Abstract
Background: As a chronic and progressive neurodegenerative disease, Parkinson’s disease (PD) still lacks effective and safe targeted drug therapy. Low-intensity focused ultrasound (LIFU), a new method to stimulate the brain and open the blood-brain barrier (BBB), has been widely concerned by PD researchers due to its non-invasive characteristics.
Methods: PubMed was searched for the past 10 years using the terms ‘focused ultrasound’, ‘transcranial ultrasound’, ‘pulse ultrasound’, and ‘Parkinson’s disease’. Relevant citations were selected from the authors’ references. After excluding articles describing high-intensity focused ultrasound or non-Parkinson’s disease applications, we found more than 100 full-text analyses for pooled analysis.
Results: Current preclinical studies have shown that LIFU could improve PD motor symptoms by regulating microglia activation, increasing neurotrophic factors, reducing oxidative stress, and promoting nerve repair and regeneration, while LIFU combined with microbubbles (MBs) can promote drugs to cross the BBB, which may become a new direction of PD treatment. Therefore, finding an efficient drug carrier system is the top priority of applying LIFU with MBs to deliver drugs.
Conclusions: This article aims to review neuro-modulatory effect of LIFU and the possible biophysical mechanism in the treatment of PD, summarize the latest progress in delivering vehicles with MBs, and discuss its advantages and limitations.
KEY MESSAGES
Neuro-modulatory effects of LIFU at the cellular or molecular level.
Opening the BBB through the combination of LIFU and MBs.
Biophysical mechanism of LIFU.
1. Introduction
The permeability of the blood-brain barrier (BBB) limits many new treatment options for Parkinson’s disease (PD). A new brain stimulation method, low-intensity focused ultrasound (LIFU), is receiving increasing attention due to its neuroregulatory effects and ability to open the BBB when combined with microbubbles (MBs). By using a specially designed transducer, focused ultrasound (FUS) can be formed by aiming ultrasonic (US) waves with a diameter of only a few millimeters at the focal point.
Transcranial FUS can be divided into High-Intensity Focused Ultrasound (HIFU) and LIFU based on intensity. The former refers to the use of intensity greater than 200 W/cm2, while the latter usually refers to the use of intensity less than 100 W/cm2 [Citation1]. In general, ‘low intensity’ is regarded be the magnitude of ultrasound intensity that is similar to or lower than the intensity commonly used in diagnostic examinations in the United States [Citation2]. Many articles refer to this technique as low-intensity pulsed ultrasound (LIPU) [Citation3,Citation4], which penetrates through the skull of the animal and focuses on a particular area of the brain. There are some LIPU transducers that are non-focusing, typically used for in vitro experiments [Citation5]. The spatial resolution of LIFU can reach the millimeter level. It can target structures beneath the cortex down to depths of more than 10 centimeters by using the intact skull [Citation2], which makes it a hot research topic for many diseases such as depression [Citation6], thrombolysis in brain ischemia [Citation7], Alzheimer’s disease (AD) [Citation8] and brain tumors [Citation9].Compared to HIFU, the impact of short-term burst of LIFU on neurons and neuronal circuits is reversible and there are no adverse events [Citation2]. This article provides a review of the clinical applications of FUS and the various preclinical studies of LIFU in the treatment of PD, summarizes the different neuroregulatory effects of LIFU in improving motor symptoms and discusses the research progress in utilizing MBs to open the BBB for the delivery of various drugs.
2. Clinical application of FUS in the treatment of PD
Currently, surgical methods available for the treatment of PD include deep brain stimulation (DBS) [Citation10,Citation11], levodopa carbidopa intestinal gel therapy [Citation12], HIFU ablation of the subthalamic nucleus [Citation13], and other methods, but these methods are highly invasive. At present, HIFU is mainly used clinically for PD, such as MRgFUS (unilateral [Citation14–16] or bilateral [Citation17,Citation18]), and unilateral FUS-STN [Citation19].The clinical efficacy of non-incisional transcranial MRgFUS will ultimately rest in appropriate target selection and optimized thermolesional coverage of the target [Citation16,Citation20]. Evidence on different targets, such as the ventral intermediate nucleus [Citation21,Citation22] and putamen nucleus [Citation16] has shown clear benefits. Other studies combining DBS with MRgFUS have found that mixed surgery improves bilateral essential tremor without any neurological deficits [Citation23]. From the above, different invasive therapies for PD can only be tested in specific patient groups, some of which have strong scientific basis.
LIFU has become a hot spot in many research fields. For example, in the field of neural research, studies have shown that low intensity ultrasound (LIUS) has an inhibitory effect on cytotoxic cerebral edema, suggesting its therapeutic potential in reducing AQP4 localization around astrocyte feet [Citation24]. LIFU-responsive nanomedicine can also shrink vulnerable plaques by triggering the vaporization of perfluorohexane [Citation25] and achieve thrombolysis via an acoustic droplet vaporization effect [Citation26]. In the field of tumor therapy, LIFU’s disruption of microtubules as a detoxifying agent for paclitaxel cytotoxicity in cells [Citation27]. And recently sonodynamic therapy, a combination of LIUS with a chemotherapeutic agent (sonosensitizer) has been explored as a promising alternative to cancer therapies [Citation28]. In the field of stem cell research, LIPU promotes the expansion, differentiation, and migration of mesenchymal stem cells [Citation29,Citation30], and it has been reported that LIFU-induced BBB regulation can promote neurogenesis through the activation of endogenous neural stem cells [Citation31]. In addition, LIPU can produce different biological effects on organs, tissues, and cells, and can be applied in fracture healing, cartilage repair [Citation32], periodontal tissue regeneration [Citation33], and alveolar bone regeneration [Citation34]. In human studies, transcranial FUS inhibits the brain electrical activity evoked by somatosensory evoked potentials (SEPs) induced by median nerve stimulation in the primary somatosensory area [Citation35,Citation36]. Combining LIFU, tLyP-1-10-HCPT-PFP NPs can undergo phase transition to phase transition to microbubbles, enhancing the tumor ultrasound molecular imaging for tumor diagnosis [Citation37]. And LIFU has been shown to safely and noninvasively mediate BBB opening in the human hippocampus/EC region [Citation38]. To date, a large amount of research evidences (including in vivo sensory and behavioral responses [Citation39], electrophysiological records [Citation40], and functional imaging data [Citation41]) have emerged and confirmed that LIFU can enhance or suppress neuronal activity and modulate the oscillations of neural networks in animals and humans. LIFU under certain conditions has been shown to reversibly permeabilize the BBB, without ablation, suggesting that LIFU-induced BBB disruption might provide an avenue for the delivery of drugs, antibodies, nanoparticles, gene therapy, and even cells to the brain, providing increasing the range of options for therapeutic macromolecules that can be used to treat PD [Citation42,Citation43]. However, there is still a lack of clinical therapeutic evidence of non-invasive targeted drug delivery through the BBB by LIFU for the treatment of PD.
