Abstract
Background
The association between inflammation and venous thromboembolism (VTE) has attracted increasing research interest. Recently, the systemic inflammation response index (SIRI) has been proposed as a novel inflammatory biomarker, but its potential association with lower extremity deep venous thrombosis (LEDVT) has not been investigated. Thus, this study aimed to explore the association between SIRI and LEDVT risk in a large sample over a 10-year period (2012–2022).
Methods
All hospitalized patients who underwent lower extremity compression ultrasonography (CUS) examinations were consecutively identified from our hospital information system database. Multivariate logistic regression analysis was used to investigate the association between SIRI and LEDVT risk. Sensitivity, restricted cubic spline and subgroup analyses were also performed.
Results
In total, 12643 patients were included, and 1346 (10.6%) LEDVT events occurred. After full adjustment, a higher SIRI level was significantly associated with an increased risk of LEDVT (odds ratio [OR] = 1.098, 95% confidence interval [CI]: 1.068–1.128, p < 0.001), and patients in quartile 4 had a 2.563-fold higher risk of LEDVT than those in quartile 1 (95% CI: 2.064–3.182, p < 0.001). A nonlinear relationship was observed (P for nonlinearity < 0.001), with an inflection point of 4.17. Below this point, each unit increase in SIRI corresponded to a 35.3% increase in LEDVT risk (95% CI: 1.255–1.458, p < 0.001). No significant difference was found above the inflection point (OR = 1.015, 95% CI: 0.963–1.069, p = 0.582). Sensitivity and subgroup analyses confirmed the robustness of the association. This association also existed in both distal and proximal LEDVT.
Conclusion
A High SIRI is significantly associated with an increased risk of LEDVT in hospitalized patients. Given that the SIRI is a readily available biomarker in clinical settings, its potential clinical use deserves further exploration.
KEY MESSAGES
A High SIRI is significantly associated with an increased risk of LEDVT in hospitalized patients.
The association between SIRI and LEDVT risk was nonlinear, with an inflection point of 4.17.
A positive association was observed below the inflection point, but no significant difference was found above this point.
Introduction
Lower extremity deep venous thrombosis (LEDVT) is a common and serious condition that affects millions of people worldwide [Citation1]. Numerous studies have indicated that hospitalization is one of the most important factors triggering DVT, and the overall annual incidence among hospitalized patients is more than 100-fold higher than that among community residents [Citation2,Citation3]. During the last two decades, an increasing trend in DVT-related hospitalizations has been observed in the United States [Citation4,Citation5], Korea [Citation6], and China [Citation7]. Once a thrombus occurs, patients may develop post-thrombotic syndrome (PTS), DVT recurrence, major bleeding, pulmonary embolism (PE), and even sudden death [Citation8–10]. Notably, the awareness and knowledge of DVT among the general public and patients are relatively limited [Citation11]. Hence, DVT is a major global public health challenge and a significant economic burden [Citation12].
In recent years, anticoagulants have been recommended by guidelines and are widely used to prevent and treat DVT [Citation13–16]. However, even with effective antithrombotic drugs, up to 50% of DVT patients develop PTS and up to 4% of PE patients develop chronic thromboembolic pulmonary hypertension [Citation17]. In addition, many well-designed randomized controlled trials (RCTs) have confirmed that anticoagulant therapy (e.g. rivaroxaban, enoxaparin, and dalteparin) cannot completely prevent thrombotic events [Citation18–21]. Together, these results imply the existence of other unresolved mechanisms that lead to undertreatment, such as systemic inflammation [Citation17,Citation22].
To date, growing evidence supports the involvement of inflammation in DVT initiation and resolution [Citation23–25]. Moreover, several inflammatory biomarkers have been found to be associated with DVT, including the neutrophil-to-lymphocyte ratio (NLR) [Citation26], monocyte-to-lymphocyte ratio (MLR) [Citation27], and systemic inflammatory index (SII) [Citation28]. These studies have shown promising results; however, none of these biomarkers has reached consensus conclusions with good clinical guidelines [Citation29]. More recently, the systemic inflammation response index (SIRI) has been considered a potential biomarker of systemic inflammation, and it results from a combination of NLR and MLR [Citation30]. Thus, this study hypothesized that SIRI levels are associated with the risk of LEDVT in hospitalized patients.
To our knowledge, existing research focusing on the relationship between SIRI and DVT risk is limited [Citation27,Citation31]. Importantly, the sample sizes of these two studies were small, and the robustness of this association was not tested in large-scale studies, thereby limiting its clinical application [Citation29]. Therefore, the first aim of this study was to explore the association between SIRI and LEDVT risk in a large sample over a 10-year period (2012–2022). Furthermore, a nonlinear relationship between SIRI and cardiovascular mortality has been described [Citation30]; however, to date, no study has explored the dose-response relationship between SIRI and LEDVT risk. Accordingly, the second aim was to investigate whether this association was linear or nonlinear.
