 10.1371/journal.pone.0043687.
- Hayley S, Du L, Litteljohn D, et al. Gender and brain regions specific differences in brain derived neurotrophic factor protein levels of depressed individuals who died through suicide. Neurosci Lett. 2015;600:12–16. doi: 10.1016/j.neulet.2015.05.052.
- Zhu B, Dong Y, Xu Z, et al. Sleep disturbance induces neuroinflammation and impairment of learning and memory. Neurobiol Dis. 2012;48(3):348–355. doi: 10.1016/j.nbd.2012.06.022.
- Manchanda S, Singh H, Kaur T, et al. Low-grade neuroinflammation due to chronic sleep deprivation results in anxiety and learning and memory impairments. Mol Cell Biochem. 2018;449(1-2):63–72. doi: 10.1007/s11010-018-3343-7.
- Patel SR, Zhu X, Storfer-Isser A, et al. Sleep duration and biomarkers of inflammation. Sleep. 2009;32(2):200–204. doi: 10.1093/sleep/32.2.200.
- Irwin MR, Carrillo C, Olmstead R. Sleep loss activates cellular markers of inflammation: sex differences. Brain Behav Immun. 2010;24(1):54–57. doi: 10.1016/j.bbi.2009.06.001.
- Irwin MR, Wang M, Campomayor CO, et al. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166(16):1756–1762. doi: 10.1001/archinte.166.16.1756.
- McAlpine CS, et al. Sleep exerts lasting effects on hematopoietic stem cell function and diversity. J Exp Med. 2022;219(11):e20220081. doi:10.1084/jem.20220081.
- Pak VM, Onen S-H, Bliwise DL, et al. Sleep disturbances in MCI and AD: neuroinflammation as a possible mediating pathway. Front Aging Neurosci. 2020;12:69. doi: 10.3389/fnagi.2020.00069.
- Benington JH, Frank MG. Cellular and molecular connections between sleep and synaptic plasticity. Prog Neurobiol. 2003;69(2):71–101. doi: 10.1016/s0301-0082(03)00018-2.
- Wang G, Grone B, Colas D, et al. Synaptic plasticity in sleep: learning, homeostasis and disease. Trends Neurosci. 2011;34(9):452–463. doi: 10.1016/j.tins.2011.07.005.
- Tononi G, Cirelli C. Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron. 2014;81(1):12–34. doi: 10.1016/j.neuron.2013.12.025.
- da Costa Souza A, Ribeiro S. Sleep deprivation and gene expression. Curr Top Behav Neurosci. 2015;25:65–90.
- Ardeshiri MR, Hosseinmardi N, Akbari E. Orexin 1 and orexin 2 receptor antagonism in the basolateral amygdala modulate long-term potentiation of the population spike in the perforant path-dentate gyrus-evoked field potential in rats. Neurobiol Learn Mem. 2018;149:98–106. doi: 10.1016/j.nlm.2018.02.024.
- Chen XY, Chen L, Du YF. Orexin-A increases the firing activity of hippocampal CA1 neurons through orexin-1 receptors. J Neurosci Res. 2017;95(7):1415–1426. doi: 10.1002/jnr.23975.
- Gao XB, Wang AH. Experience-dependent plasticity in hypocretin/orexin neurones: re-setting arousal threshold. Acta Physiol. 2010;198(3):251–262. doi: 10.1111/j.1748-1716.2009.02047.x.
- Gotter AL, et al. Orexin receptors as therapeutic drug targets. Prog Brain Res. 2012;198:163–188.
- Butterick TA, Nixon JP, Billington CJ, et al. Orexin a decreases lipid peroxidation and apoptosis in a novel hypothalamic cell model. Neurosci Lett. 2012;524(1):30–34. doi: 10.1016/j.neulet.2012.07.002.
- Kooshki R, Abbasnejad M, Esmaeili-Mahani S, et al. The effect of CA1 administration of orexin-A on hippocampal expression of COX-2 and BDNF in a rat model of orofacial pain. Arq Neuropsiquiatr. 2018;76(9):603–608. doi: 10.1590/0004-282X20180099.
- Xiong X, White RE, Xu L, et al. Mitigation of murine focal cerebral ischemia by the hypocretin/orexin system is associated with reduced inflammation. Stroke. 2013;44(3):764–770. doi: 10.1161/STROKEAHA.112.681700.
- Duffy CM, Yuan C, Wisdorf LE, et al. Role of orexin a signaling in dietary palmitic acid-activated microglial cells. Neurosci Lett. 2015;606:140–144. doi: 10.1016/j.neulet.2015.08.033.
- Modi HR, Wang Q, Gd S, et al. Intranasal post-cardiac arrest treatment with orexin-A facilitates arousal from coma and ameliorates neuroinflammation. PLoS One. 2017;12(9):e0182707. doi: 10.1371/journal.pone.0182707.
- Cohen S, Matar MA, Vainer E, et al. Significance of the orexinergic system in modulating stress-related responses in an animal model of post-traumatic stress disorder. Transl Psychiatry. 2020;10(1):10. doi: 10.1038/s41398-020-0698-9.
- Yamada N, Katsuura G, Tatsuno I, et al. Orexins increase mRNA expressions of neurotrophin-3 in rat primary cortical neuron cultures. Neurosci Lett. 2009;450(2):132–135. doi: 10.1016/j.neulet.2008.11.028.
- Toor B, et al. Sleep, orexin and cognition. Front Neurol Neurosci. 2021;45:38–51.
- Lonstein JS, Linning-Duffy K, Tang Y, et al. Impact of daytime light intensity on the Central orexin (hypocretin) system of a diurnal rodent (Arvicanthis niloticus). Eur J Neurosci. 2021;54:4167–4181. doi: 10.1111/ejn.15248.