3. Optimal sonication parameters in LIFU
Ultrasound has been shown to be an effective neuromodulator in rodents [Citation44], monkeys [Citation45] and humans [Citation46], ultrasound that is below FDA standards has the potential to create a noninvasive treatment for patients with PD who are unwilling or unfit for routine surgery [Citation47]. In 2016, Lee and colleagues used ultrasound energy doses above the limits previously described by the FDA to stimulate the V1 site in humans, underscoring the safety and accuracy of FUS [Citation48]. Downs et al. used FUS to open the BBB in the shell and caudate nucleus of primates, monitoring safety through vital signs, physiological changes, MRI analysis, and behavioral testing. No evidence of bleeding was found, the edema in a few subjects was also completely reversible [Citation1]. Unlike HIFU, none of the studies using LIFU showed any problems with histology, the BBB, or behavioral data [Citation49]. There were few signs of apoptosis or ischemia when LIFU destroyed the BBB, and only mild inflammatory reaction was found in the lesion. As with any emerging technology, long-term follow-up security research remains to be done. Clinically, FUS has the advantage of being non-invasive, but it also has the disadvantage that ultrasound energy will be attenuated by skull, resulting in the difference of energy efficiency between different patients. Different acoustic parameters can induce a variety of biological effects, depending on the cell type and tissue structure [Citation50].There is still a gap in our knowledge of the mechanism and optimal spatiotemporal parameters of ultrasound-induced stimulation in vivo. Currently, there are no clear display standards for the parameters used in LIFU treatment for PD as reported in the text, leading to a wide variety of types being used. The most commonly used ultrasound parameters are sound intensity, fundamental frequency (FF), pulse repetition frequency (PRF), duty cycle (DC), burst duration (TBD), and stimulation duration (SD) [Citation5,Citation51–53] (). There are three main types of sound intensity, namely the spatial peak pulse average intensity (Isppa), the spatial peak time average intensity (Ispta) and the spatial average time average intensity (Isata). Ispta is the best measure of heat delivered to tissue by ultrasound. The Food and Drug Administration in the United States, along with other regulatory agencies, has clearly defined the conditions for exposure to ultrasonic waves to ensure safety [Citation54]. As follows, the thermal index (TI) should generally be maintained below 6.0, the mechanical index (MI) should not exceed 1.9, the maximum limit of Ispta should be below 720 mW/cm2, and Isppa should be below 190 mW/cm2. According to Kim and colleagues, the optimal ‘sweet spot’ for rat neuroregulatory experiments is DC of 50% and SD of 300 ms [Citation55]. In vivo experiments have shown that low frequency (500KHz, 800KHz), relatively low DC voltage (5%, 10%, 20%), and greater variations in SD (50 ms, 6s) are necessary to produce neuro-regulation effects in C57BL/6 mice through LIFU stimulation [Citation51,Citation52,Citation56].
Table 1. Summary of the neuro-modulatory effects of LIFU/LIPU in PD model.
But Hui Zhou and her team treated PD mices with higher ultrasound frequencies (3.8 MHz) and SD (50%) to improve motor symptoms and exert neuro-modulatory effects [Citation57]. In vitro studies, low intensity ultrasound (LIU) (50mW/cm2, 100mW/cm2) can alleviate parkinsonian mimetic MPP(+)-induced neurotoxicity in PC12 cells, using FF of 1 MHz, DC of 20% [Citation4,Citation5,Citation53]. The proportion of the effect of neural modulation is closely related to the intensity, frequency, SD, and DC of FUS stimulation, and further related research is needed to determine the optimal ‘sweet spot’ for treating PD.
4. Potential neuro-modulatory effects of LIFU at the cellular or molecular level
4.1. Release and expression of neurotrophic factors
Neurotrophic factors are a category of proteins that play important roles in the development, survival, and apoptosis of neurons, and are considered potential therapeutic targets for neurodegenerative diseases such as PD, AD, and Huntington’s disease [Citation58]. Tyler et al. showed that focusing the LIPU on deep brain regions can stimulate intact brain circuits and the activity of endogenous brain-derived neurotrophic factor (BDNF) with potential therapeutic effects [Citation59]. Lins et al. found in an in vivo rat model of AD that LIPU (1 MHz FF, 1 Hz PRF, 50 ms SD, 5% DC) significantly increased the expression of BDNF, glial-derived neurotrophic factor (GDNF), and vascular endothelial growth factor in the lateral hippocampus after 15 min of stimulation [Citation60]. Astrocytes play a protective and even regenerative role in developing neurons by secreting neurotrophic factors [Citation61]. In vitro experiments have shown that LIPU (1 MHz FF, 10 Hz PRF, 50 ms SD, 50% DC) can directly stimulate astrocytes and increase the level of neurotrophic factors [Citation3]. Moreover, in vivo experiments have demonstrated that ultrasound (1 MHz FF, 1 Hz PRF, 50 ms SD, 5% DC) activates NF-κB in astrocytes through the TrkB/PI3K/Akt and calcium/CaMK signaling pathways, thereby enhancing the expression of BDNF [Citation62]. LIPU (1 MHz FF, 100 Hz PRF, 30 or 50 mW/cm2 Ispta, 20% DC, 10 min per day) can also activate the PI3K/Akt and erk-creb-trx-1 signaling pathways through ion channels activated by stretching, enhancing Neurotrophic Factor-induced neuronal axon growth, and this method can be used to treat neurodegenerative diseases [Citation4]. Repetitive transcranial ultrasound stimulation can modulate the human motor cortex [Citation63], while LIFU can safely modulate the neural circuits within the central nervous system (CNS) associated with movement-related clinical targets such as the motor cortex-spinal cord and thalamocortical pathways [Citation64]. The motor cortex, substantia nigra, and striatum participate in basal ganglia circuits that influence the modulation of behavioral responses [Citation65]. LIFU stimulates astrocytes in the subthalamic nucleus (STN)-motor cortex to promote the expression and release of neurotrophic factors through corresponding signaling pathways. Further research is needed to determine which specific areas of the brain are stimulated by LIFU and which signaling pathways promote the expression of neurotrophic factors.