Materials and methods
Study population
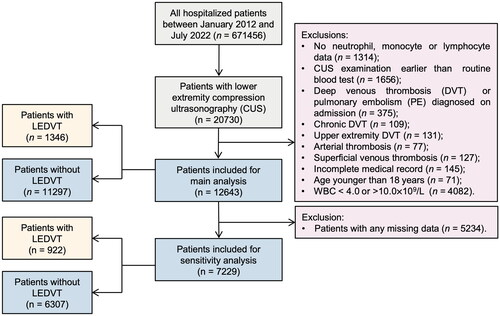
This retrospective observational study was conducted at the People’s Hospital of Deyang City, a 1838-bed tertiary teaching hospital in southwest China that provides comprehensive medical services to approximately 3.5 million residents. The study protocol was reviewed and approved by our Institutional Review Board (No. 2021-04-019-K01). The requirement for patient informed consent was waived because of the retrospective and anonymous nature of the study. The study population has been described in our previous study [Citation28]. Briefly, all medical and surgical hospitalized patients undergoing lower extremity compression ultrasonography (CUS) examinations, between 1 January 2012 and 31 July 2022, were consecutively identified from our hospital information system (HIS) database. Subsequently, patients were excluded if they met any of the following criteria: 1) no neutrophil, monocyte, or lymphocyte data; 2) CUS examination earlier than routine blood test; 3) DVT or PE diagnosed on admission; 4) chronic DVT; 5) upper extremity DVT; 6) arterial thrombosis; 7) superficial venous thrombosis; 8) incomplete medical records; and 9) age younger than 18 years. Since significantly elevated or decreased white blood cell (WBC) counts may indicate a current infection, patients with leukocytosis (> 10.0 × 109/L) or leukopoenia (< 4.0 × 109/L) were further excluded to avoid the effect of acute infection on the inflammatory status [Citation32]. The flowchart of the study is presented in . Ultimately, 12643 patients were included in the main analyses.
Data collection
All data were extracted from electronic medical records, including patient characteristics (age, sex, body mass index [BMI], smoking, and drinking history), comorbidities, and laboratory findings on admission. Obesity was defined as BMI ≥28.0 kg/m2, using the Working Group on Obesity in China criteria [Citation33]. Smoking and drinking statuses were classified as never, former, or current. Comorbidities were assessed using the International Classification of Diseases-10 (ICD-10) for discharge diagnoses, including hypertension (codes: I10-I13, I15), diabetes mellitus (codes: E11-E14), chronic obstructive pulmonary disease (COPD; codes: J42-J44), atrial fibrillation (code: I48), heart failure (code: I50), stroke (codes: I60-I64), hepatic insufficiency (codes: K72.0-K72.1, K72.9), renal insufficiency (codes: N17-N19), and cancer (codes: C00-C97, D00-D09) [Citation34].
At our institute, peripheral venous blood samples were routinely collected from patients on admission or the second day after admission. Immediately thereafter, routine blood tests were performed using automatic haematology analyzers (Sysmex XN2000, Kobe, Japan). The SIRI was calculated using the first blood tests after admission: SIRI = neutrophil (reference range: 1.8–6.3 × 109/L) × monocyte (reference range: 0.1–0.6 × 109/L)/lymphocyte (reference range: 1.1–3.2 × 109/L). The SIRI unit is expressed as 109/L. In this study, patients were categorized into four groups based on SIRI quartiles: Q1: < 0.84, Q2: 0.84–1.38, Q3: 1.39–2.43, Q4: ≥ 2.44. Moreover, several hematological indicators closely associated with DVT were collected, including WBC (reference range: 3.5–9.5 × 109/L) [Citation35], red blood cells (RBC, reference range: 3.8–5.1 × 109/L) [Citation36], haemoglobin (reference range: 115.0-150.0 g/L) [Citation37], and platelets (reference range: 101.0–320.0 × 109/L) [Citation38].
Study outcomes
The primary outcome was the association between SIRI and all LEDVT events during the hospital stay, which were confirmed by CUS reports and discharge diagnoses (codes: I80.1, I80.2, I80.3, I80.8, I80.9, I82.8 and I82.9). Distal and proximal LEDVT often differ substantially in terms of demographics, risk factors, comorbidities, mortality and inflammation level [Citation39,Citation40]. Therefore, the secondary outcomes were the association between SIRI and distal LEDVT (thrombus below the popliteal vein) and proximal LEDVT (thrombus in the popliteal vein and/or above). As LEDVT is thought to progress from the distal to the proximal location, patients with both distal and proximal thrombi were regarded as having proximal LEDVT [Citation41].
Statistical analysis
Prior to the analysis, missing values were detected, and 31.3% of obesity (n = 3961), 4.3% of smoking status (n = 545), and 15.5% of drinking status (n = 1964) were missing. To address this missing data, the missing indicator method was used to create a separate category [Citation42].
For continuous variables, normality was assessed using the Shapiro-Wilk test, and none of the variables followed a normal distribution. Continuous variables are presented as median (interquartile range [IQR]), whereas other categorical variables are reported as numbers (percentages). Differences among the SIRI quartile groups were compared using the Wilcoxon rank-sum test or Pearson’s chi-squared test. In addition, the SIRI levels between patients with and without LEDVT were visualized using a violin plot.
Before multivariate analyses, the linearity assumption was checked using the Box-Tidwell test [Citation43], and continuous variables (age, WBC, RBC, haemoglobin, and platelet) were violated. These data were transformed into categorical variables using receiver operating characteristic (ROC) curve analyses (Supplementary Table 1, Supplementary Figure 1). On the other hand, multicollinearity between independent variables was evaluated by variance inflation factor (VIF), with a VIF ≥5 indicating multicollinearity [Citation44]. No multicollinearity was observed among the variables (Supplementary Table 2).
The association between the SIRI and LEDVT risk was examined using a series of multivariate logistic regression models. Model 1 was adjusted for patient characteristics (age, sex, obesity, smoking, and drinking status); model 2 was adjusted for patient characteristics and comorbidities (hypertension, diabetes mellitus, chronic obstructive pulmonary disease, atrial fibrillation, heart failure, stroke, hepatic insufficiency, renal insufficiency and cancer); and model 3 was adjusted for patient characteristics, comorbidities and laboratory findings (WBC, RBC, haemoglobin and platelet). The SIRI was included in these models in the form of categorical and continuous variables. The lowest quartile (Q1) was used as the reference. For trend tests, the median value was assigned to each category (Q1: 0.61, Q2: 1.08, Q3: 1.80, Q4: 3.71) and modelled as a continuous variable. Moreover, we performed sensitivity analyses to determine the effects of missing data on observed associations. Odds ratios (OR) and 95% confidence intervals (CI) were calculated to estimate the risk of developing LEDVT.
To further explore the dose-response relationship between SIRI and LEDVT risk, restricted cubic spline (RCS) regression with five knots was applied. Nonlinearity was tested using a likelihood ratio test, comparing the spline model to a linear model. The inflection point was estimated using two-piecewise linear regression. Finally, we evaluated the association in subgroups stratified by age, obesity, smoking, diabetes mellitus, atrial fibrillation, heart failure, stroke, hepatic insufficiency, cancer, WBC, RBC, haemoglobin, and platelet, because these factors were found to be significantly associated with LEDVT (Supplementary Table 3). Potential interactions between the stratification factors and SIRI were also tested. Considering the sample size of each subgroup, subgroup analyses were limited to the primary outcome only and adjusted for all variables, except for the one defining the subgroup.
All reported P values were two-sided, and p < 0.05. All analyses were performed using the JMP Pro software (version 16.0.0; SAS Institute Inc., Cary, NC, USA) and GraphPad Prism (version 9.1.1; GraphPad Software, San Diego, California, USA).
Results
Patient characteristics
summarizes the patient characteristics. The median age was 66.0 years and 52.5% were men. Among the comorbidities, diabetes mellitus (40.5%) and hypertension (40.0%) were the most common. Of these patients, 1346 (10.6%) were diagnosed with ultrasound-confirmed LEDVT, including 1018 (75.6%) with distal and 328 (24.4%) with proximal LEDVT. The median SIRI level was 1.39 (0.84, 2.44), and the median time from CUS examination to routine blood test was 3.03 (1.25, 5.65) days. Overall, the differences among the SIRI quartiles were statistically significant for all the patient characteristics (p < 0.01). In addition, patients in the higher quartiles of SIRI exhibited a higher incidence of LEDVT (p < 0.001). Consistently, patients with LEDVT had a significantly higher SIRI level than those without LEDVT (2.08 [1.26, 3.61] vs. 1.33 [0.81, 2.30], p < 0.001, ). This elevation was also observed in patients with distal (2.00 [1.20, 3.53], p < 0.001) and proximal LEDVT (2.36 [1.39, 3.73], p < 0.001).
Figure 2. Violin plot of SIRI levels in different groups. The dashed lines within each violin indicate medians, and solid lines indicate interquartile range. SIRI, systemic inflammation response index; LEDVT, lower extremity deep venous thrombosis.
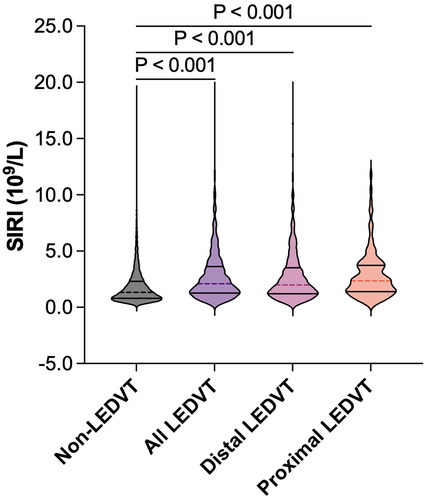
Table 1. Patient characteristics according to quartiles (Q1-Q4) of systemic inflammation response index (SIRI).