4.2. Bidirectional regulation of neuroinflammation by microglia
Neuroinflammation has long been associated with CNS disorders, including PD and multiple system atrophy in alpha-synucleosis, but its key role in pathogenicity is not yet fully understood [Citation66]. In the rat model of cerebral ischemic injury, the use of LIPU (1.5 kHz PRF, Isata 86mW/cm2) can alleviate brain inflammation [Citation67]. Under low intensity and low frequency ultrasound radiation (27 kHz FF, 0.25 W/cm2), endothelial cells can achieve vasodilation and anti-inflammatory effects by upregulating eNOS and increasing the production of NO. However, this effect is transient and reversible [Citation68]. Microglia are resident macrophages in the brain with neuroprotective and neurotoxic functions, and their activity in CNS is considered the most important neuroinflammatory signature marker in PD [Citation69]. Zhou et al. performed continuous ultrasound deep brain stimulation on the STN or GP for 5 weeks after MPTP formation (3.8 MHz FF, 1 kHz PRF, 50% DC, 30 min/day). The results demonstrated for the first time that US improved motor function and reduced chronic inflammatory responses of microglia and astrocytes [Citation57]. The results were consistent with the study by Chen et al. suggesting that DBS of STN can inhibit microglial activation in SN and normalize the expression of NF-κB and IL-1β in an animal model of PD [Citation70]. On the contrary, MRI-guided pulsed focused ultrasound (589.636 kHz FF, 1% DC) combined with ultrasound contrast agent MBs can lead to a sustained increase in IL-1, IL-18, TNFα, and heat shock protein 70 for 24 h, and activated astrocytes and microglia increased after 24 h [Citation71]. After the ultrasound treatment combined with MBs, the expression of anti-inflammatory cytokines (IL4, IL10, and IL13) in the CNS was increased. This can limit the degree of inflammation and demonstrate a new model for regulating brain inflammation, thus promoting the survival of neurons and inducing apoptosis in microglial cells [Citation72–74]. Interestingly, activated microglia clear dead cells from the CNS and secrete a variety of neurotrophic factors to promote neuronal survival [Citation75]. Bobola et al. performed transcranial focused ultrasound (tFUS) (2.0 MHz FF, 40 Hz PRF, 400 ms pulse, 190 W/cm2 Isppa) on 5XFAD mice in a sedated state, and the activation of microglia coexisting with Aβ plaques and the removal of nearly 50% of Aβ plaques by 5-day tFUS [Citation76]. Therefore, it remains controversial whether FUS improves PD motor function by inhibiting or activating microglia activation.
4.3. Reduce ROS production and oxidative stress
Mitochondrial dysfunction and oxidative stress are also considered as one of the pathogenesis of PD. The pathological and clinical manifestations of MPTP in humans, monkeys and mice are consistent with clinical and idiopathic Parkinson’s syndrome [Citation77]. MPTP is converted to MPP + by the catalysis of monoamine oxidase B, which accumulates in DAergic neurons through the DA reuptake pathway and then actively transports into mitochondria, selectively inhibiting the activity of NADHCoQ reductase complex I in the respiratory chain and interfering with ATP synthesis. In in vivo experiments of Parkinson’s MPTP model, LIFU stimulation of the motor cortex for 7 days (800 kHz FF, 100 Hz PRF, 10%DC, 40 min/day) can reverse the reduced levels of T-SOD and GSH-PX caused by MPTP, and improve Parkinson’s motor dysfunction by exerting an antioxidative effect [Citation52]. In the rat model of brain injury induced by AlCl3, LIFU (1 Hz FF, 528 mW/cm2 Ispta, 5% DC, 50 ms SD) also restored the antioxidant enzyme activity decreased by AlCl3, as well as the increased SOD, GSH and GSH-Px activity, thus reducing the oxidative stress in the hippocampus treated with AlCl3 [Citation78]. According to reports, in vitro PC12 cells induced by MPP+, LIPU (1 MHz FF, 100 Hz PRF, 50 mW/cm2 Ispta, 20% DC, 10 min) [Citation5] or LIFU (30, 50 and 100 mW/cm2) [Citation53] can inhibit the production of ROS and mitochondrial dysfunction in PC12 cells. The mechanism of action is the regulation of antioxidant proteins or the enhancement of mitochondrial complex I activity. Mitochondrial complex I deficiency has been widely demonstrated in the brains of PD patients [Citation79]. However, this view is also controversial. Some articles have shown that the death of dopaminergic neurons induced by rotenone, MPP + or paraquat does not require inhibition of mitochondrial complex I [Citation80]. Normal mitochondrial membrane potential is a prerequisite for maintaining mitochondrial function [Citation81]. LIPU (1 MHz FF, 1000 Hz PRF, 20% DC, 10 min) also inhibits the reduction of mitochondrial membrane potential caused by MPP+ [Citation56]. Whether LIFU protects dopaminergic neurons by reducing MPP + inhibition of mitochondrial complex 1 activity or maintaining mitochondrial membrane potential warrants further studying.
4.4. Promotes neuron regeneration and repair
The most intuitive therapeutic effect of LIFU is the improvement of neural plasticity. This may be because, at the molecular level, ultrasound can provide a favorable microenvironment for neural regeneration and repair by promoting gradients of local cell factors, growth factors, and adhesion molecules. Many experiments have shown that low frequency ultrasound (LFU) can affect the regeneration and repair of nerves in vitro and in vivo [Citation82,Citation83]. Xu et al. showed that after 10-day US treatment at the intensity of 0.3 W/cm2, neuronal repair occurred in the bilateral substantia nigra section, and the vacuoles caused by MPTP-induced nerve necrosis were significantly reduced [Citation47]. And they believed that the release of dopamine induced by LIU can be attributed to the combination of neuronal regeneration and improved membrane permeability produced by ultrasound mechanics [Citation84].Moreover, post-exposure neurite outgrowth and neuritogenesis can be respectively observed after LIFU stimulation (500 Hz PRF, 1.168 W/cm2 Ispta, 0.84 MPa peak acoustic pressure, 100 cycle pulse duration) in neurite-bearing and neurite-less neuronal cells [Citation85]. Some reports suggest that the application of LIPU can accelerate the regeneration of peripheral nerves, including Schwann cells (SCs) and damaged nerves, promote the expression of the NT-3 gene, and involve changes in the expression of the BDNF gene [Citation86,Citation87]. Random treatment of autologously transplanted peripheral nerves with LIPU of 250 mW/cm2, 500 mW/cm2, or 750 mW/cm2, functional and pathological results showed that LIPU of 250 mW/cm2 significantly induced faster axonal regeneration rates [Citation88]. LIPU (1 MHz FF, 0.3 W/cm2 Ispta, 5 min daily) can also promote the permeability and selectivity of cell membranes and cell walls, increase the absorption of nutrients, thereby improving the survival and proliferation rate of SCs, which is crucial for peripheral nerve regeneration [Citation82]. In addition, researchers have found that LIPU can induce nerve growth and regulate the production of nitric oxide, promote the differentiation of neural stem/progenitor cells into neurons, enhance the vitality and proliferation of iPSCs-derived neural crest stem cells (iPSCs-NCSC), and affect the multipotent differentiation of mesenchymal stem and progenitor cell lineages through the ROCK-Cot/Tpl2-MEK-ERK signaling pathway [Citation89]. Lv et al. indicated that at an intensity of 500 mW/cm2, LIPU promoted the differentiation of iPSCs-NSCs into neurons and Schwann cells, while also promoting gene and protein expression [Citation90]. Similarly, LIU and LIPU increased mesenchymal stem cell proliferation by activating the MAPK/ERK and PI3K/Akt signaling pathways, resulting in upregulation of multiple cell cycle proteins [Citation91,Citation92].