Association between SIRI and LEDVT risk
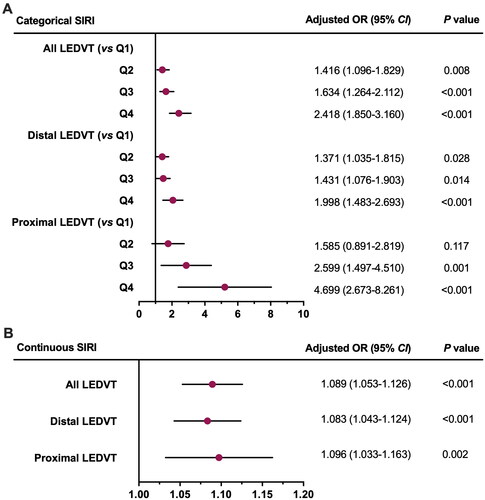
As shown in , a higher SIRI level (continuous variable) was significantly associated with an increased risk of LEDVT in the crude model. After multivariate adjustment, the results remained robust and statistically significant, with model 1 (OR = 1.168, 95% CI: 1.141–1.195, p < 0.001), model 2 (OR = 1.148, 95% CI: 1.121–1.176, p < 0.001), and model 3 (OR = 1.098, 95% CI: 1.068–1.128, p < 0.001). Similar results were observed when the SIRI was modelled as a categorical variable (p ≤ 0.001). In terms of trends, LEDVT risk increased across the SIRI quartiles, especially in the fourth quartile, and the tests for trend reached significance (p < 0.001). Moreover, a positive association between SIRI (either as a continuous or categorical variable) and distal and proximal LEDVT risk still existed.
Table 2. Association between SIRI and LEDVT risk.
After excluding 5234 patients with missing data, 7229 patients were available for further analyses, including 716 (9.9%) with distal and 206 (2.9%) with proximal LEDVT (). The results of sensitivity analyses were consistent with the main findings ().
Figure 3. Forest Plots of sensitivity analysis for the association between SIRI and LEDVT risk. SIRI was included in multivariate analysis as a categorical variable (A) or continuous variable (B). dots and horizontal lines represent adjusted odds ratio (or) and 95% confidence interval (CI), respectively. SIRI, systemic inflammation response index; LEDVT, lower extremity deep venous thrombosis.
Dose-response relationship between SIRI and LEDVT risk
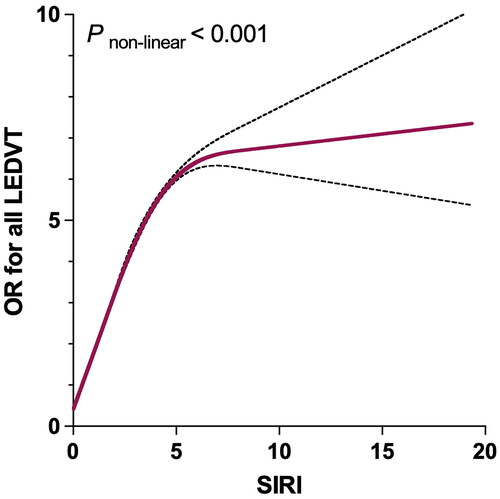
As seen in , the relationship between SIRI and LEDVT risk was nonlinear (P for nonlinearity < 0.001). More specifically, the LEDVT risk increased significantly with increasing SIRI level, reached a positive maximum, and then slowly increased. The inflection point identified using the two-piecewise linear regression model was 4.17. Below this point, each unit increase in SIRI corresponded to a 35.3% increase in LEDVT risk (95% CI: 1.255–1.458, p < 0.001). However, no significant difference was observed above the inflection point (OR = 1.015, 95% CI: 0.963–1.069, p = 0.582).
Figure 4. Restricted cubic spline plot between SIRI and adjusted odds ratio (or) of all LEDVT events. The associations (ORs) were adjusted for patient characteristics (age, sex, obesity, smoking and drinking status), comorbidities (hypertension, diabetes mellitus, chronic obstructive pulmonary disease, atrial fibrillation, heart failure, stroke, hepatic insufficiency, renal insufficiency and cancer), and laboratory findings (white blood cell, red blood cell, haemoglobin and platelet). SIRI, systemic inflammation response index; LEDVT, lower extremity deep venous thrombosis.
Subgroup analyses
To further verify the robustness of the results, a series of subgroup analyses were performed (). Consistent with the main findings, patients with a high SIRI (≥ 4.17) had significantly higher adjusted ORs in nearly all subgroups (OR range: 1.314–2.280, p < 0.05), except for patients with atrial fibrillation (p = 0.397) and heart failure (p = 0.420). In addition, the interactions between SIRI, heart failure, and RBC count were significant (p for interaction < 0.01).
Table 3. Subgroup analysis of the association between SIRI and LEDVT risk.
Discussion
In this large sample study of 12643 hospitalized patients, even after multivariate adjustment for patient characteristics, comorbidities, and laboratory findings, each increase in SIRI level was associated with a 9.8% increase in LEDVT risk. To further verify the observed trend, patients were categorized into four groups based on SIRI quartiles, and patients in Q2, Q3 and Q4 had a 1.419-fold, 1.789-fold and 2.563-fold higher risk of LEDVT when compared with those in Q1. Moreover, the tests for trends reached significance. Consistent with this finding, the existing studies divided patients into high and low SIRI groups according to ROC analysis, and reported that patients with high SIRI had a 9.28-fold higher risk of DVT in COVID-19 patients and a 10.86-fold increased risk of DVT in patients undergoing total knee arthroplasty [Citation27,Citation31]. In addition, a high SIRI level is significantly associated with left atrial thrombus in patients with valvular atrial fibrillation [Citation45], myocardial infarction and stroke [Citation46,Citation47]. However, the diagnostic efficiency of SIRI for DVT was not high, with an AUC of 0.650, sensitivity of 72.9%, specificity of 49.8%, and accuracy of 52.2% (Supplementary Figure 1). This implies that SIRI alone is not sufficient for clinical diagnosis, and other clinical indicators should be combined to improve diagnostic performance. Given that SIRI is a readily available biomarker in clinical settings, the additional diagnostic value of combining SIRI with other biomarkers is still worth exploring.
Considering the obvious differences between distal and proximal LEDVT [Citation39,Citation40], we further explored the association based on the thrombus location. Consistently, elevated SII levels (categorical or continuous variables) were significantly associated with a higher risk of distal and proximal LEDVT. For instance, patients in the highest quartile of SIRI had a 2.101-fold and 4.734-fold increased risk of distal and proximal LEDVT, respectively, compared with those in the lowest quartile. To the best of our knowledge, except for our previous study [Citation28], no prior study has investigated the association between any inflammation biomarker and distal or proximal LEDVT risk. In addition, sensitivity and subgroup analyses yielded similar findings, suggesting that the association was robust and reliable.
Moreover, RCS analysis showed the association to be nonlinear, which was first reported for LEDVT. More specifically, the LEDVT risk increased significantly with increasing SIRI level, reached a positive maximum, and then slowly increased. A similar nonlinear relationship was also observed between SIRI and all-cause mortality, and cardiovascular mortality [Citation30]. In this study, the inflection point identified using a two-piecewise linear regression model was 4.17, which was within the range of 3.25 to 6.91 reported in the literature [Citation27,Citation31]. Below this point, the LEDVT risk increased by 35.3% for each unit increment in SIRI. Of note, above this point, there was a trend for an increased risk of LEDVT, but this was not statistically significant. A possible reason for this may be the following: this study excluded patients with leukocytosis (WBC > 10.0 × 109/L) and led to a relatively small sample size (n = 1234) and LEDVT events (n = 256) above the inflection point, which may limit the statistical power.
Although the mechanism for this association is unclear, this study provides robust clinical evidence of a potential link between inflammation and DVT. A strong link has been established between venous thromboembolism (VTE) and inflammatory diseases (e.g. ulcerative colitis, Crohn’s disease, rheumatoid arthritis, and psoriatic arthritis) [Citation48]. In turn, a recent study showed that consumption of foods with high anti-inflammatory potential exhibited a lower risk of VTE in current and past smokers [Citation49]. Recently, some researchers have proposed that prevention and treatment of VTE should include both anticoagulant and anti-inflammatory agents, and subtherapeutic doses of both drugs may improve the efficacy of VTE management without increasing the risk of bleeding [Citation17,Citation22,Citation50].
This study has several strengths. First, the relatively large sample size provided sufficient statistical power to detect any potential associations. Moreover, the study population was consecutively included and derived from a CUS examination population, thereby reducing the potential for selection bias. Second, all thrombotic events were confirmed by CUS examinations, thus ensuring reliable identification of thrombus location (distal and proximal LEDVT). Third, a series of statistical analyses (e.g. multivariate, sensitivity, and subgroup analyses) were conducted and yielded similar results, indicating that the results were stable and reliable.