5. Opening the BBB to deliver drug vehicles through the combination of LIFU and MBs
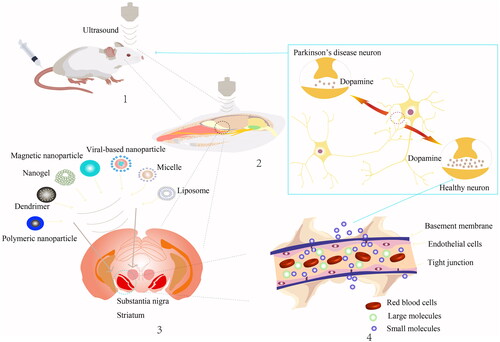
The site of the lesion in PD is focal, and early intervention through LIFU-mediated opening of the BBB and MBs is an important breakthrough in PD research [Citation93]. Targeted gene therapy aimed at specific brain regions could potentially halt the spread of Lewy bodies, while changes in early PD biomarkers could assist in early diagnosis [Citation94]. As a forward-thinking concept in early treatment of PD, the combined use of LIFU and MBs can help deliver drugs or carriers to the correct brain region for enhancing neuronal function. Specific mechanisms depend on the diversity of drugs and carriers used ().
Figure 1. The strategy and effect of low intensity focused ultrasound in the treatment of PD. 1. LIFU combined with MBs opens the BBB. 2. LIFU acts on specific parts of the mice brain, such as the motor cortex, substantia nigra, striatum and other specific sites. 3. Microvesicles or nanoparticles deliver relevant genes or therapeutic drugs such as polymeric nanoparticle, dendrimer, nanogel, magnetic nanoparticle, viral-based nanoparticle, micelle and liposome. 4. Therapeutic substances typically pass through an open BBB in two ways: the paracellular route and the transcellular route.
5.1. Pharmacological therapies
The molecular weight of BDNF (27 kDa) exceeds the ∼180 Da limit for BBB permeable ionized water-soluble drugs [Citation95], and Baseri et al. showed for the first time that exogenous BDNF can cross the BBB for noninvasive destruction, maintain biological activity, and trigger neuronal downstream signaling effects in highly localized regions of the brain [Citation96], and GDNF family member Neurturin was also detected in the caudate putamen and SN within 1 h after intravenous administration [Citation97]. However, the administration of neurotrophic factors via intravenous injection has not fully achieved therapeutic levels to reverse the disease. Current issues include shortening the half-life in the bloodstream and generating a large amount of systemic side effects [Citation98]. Attaching GDNF to the MB surface by a biotin-affinin method allows the highest aggregation in brain tissue, with better results than bare GDNF [Citation99]. It has also been shown that FUS-induced active pumping enhanced BDNF release with intranasal administration in combination with FUS [Citation100].
In addition to neurotrophic factors, other large molecules can also cross the BBB when opened by LIFU. Lipid-based PLGA nanoparticles encapsulated with curcumin are combined with LIFU to deliver the curcumin locally to deep brain nuclei, enhancing its efficacy [Citation101]. Fibroblast growth factor-20 (FGF20) is a paracrine member of the FGF family and is preferentially expressed in the dense fraction of the substantia nigra (SNpc). Compared to rhFGF20 or rhFGF20 liposomes, FUS-liposome-rhFGF20 significantly improved apomorphine-induced behavioral disorders and prevented the loss of dopaminergic neurons in SNpcs [Citation102]. The BBB opening mediated by FUS can also non-invasively transport neurotransmitter chemical substances to the target location in the brain, such as inhibitory neurotransmitter γ-aminobutyric acid transported to the right somatosensory cortex area of the rat brain, thereby further reducing the blood oxygen level-dependent signal [Citation103]. Using MRI-guided ultrasound beam positioning, neural stem cells expressing green fluorescent protein (GFP) were specifically delivered to the striatum and hippocampus of rats, and these cells were able to express doublecortin [Citation104]. From the above, we can believe that the combination of ultrasound and minimally invasive ultrasound system will provide greater prospects for the treatment of PD.
5.2. Gene therapies
The combined application of LIFU and MB to help efficiently deliver gene vectors to specific brain regions () is currently a viable therapeutic strategy for patients with PD [Citation105]. These vectors can be broadly divided into four types: DNA-loaded MBs, DNA-NPs, gene-liposome-MBs complexes, and gene-AAV vectors ().
Table 2. Summary of different strategies for FUS-mediated Permeabilization of the BBB in PD model.
5.2.1. DNA-loaded MBs
DNA-loaded MBs are a more novel way of administration than MBs and DNA injections separately. Studies have shown that the combination of ultrasound and pBDNF-EGFP loaded MB significantly enhances BDNF expression [Citation106]. Cationic methyl bromide (cMB) has a better binding ability to GDNF plasmids than neutral methyl bromide. A cationic MB platform loaded with GDNF plasmids triggered by focused ultrasound has a higher therapeutic effect than injecting high-titer GDNF genes into the brain [Citation107].
5.2.2. DNA-NPs
It has long been reported that the use of LIFU is able to activate stable MB oscillations to trigger the release of drugs in specific target regions such as pons [Citation108]. MRI-guided FUS delivery of nano-MBs improved the transfection efficiency of the Nrf2 gene [Citation109]. Brain penetration of nanoparticles (BPNs) coated with dense polyethylene glycol corona can also overcome BTB/BBB. Elizabeth Nance et al. demonstrated that systemic administration of FUS and DNA-BPN in areas treated with FUS can result in dose-dependent transgene expression [Citation110]. Research has shown that GDNF-BPN can achieve therapeutic levels in the rat striatum localization area through the FUS delivery pathway, with a duration of up to 10 weeks [Citation111]. Currently, there is limited research on combining nanosystems with FUS for gene delivery, mainly because many nanosystems can directly penetrate the BBB, reducing damage to dopamine neurons in PD models and reversing their neurological behavioral deficits. For example, gold nanoparticles [Citation112], gelatin nanoparticles loaded with neuropeptide substance P [Citation113], GDNF genes packaged with vascular iopep-2 modified NP [Citation114], and brain-targeted peptide rabies virus glycoprotein 29 modified polymer nanoparticle systems [Citation115]. Perhaps these nanosystems combined with LIFU to achieve gene transmission can further improve the level of drug targeting and therapeutic effect, which has broad prospects.
5.2.3. Gene-liposome-MBs complexes
Polyethylene glycol (PEG) liposomes (PLs) are promising and valuable drug carriers [Citation116,Citation117]. Using MRI-guided FUS to deliver PLs-GDNF-MBs to the brain is beneficial in reducing neuronal loss and behavioral deficits in the PD mouse model [Citation118]. Liposomal plasmid DNA-MBs (gene-liposome-MBs) complexes are superior to LpDNA-MBs administration alone in terms of gene delivery and expression [Citation119]. Lin et al. combined liposomes carrying BDNF or GDNF genes with MB to restore normal secretion of FUS and restore motor behavior in a PD model [Citation120].
5.2.4 gene-AAV vectors
Bartus et al. reported that the clinical gene therapy trial using viral vectors to deliver neurotrophic factors did not achieve the primary therapeutic outcome, primarily due to incomplete delivery to the target structure and invasive surgical strategies [Citation121]. MRI-guided FUS efficiently and noninvasively delivers gene viral vectors (including adeno-associated virus 9 (AAV9) [Citation94,Citation122], AAV2/1 [Citation123], rAAV [Citation124]) to neurons, astrocytes, and oligodendrocytes in multiple brain regions to express transgenes. Clinically scalable microbubble drug complexes (MDCs) used to carry viral genes, such as AAV.SIRT3-myc (AAV expressing myc-tagged SIRT3), have potential as disease-modifying strategies for treating PD and other neurodegenerative diseases [Citation125]. It is anticipated that the binding of MDC to other viral genes may be useful in the treatment of PD, although significant limitations exist, such as safety issues, high production costs, and limited packaging capabilities of the virus [Citation126].
6 Biophysical mechanism of LIFU
6.1. Biophysical mechanism of LIFU exerting neuro-modulatory effects
Currently, the debate on the biophysical mechanisms of LIFU is ongoing, and three possible physical mechanisms - heat generation, cavitation, and mechanical agitation - represent ways in which it can exert its biological effects. We are all aware that the biological effect of HIFU mainly involves thermal ablation of tissue [Citation127], whereas the neural modulation effect of LIFU is only observed to cause mild temperature increase (below 0.1 °C) [Citation128]. Surprisingly, Darrow et al. demonstrated that LIFU primarily reversibly inhibits somatic sensory evoked potentials (SSEPs), resulting in neuro-suppression [Citation129], which is achieved at temperature changes of approximately 2 °C [Citation64], depending on ultrasonic intensity rather than pressure wave amplitude or DC. However, further studies have shown that the effect of LIFU is mechanical agitation. Mechanosensitive channels are a type of transmembrane protein that can respond to mechanical force or stress [Citation130]. The mechanical forces induced by the US on the plasma membrane activate the mechanosensitive channels in the CNS, leading to extensive morphological changes [Citation131]. These mechanosensitive ion channels are typically present in potassium and sodium mechanosensitive ion channels [Citation132–134]. Mechanically induced stress by LIFU converts the stimulated permeation stress into an ion flux on the voltage-gated channel, further depolarizing and producing an action potential [Citation135]. For example, LIFU attenuated evoked potentials in rat hippocampus [Citation136]. Mihran et al. also found in their study that short-term (0.5 ms) focused ultrasound (FUS) can excite the sciatic prepatellar nerve in frogs while lowering its action potential [Citation137].Neural regulation induced by LIFU may also occur through mechanical stretching of lipid bilayers, and activated voltage-gated sodium channels can trigger exocytosis and synaptic transmission in the hippocampal circuit [Citation133]. Therefore, the mechanical forces generated by LIFU not only affect the conformational activity of ion channels [Citation138], but also on action potentials and synaptic vesicle release [Citation139]. It is believed that the cell membrane is capable of absorbing the mechanical energy of ultrasound and converting it into contractions and expansions within the intracellular space [Citation44]. This refers to the acoustic cavitation effect, also known as the ‘dual-tone sound carrier’, which is another method of US neuro-regulation [Citation140]. It is able to regulate the influx and efflux of Ca2+, K+, and Na + ions, as well as produce action potentials in neuronal axons [Citation141]. However, Wall and his colleagues’ early research suggests that cavitation is not the primary cause of ultrasound-induced neuromodulation [Citation142], but that the BBB can be temporarily opened [Citation143]. The biophysical mechanisms underlying low-intensity focused ultrasound have not been fully understood, and it may not be possible to attribute it to just one mechanism as they may interact with each other.
6.2. Routes that cross BBB mediated by LIFU and MBs
The combination of LIFU and MB induces targeted drug delivery through two main pathways: one is through the intracellular pathway, while the other is through the extracellular pathway. On one hand, using sulforhodamine B and rhodamine B hexyl ester as markers of intercellular permeation in the stratum corneum, it has been shown that intercellular permeation pathways are utilized in the delivery of LIU and LIU/SLS treated skin [Citation144]. When the FUS wave is activated, MBs with a diameter of 1-4μm expand or contract under low pressure, and this is stable cavitation [Citation145]. The mechanically stretched local blood vessel walls of brain tissue from rapidly expanding but not yet ruptured bubbles produce fluid shear forces and circumferential stress on capillary walls, thereby enlarging the tight junctions between various microcirculation endothelial cells, increasing endothelial cell membrane pores, and triggering intercellular phagocytosis [Citation146]. The joint stimulation of LFU and MBs is able to upregulate the expression of caveolin-1 and caveolin-2, which triggers an active transport process and creates a brief window of time (approximately 4-6 h) that can be activated by the fluctuation of microvesicles to promote intercellular transport through the blood-tumor barrier [Citation147] and activate endothelial cell receptors to promote molecular intercellular transport [Citation148]. Microfluidic and shear stress can also promote fluid-phase endocytosis in cells [Citation149]. The mechanical force generated by MBs can cause deformation of the cell membrane or rearrangement of the cell cytoskeleton, thereby changing the tension of the cell membrane and stimulating the mechanical sensors located on the cell membrane, thereby regulating endocytosis [Citation150]. The presence of vesicles and cytoplasmic channels within cerebral endothelial cells also implies an increase in endocytic activity [Citation151]. On the other hand, studies have shown that within several hours after the combination of ultrasound and MBs (USMB), the bypass diffusion of compounds increases [Citation152,Citation153].