However, this study has certain limitations. First, this was an observational study, and the results can only imply an association and not causation. In addition, this was a retrospective study with inherent limitations, some important variables related to VTE could not be accessed, including other risk factors (e.g. prior VTE, immobility, trauma), thrombosis risk assessment models (e.g. Padua, Caprini, IMPROVE), anticoagulation and antiplatelet use before hospital admission. Also, drug treatment (e.g. anticoagulants, antiaggregants, antibiotics, antiviral drugs, non-steroidal anti-inflammatory drugs) after admission were not obtained due to lack of detailed medication regimen in the database. Further prospective studies are required to confirm our findings. Second, this was a single-center study in China, and the study population was restricted to hospitalized patients, which may have limited the generalizability of the results. Third, SIRI level is prone to change over time, and its dynamic status has been found to be associated with risk of cardiovascular diseases [Citation46]. In this study, the SIRI level was assessed only once at admission and its fluctuations were not measured or analyzed during hospitalization. This may have led to underestimation of the association.
Conclusions
This study revealed that a high SIRI is significantly associated with an increased risk of LEDVT in hospitalized patients. Given that the SIRI is a readily available biomarker in clinical settings, its potential clinical use deserves further exploration.
Ethical approval and consent to participate
Studies involving human participants were reviewed and approved by the Institutional Review Board of the People’s Hospital of Deyang City (No. 2021-04-019-K01). The requirement for patient informed consent was waived because of the retrospective and anonymous nature of the study.
Authors’ contributions
ZW conceived and designed the study. YL and ML collected the data. HL, XC, and ZW performed statistical analyses. HL and XC wrote the manuscript. ZW supervised the study and approved the final manuscript. All authors have contributed to the manuscript and approved the submitted version.
Acknowledgements
We thank Xiaohong Qin from Shanghai ClinBrain Information Technology Co., Ltd., for assistance with data extraction, and Yuhang Cai for assistance with data cleaning.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data generated and/or analysed during the current study are available upon request by the corresponding author.
Additional information
Funding
References
- Chopard R, Albertsen IE, Piazza G. Diagnosis and treatment of lower extremity venous thromboembolism: a review. JAMA. 2020;324(17):1–11. doi: 10.1001/jama.2020.17272.
- Heit JA, Melton LJ, 3rd, Lohse CM, et al. Incidence of venous thromboembolism in hospitalized patients vs community residents. Mayo Clin Proc. 2001;76(11):1102–1110. doi: 10.4065/76.11.1102.
- Bjøri E, Johnsen HS, Hansen JB, et al. Hospitalization as a trigger for venous thromboembolism - Results from a population-based case-crossover study. Thromb Res. 2019;176:115–119. doi: 10.1016/j.thromres.2019.02.024.
- Mehta KD, Siddappa Malleshappa SK, Patel S, et al. Trends of inpatient venous thromboembolism in United States before and after the surgeon general’s call to action. Am J Cardiol. 2019;124(6):960–965. doi: 10.1016/j.amjcard.2019.06.015.
- Neeman E, Liu V, Mishra P, et al. Trends and risk factors for venous thromboembolism among hospitalized medical patients. JAMA Netw Open. 2022;5(11):e2240373. doi: 10.1001/jamanetworkopen.
- Hwang HG, Lee JH, Kim SA, et al. Incidence of venous thromboembolism: the 3rd Korean nationwide study. J Korean Med Sci. 2022;37(17):e130. doi: 10.3346/jkms.2022.37.e130.
- Zhang Z, Lei J, Shao X, et al. Trends in hospitalization and in-hospital mortality from VTE, 2007 to 2016, in China. Chest. 2019;155(2):342–353. doi: 10.1016/j.chest.2018.10.040.
- Izcovich A, Criniti JM, Popoff F, et al. Thrombolytics for venous thromboembolic events: a systematic review with meta-analysis. Blood Adv. 2020;4(7):1539–1553. doi: 10.1182/bloodadvances.2020001513.
- Wang X, Ma Y, Hui X, et al. Oral direct thrombin inhibitors or oral factor Xa inhibitors versus conventional anticoagulants for the treatment of deep vein thrombosis. Cochrane Database Syst Rev. 2023;4(4):Cd010956. doi: 10.1002/14651858.CD010956.pub3.
- Broderick C, Watson L, Armon MP. Thrombolytic strategies versus standard anticoagulation for acute deep vein thrombosis of the lower limb. Cochrane Database Syst Rev. 2021;1(1):Cd002783. doi: 10.1002/14651858.CD002783.pub5.
- Malhotra K, Bawa A, Goyal K, et al. Global impact of deep vein thrombosis awareness month: challenges and future recommendations. Eur Heart J. 2022;43(36):3379–3381. doi: 10.1093/eurheartj/ehac252.
- ISTH Steering Committee for World Thrombosis Day. Thrombosis: a major contributor to the global disease burden. J Thromb Haemost. 2014;12(10):1580–1590. doi: 10.1111/jth.12698.
- Ortel TL, Neumann I, Ageno W, et al. American society of hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism. Blood Adv. 2020;4(19):4693–4738. doi: 10.1182/bloodadvances.2020001830.