In an in vitro transwell cell model, USMB increased the cell paracellular permeability of anti-CXCR4 nanobodies by two-fold [Citation154]. It is speculated that the downregulation of tight junction proteins or damage to the endothelial cell wall may allow for the transport of substances through the BBB via transcellular transport [Citation155]. The combined application of LIFU and MBs has opened up a new approach for the treatment of PD, but its mechanism still poses numerous questions. Therefore, controlling the energy of LIFU to stably excite MBs in a cavitation state is particularly crucial.
7. Discussion
PD is one of the most common neurodegenerative diseases, with an annual incidence of 1.1-1.9‰ and prevalence of 10.8-25.7‰ in Europe [Citation156]. This disease not only has a significant negative impact on the quality of life of patients, but also places a heavy burden on society and the economy. However, due to the complex etiology, there is currently a lack of safe and effective treatment options for PD.
As a new intervention method, LIFU can exert a wide range of neuroregulatory effects on various cascading reactions caused by thermogenesis [Citation129], cavitation [Citation131], and mechanical agitation [Citation44], including promoting the generation of neurotrophic factors, regulating microglia [Citation68], reducing oxidative stress [Citation52], and promoting the regeneration and repair of neurons [Citation83]. More importantly, the safety profile of MRI-guided FUS enables multiple treatments, and the duration and spatial properties of BBB opening can be adjusted by changing ultrasound parameters and the size and dose of MBs (MB) so that multiple pharmacological substances or gene vectors can be delivered to the region of interest [Citation157]. The above technical characteristics of LIFU provide a theoretical basis for the formulation of a new therapy for PD. Compared with traditional drug or surgical therapies, LIFU combined with MBs auxiliary specific pharmacological/gene therapies has predictable advantages in terms of targeting specificity, safety, and efficacy. Moreover, these therapeutic methods can also be widely applied to other neurodegenerative diseases, such as AD [Citation38] and motor neuron diseases [Citation158]. Although the broad prospect of LIFU in clinical application is close at hand some key problems still need to be solved. Firstly, the use of low-frequency piezoelectric transducers in LIFU entails significant spatial limitations in terms of the excitation volume, typically with a diameter exceeding several millimeters, thereby curtailing its application in wearable devices. According to reports, using a new photoacoustic transducer (with a diameter of 600 μm) can activate neurons within a radius of 500 μm around the tip of the optical fiber, providing better spatial resolution than traditional piezoelectric low-frequency sensors [Citation159]. In addition, the skull weakens ultrasound energy which leads to variations in energy efficiency among different patients. Through the use of 3D printing technology, the correlation between skull composition and ultrasound energy can be determined and compared to actual skull components for determining the optimal ultrasound parameters required for clinical research [Citation160]. Furthermore, there is potential hidden danger associated with LIFU when opening the BBB. ‘Highly repetitive’ LIFU treatment may cause edema, microbleeds, and acute inflammation in fragile brain tissue.
8. Conclusion
Therefore, long-term longitudinal safety studies are still needed for the clinical application of LIFU, with a focus on examining various parameters (including intensity, frequency, SD, and DC) to ensure its safety. Undoubtedly, in the clinical development of LIFU, developing a procedural and standardized ultrasound paradigm and improving personalized treatment strategies will become the inevitable research focus.
Authors contributions
Ling Long directed the conception and drafting of the review; Yun-Xiao Zhong and Jin-Chi Liao participated in the conception and drafting of the review; Xv Liu, Hao Tian and Li-Ren Deng made critical revisions to the knowledge content; Long Ling finally approved the version to be published; and that all authors agree to be accountable for all aspects of the work.
Disclosure statement
The authors have no conflicts of interest to declare.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials. Data sharing is not applicable to this article as no new data were created or analyzed in this study. The source of the figure and table provided in the article were original.
Additional information
Funding
References
- Bowary P, Greenberg BD. Noninvasive focused ultrasound for neuromodulation: a review. Psychiatr Clin North Am. 2018;41(3):1–15. doi:10.1016/j.psc.2018.04.010.
- Baek H, Pahk KJ, Kim H. A review of low-intensity focused ultrasound for neuromodulation. Biomed Eng Lett. 2017;7(2):135–142. doi:10.1007/s13534-016-0007-y.
- Yang F-Y, Lu W-W, Lin W-T, et al. Enhancement of neurotrophic factors in astrocyte for neuroprotective effects in brain disorders using low-intensity pulsed ultrasound stimulation. Brain Stimul. 2015;8(3):465–473. doi:10.1016/j.brs.2014.11.017.
- Zhao L, Feng Y, Hu H, et al. Low-intensity pulsed ultrasound enhances nerve growth factor-induced neurite outgrowth through mechanotransduction-mediated ERK1/2-CREB-Trx-1 signaling. Ultrasound Med Biol. 2016;42(12):2914–2925. doi:10.1016/j.ultrasmedbio.2016.07.017.
- Zhao L, Feng Y, Shi A, et al. Neuroprotective effect of low-intensity pulsed ultrasound against MPP-induced neurotoxicity in PC12 cells: involvement of K2P channels and stretch-activated ion channels. Ultrasound Med Biol. 2017;43(9):1986–1999. doi:10.1016/j.ultrasmedbio.2017.04.020.
- Zhang D, Li H, Sun J, et al. Antidepressant-like effect of low-intensity transcranial ultrasound stimulation. IEEE Trans Biomed Eng. 2019;66(2):411–420. doi:10.1109/TBME.2018.2845689.
- Daffertshofer M, Gass A, Ringleb P, et al. Transcranial low-frequency ultrasound-mediated thrombolysis in brain ischemia: increased risk of hemorrhage with combined ultrasound and tissue plasminogen activator: results of a phase II clinical trial. Stroke. 2005;36(7):1441–1446. doi:10.1161/01.STR.0000170707.86793.1a.
- Meng Y, Volpini M, Black S, et al. Focused ultrasound as a novel strategy for alzheimer disease therapeutics. Ann Neurol. 2017;81(5):611–617. doi:10.1002/ana.24933.
- Krishna V, Sammartino F, Rezai A. A review of the current therapies, challenges, and future directions of transcranial focused ultrasound technology: advances in diagnosis and treatment. JAMA Neurol. 2018;75(2):246–254. doi:10.1001/jamaneurol.2017.3129.