- Neumann I, Izcovich A, Aguilar R, et al. ASH, ABHH, ACHO, grupo CAHT, grupo CLAHT, SAH, SBHH, SHU, SOCHIHEM, SOMETH, sociedad panameña de hematología, SPH, and SVH 2021 guidelines for management of venous thromboembolism in Latin America. Blood Adv. 2021;5(15):3032–3046. doi: 10.1182/bloodadvances.2021004267.
- Tran HA, Gibbs H, Merriman E, et al. New guidelines from the thrombosis and haemostasis society of Australia and New Zealand for the diagnosis and management of venous thromboembolism. Med J Aust. 2019;210(5):227–235. doi: 10.5694/mja2.50004.
- Stevens SM, Woller SC, Kreuziger LB, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. Chest. 2021;160(6):e545–e608. doi: 10.1016/j.chest.2021.07.055.
- Weitz JI, Chan NC. Novel antithrombotic strategies for treatment of venous thromboembolism. Blood. 2020;135(5):351–359. doi: 10.1182/blood.2019000919.
- Ageno W, Bertù L, Bucherini E, et al. Rivaroxaban treatment for six weeks versus three months in patients with symptomatic isolated distal deep vein thrombosis: randomised controlled trial. BMJ. 2022;379:e072623. doi: 10.1136/bmj-2022-072623.
- Sidhu VS, Kelly TL, Pratt N, et al. Effect of aspirin vs enoxaparin on symptomatic venous thromboembolism in patients undergoing hip or knee arthroplasty: the CRISTAL randomized trial. JAMA. 2022;328(8):719–727. doi: 10.1001/jama.2022.13416.
- Planquette B, Bertoletti L, Charles-Nelson A, et al. Rivaroxaban vs dalteparin in cancer-associated thromboembolism: a randomized trial. Chest. 2022;161(3):781–790. doi: 10.1016/j.chest.2021.09.037.
- Becattini C, Pace U, Pirozzi F, et al. Rivaroxaban vs placebo for extended antithrombotic prophylaxis after laparoscopic surgery for colorectal cancer. Blood. 2022;140(8):900–908. doi: 10.1182/blood.2022015796.
- Stark K, Massberg S. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat Rev Cardiol. 2021;18(9):666–682. doi: 10.1038/s41569-021-00552-1.
- Budnik I, Brill A. Immune factors in deep vein thrombosis initiation. Trends Immunol. 2018;39(8):610–623. doi: 10.1016/j.it.2018.04.010.
- Mukhopadhyay S, Johnson TA, Duru N, et al. Fibrinolysis and inflammation in venous thrombus resolution. Front Immunol. 2019;10:1348. doi: 10.3389/fimmu.2019.01348.
- Nicklas JM, Gordon AE, Henke PK. Resolution of deep venous thrombosis: proposed immune paradigms. Int J Mol Sci. 2020;21(6):2080. doi: 10.3390/ijms21062080.
- Selvaggio S, Brugaletta G, Abate A, et al. Platelet‑to‑lymphocyte ratio, neutrophil‑to‑lymphocyte ratio and monocyte‑to‑HDL cholesterol ratio as helpful biomarkers for patients hospitalized for deep vein thrombosis. Int J Mol Med. 2023;51(6):52. doi: 10.3892/ijmm.2023.5255.
- Mureșan AV, Hălmaciu I, Arbănași EM, et al. Prognostic nutritional index, controlling nutritional status (CONUT) score, and inflammatory biomarkers as predictors of deep vein thrombosis, acute pulmonary embolism, and mortality in COVID-19 patients. Diagnostics . 2022;12(11):2757. doi: 10.3390/diagnostics12112757.
- Chen X, Ou Y, Wang Z, et al. Association between systemic immune-inflammation index and risk of lower extremity deep venous thrombosis in hospitalized patients: a 10-year retrospective analysis. Front Cardiovasc Med. 2023;10:1211294. doi: 10.3389/fcvm.2023.1211294.
- Xue J, Ma D, Jiang J, et al. Diagnostic and prognostic value of immune/inflammation biomarkers for venous thromboembolism: is it reliable for clinical practice? J Inflamm Res. 2021;14:5059–5077. doi: 10.2147/JIR.S327014.
- Zhao S, Dong S, Qin Y, et al. Inflammation index SIRI is associated with increased all-cause and cardiovascular mortality among patients with hypertension. Front Cardiovasc Med. 2022;9:1066219. doi: 10.3389/fcvm.2022.1066219.
- Melinte RM, Arbănași EM, Blesneac A, et al. Inflammatory biomarkers as prognostic factors of acute deep vein thrombosis following the total knee arthroplasty. Medicina. 2022;58(10):1502. doi: 10.3390/medicina58101502.
- Wei Y, Wang T, Li G, et al. Investigation of systemic immune-inflammation index, neutrophil/high-density lipoprotein ratio, lymphocyte/high-density lipoprotein ratio, and monocyte/high-density lipoprotein ratio as indicators of inflammation in patients with schizophrenia and bipolar disorder. Front Psychiatry. 2022;13:941728. doi: 10.3389/fpsyt.2022.941728.
- Zhou B, Meta C. Analysis group of China obesity task force. [predictive values of body mass index and waist circumference to risk factors of related diseases in Chinese adult population]. Zhonghua Liu Xing Bing Xue Za Zhi. 2002;23(1):5–10. doi: 10.3760/j.issn:0254-6450.2002.01.003.
- Glise Sandblad K, Rosengren A, Sörbo J, et al. Pulmonary embolism and deep vein thrombosis-comorbidities and temporary provoking factors in a register-based study of 1.48 million people. Res Pract Thromb Haemost. 2022;6(4):e12714. doi: 10.1002/rth2.12714.
- Wang G, Zhao W, Zhao Z, et al. Leukocyte as an independent predictor of lower-extremity deep venous thrombosis in elderly patients with primary intracerebral hemorrhage. Front Neurol. 2022;13:899849. doi: 10.3389/fneur.2022.899849.
- Liu X, Li T, Xu H, et al. Hyperglycemia may increase deep vein thrombosis in trauma patients with lower limb fracture. Front Cardiovasc Med. 2022;9:944506. doi: 10.3389/fcvm.2022.944506.
- Hultcrantz M, Modlitba A, Vasan SK, et al. Hemoglobin concentration and risk of arterial and venous thrombosis in 1.5 million swedish and danish blood donors. Thromb Res. 2020;186:86–92. doi: 10.1016/j.thromres.2019.12.011.
- Edvardsen M, Hansen E, Hindberg K, et al. Combined effects of plasma von Willebrand factor and platelet measures on the risk of incident venous thromboembolism. Blood. 2021;138(22):2269–2277. doi: 10.1182/blood.2021011494.
- Bikdeli B, Caraballo C, Trujillo-Santos J, et al. Clinical presentation and short- and long-term outcomes in patients with isolated distal deep vein thrombosis vs proximal deep vein thrombosis in the RIETE registry. JAMA Cardiol. 2022;7(8):857–865. doi: 10.1001/jamacardio.2022.1988.
- Kuplay H, Erdoğan SB, Bastopcu M, et al. The neutrophil-lymphocyte ratio and the platelet-lymphocyte ratio correlate with thrombus burden in deep venous thrombosis. J Vasc Surg Venous Lymphat Disord. 2020;8(3):360–364. doi: 10.1016/j.jvsv.2019.05.007.
- Ma J, Du P, Qin J, et al. Incidence and risk factors predicting deep venous thrombosis of lower extremity following spinal fractures. Sci Rep. 2021;11(1):2441. doi: 10.1038/s41598-021-82147-x.
- Choi J, Dekkers OM, Le Cessie S. A comparison of different methods to handle missing data in the context of propensity score analysis. Eur J Epidemiol. 2019;34(1):23–36. doi: 10.1007/s10654-018-0447-z.
- Thompson WK, Xie M, White HR. Transformations of covariates for longitudinal data. Biostatistics. 2003;4(3):353–364. doi: 10.1093/biostatistics/4.3.353.
- Kim JH. Multicollinearity and misleading statistical results. Korean J Anesthesiol. 2019;72(6):558–569. doi: 10.4097/kja.19087.
- Zhou Y, Song X, Ma J, et al. Association of inflammation indices with left atrial thrombus in patients with valvular atrial fibrillation. BMC Cardiovasc Disord. 2023;23(1):9. doi: 10.1186/s12872-023-03036-x.
- Li J, He D, Yu J, et al. Dynamic status of SII and SIRI alters the risk of cardiovascular diseases: evidence from kailuan cohort study. J Inflamm Res. 2022;15:5945–5957. doi: 10.2147/JIR.S378309.
- Jin Z, Wu Q, Chen S, et al. The associations of two novel inflammation indexes, SII and SIRI with the risks for cardiovascular diseases and all-cause mortality: a ten-year follow-up study in 85,154 individuals. J Inflamm Res. 2021;14:131–140. doi: 10.2147/JIR.S283835.
- Galloway J, Barrett K, Irving P, et al. Risk of venous thromboembolism in immune-mediated inflammatory diseases: a UK matched cohort study. RMD Open. 2020;6(3):e001392. doi: 10.1136/rmdopen-2020-001392.
- Yuan S, Bruzelius M, Damrauer SM, et al. Anti-inflammatory diet and venous thromboembolism: two prospective cohort studies. Nutr Metab Cardiovasc Dis. 2021;31(10):2831–2838. doi: 10.1016/j.numecd.2021.06.021.
- Poredos P, Poredos P. Involvement of inflammation in venous thromboembolic disease: an update in the age of COVID-19. Semin Thromb Hemost. 2022;48(1):93–99. doi: 10.1055/s-0041-1732372.