- Neumann W-J, Horn A, Kühn AA. Insights and opportunities for deep brain stimulation as a brain circuit intervention. Trends Neurosci. 2023;46(6):472–487. doi:10.1016/j.tins.2023.03.009.
- Cavallieri F, Campanini I, Gessani A, et al. Long-term effects of bilateral subthalamic nucleus deep brain stimulation on gait disorders in Parkinson’s disease: a clinical-instrumental study. J Neurol. 2023;270(9):4342–4353. doi:10.1007/s00415-023-11780-5.
- Fasano A, García-Ramos R, Gurevich T, et al. Levodopa-carbidopa intestinal gel in advanced Parkinson’s disease: long-term results from COSMOS. J Neurol. 2023;270(5):2765–2775. doi:10.1007/s00415-023-11615-3.
- Martínez-Fernández R, Máñez-Miró JU, Rodríguez-Rojas R, et al. Randomized trial of focused ultrasound subthalamotomy for Parkinson’s disease. N Engl J Med. 2020;383(26):2501–2513. doi:10.1056/NEJMoa2016311.
- Martínez-Fernández R, Rodríguez-Rojas R, Del Álamo M, et al. Focused ultrasound subthalamotomy in patients with asymmetric Parkinson’s disease: a pilot study. Lancet Neurol. 2018;17(1):54–63. doi:10.1016/S1474-4422(17)30403-9.
- Eisenberg HM, Krishna V, Elias WJ, et al. MR-guided focused ultrasound pallidotomy for Parkinson’s disease: safety and feasibility. J Neurosurg. 2020;135(3):792–798.
- Foffani G, Trigo-Damas I, Pineda-Pardo JA, et al. Focused ultrasound in Parkinson’s disease: a twofold path toward disease modification. Mov Disord. 2019;34(9):1262–1273. doi:10.1002/mds.27805.
- Gallay MN, Moser D, Magara AE, et al. Bilateral MR-guided focused ultrasound pallidothalamic tractotomy for Parkinson’s disease with 1-year follow-up. Front Neurol. 2021;12:601153. doi:10.3389/fneur.2021.601153.
- Stieglitz LH, Mahendran S, Oertel MF, et al. Bilateral focused ultrasound pallidotomy for Parkinson-related facial dyskinesia-a case report. Mov Disord Clin Pract. 2022;9(5):647–651. doi:10.1002/mdc3.13462.
- Martínez-Fernández R, Natera-Villalba E, Máñez Miró JU, et al. Prospective long-term follow-up of focused ultrasound unilateral subthalamotomy for Parkinson disease. Neurology. 2023;100(13):e1395–e1405. doi:10.1212/WNL.0000000000206771.
- Gallay MN, Moser D, Jeanmonod D. Safety and accuracy of incisionless transcranial MR-guided focused ultrasound functional neurosurgery: single-center experience with 253 targets in 180 treatments. J Neurosurg. 2018;130(4):1234–1243. doi:10.3171/2017.12.JNS172054.
- Xiong Y, Lin J, Pan L, et al. Pretherapeutic functional connectivity of tractography-based targeting of the ventral intermediate nucleus for predicting tremor response in patients with Parkinson’s disease after thalamotomy with MRI-guided focused ultrasound. J Neurosurg. 2022;18;1–10. doi:10.3171/2022.1.JNS212449
- Magara A, Bühler R, Moser D, et al. First experience with MR-guided focused ultrasound in the treatment of Parkinson’s disease. J Ther Ultrasound. 2014;2(1):11. doi:10.1186/2050-5736-2-11.
- Sharma VD, Patel M, Miocinovic S. Surgical treatment of Parkinson’s disease: devices and lesion approaches. Neurotherapeutics. 2020;17(4):1525–1538. doi:10.1007/s13311-020-00939-x.
- Karmacharya MB, Kim KH, Kim SY, et al. Low intensity ultrasound inhibits brain oedema formation in rats: potential action on AQP4 membrane localization. Neuropathol Appl Neurobiol. 2015;41(4):e80–e94. doi:10.1111/nan.12182.
- Hou J, Zhou J, Chang M, et al. LIFU-responsive nanomedicine enables acoustic droplet vaporization-induced apoptosis of macrophages for stabilizing vulnerable atherosclerotic plaques. Bioact Mater. 2022;16:120–133. doi:10.1016/j.bioactmat.2022.02.022.
- Yang A, Qiao B, Strohm EM, et al. Thrombin-responsive engineered nanoexcavator with full-thickness infiltration capability for pharmaceutical-free deep venous thrombosis theranostics. Biomater Sci. 2020;8(16):4545–4558. doi:10.1039/d0bm00917b.
- Amaya C, Smith ER, Xu X-X. Low intensity ultrasound as an antidote to taxane/paclitaxel-induced cytotoxicity. J Cancer. 2022;13(7):2362–2373. doi:10.7150/jca.71263.
- Yin T, Chen H, Ma A, et al. Cleavable collagenase-assistant nanosonosensitizer for tumor penetration and sonodynamic therapy. Biomaterials. 2023;293:121992. doi:10.1016/j.biomaterials.2022.121992.
- Hua Z, Li S, Liu Q, et al. Low-intensity pulsed ultrasound promotes osteogenic potential of iPSC-derived MSCs but fails to simplify the iPSC-EB-MSC differentiation process. Front Bioeng Biotechnol. 2022;10:841778. doi:10.3389/fbioe.2022.841778.
- Min S, Byeon Y, Kim M, et al. Production enhancement of human adipose-derived mesenchymal stem cells by low-intensity ultrasound stimulation. Sci Rep. 2022;12(1):22041. doi:10.1038/s41598-022-24742-0.
- Seo Y, Han S, Song B-W, et al. Endogenous neural stem cell activation after low-intensity focused ultrasound-induced blood-brain barrier modulation. Int J Mol Sci. 2023;24(6):5712. doi: 10.3390/ijms24065712.
- Liu X, Zou D, Hu Y, et al. Research progress of low-intensity pulsed ultrasound in the repair of peripheral nerve injury. Tissue Eng Part B Rev. 2023;29(4):414–428. doi:10.1089/ten.TEB.2022.0194.
- Wang Y, Xiao Q, Zhong W, et al. Low-intensity pulsed ultrasound promotes periodontal regeneration in a beagle model of furcation involvement. Front Bioeng Biotechnol. 2022;10:961898. doi:10.3389/fbioe.2022.961898.
- Wang Y, Li J, Zhou J, et al. Low-intensity pulsed ultrasound enhances bone marrow-derived stem cells-based periodontal regenerative therapies. Ultrasonics. 2022;121:106678. doi:10.1016/j.ultras.2021.106678.
- Mueller J, Legon W, Opitz A, et al. Transcranial focused ultrasound modulates intrinsic and evoked EEG dynamics. Brain Stimul. 2014;7(6):900–908. doi:10.1016/j.brs.2014.08.008.
- Legon W, Sato TF, Opitz A, et al. Transcranial focused ultrasound modulates the activity of primary somatosensory cortex in humans. Nat Neurosci. 2014;17(2):322–329. doi:10.1038/nn.3620.
- Zhu L, Zhao H, Zhou Z, et al. Peptide-functionalized phase-transformation nanoparticles for low intensity focused ultrasound-assisted tumor imaging and therapy. Nano Lett. 2018;18(3):1831–1841. doi:10.1021/acs.nanolett.7b05087.
- Rezai AR, Ranjan M, D’Haese P-F, et al. Noninvasive hippocampal blood-brain barrier opening in Alzheimer’s disease with focused ultrasound. Proc Natl Acad Sci U S A. 2020;117(17):9180–9182. doi:10.1073/pnas.2002571117.
- Yoo S-S, Bystritsky A, Lee J-H, et al. Focused ultrasound modulates region-specific brain activity. Neuroimage. 2011;56(3):1267–1275. doi:10.1016/j.neuroimage.2011.02.058.
- Yu K, Sohrabpour A, He B. Electrophysiological source imaging of brain networks perturbed by low-intensity transcranial focused ultrasound. IEEE Trans Biomed Eng. 2016;63(9):1787–1794. doi:10.1109/TBME.2016.2591924.
- Wang X, Yan J, Wang Z, et al. Neuromodulation effects of ultrasound stimulation under different parameters on mouse motor cortex. IEEE Trans Biomed Eng. 2020;67(1):291–297. doi:10.1109/TBME.2019.2912840.
- Ellis JM, Fell MJ. Current approaches to the treatment of Parkinson’s disease. Bioorg Med Chem Lett. 2017;27(18):4247–4255. doi:10.1016/j.bmcl.2017.07.075.
- Xie J, Shen Z, Anraku Y, et al. Nanomaterial-based blood-brain-barrier (BBB) crossing strategies. Biomaterials. 2019;224:119491. doi:10.1016/j.biomaterials.2019.119491.
- Krasovitski B, Frenkel V, Shoham S, et al. Intramembrane cavitation as a unifying mechanism for ultrasound-induced bioeffects. Proc Natl Acad Sci U S A. 2011;108(8):3258–3263. doi:10.1073/pnas.1015771108.
- Durand E, Petit O, Tremblay L, et al. Social behavioral changes in MPTP-treated monkey model of Parkinson’s disease. Front Behav Neurosci. 2015;9:42. doi:10.3389/fnbeh.2015.00042.
- Lee W, Kim H, Jung Y, et al. Image-guided transcranial focused ultrasound stimulates human primary somatosensory cortex. Sci Rep. 2015;5(1):8743. doi:10.1038/srep08743.
- Xu T, Lu X, Peng D, et al. Ultrasonic stimulation of the brain to enhance the release of dopamine - A potential novel treatment for Parkinson’s disease. Ultrason Sonochem. 2020;63:104955. doi:10.1016/j.ultsonch.2019.104955.
- Lee W, Kim H-C, Jung Y, et al. Transcranial focused ultrasound stimulation of human primary visual cortex. Sci Rep. 2016;6(1):34026. doi:10.1038/srep34026.
- Bystritsky A, Korb AS, Douglas PK, et al. A review of low-intensity focused ultrasound pulsation. Brain Stimul. 2011;4(3):125–136. doi:10.1016/j.brs.2011.03.007.
- Kim H, Park MY, Lee SD, et al. Suppression of EEG visual-evoked potentials in rats through neuromodulatory focused ultrasound. Neuroreport. 2015;26(4):211–215. doi:10.1097/WNR.0000000000000330.
- Yuan Y, Zhao Z, Wang Z, et al. The effect of low-intensity transcranial ultrasound stimulation on behavior in a mouse model of Parkinson’s disease induced by MPTP. IEEE Trans Neural Syst Rehabil Eng. 2020;28(4):1017–1021. doi:10.1109/TNSRE.2020.2978865.
- Zhou H, Niu L, Xia X, et al. Wearable ultrasound improves motor function in an MPTP mouse model of Parkinson’s disease. IEEE Trans Biomed Eng. 2019;66(11):3006–3013. doi:10.1109/TBME.2019.2899631.
- Karmacharya MB, Hada B, Park SR, et al. Low-intensity ultrasound decreases α-synuclein aggregation via attenuation of mitochondrial reactive oxygen species in MPP(+)-treated PC12 cells. Mol Neurobiol. 2017;54(8):6235–6244. doi:10.1007/s12035-016-0104-z.
- Naor O, Hertzberg Y, Zemel E, et al. Towards multifocal ultrasonic neural stimulation II: design considerations for an acoustic retinal prosthesis. J Neural Eng. 2012;9(2):026006. doi:10.1088/1741-2560/9/2/026006.
- Kim H, Chiu A, Lee SD, et al. Focused ultrasound-mediated non-invasive brain stimulation: examination of sonication parameters. Brain Stimul. 2014;7(5):748–756. doi:10.1016/j.brs.2014.06.011.
- Chen X, Wang D, Zhang L, et al. Neuroprotective effect of low-intensity pulsed ultrasound on the mouse MPTP/MPP model of dopaminergic neuron injury. Ultrasound Med Biol. 2021;47(8):2321–2330. doi:10.1016/j.ultrasmedbio.2021.03.034.
- Zhou H, Meng L, Xia X, et al. Transcranial ultrasound stimulation suppresses neuroinflammation in a chronic mouse model of Parkinson’s disease. IEEE Trans Biomed Eng. 2021;68(11):3375–3387. doi:10.1109/TBME.2021.3071807.
- Allen SJ, Watson JJ, Shoemark DK, et al. GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol Ther. 2013;138(2):155–175. doi:10.1016/j.pharmthera.2013.01.004.
- Tufail Y, Matyushov A, Baldwin N, et al. Transcranial pulsed ultrasound stimulates intact brain circuits. Neuron. 2010;66(5):681–694. doi:10.1016/j.neuron.2010.05.008.
- Lin W-T, Chen R-C, Lu W-W, et al. Protective effects of low-intensity pulsed ultrasound on aluminum-induced cerebral damage in Alzheimer’s disease rat model. Sci Rep. 2015;5(1):9671. doi:10.1038/srep09671.
- Müller HW, Junghans U, Kappler J. Astroglial neurotrophic and neurite-promoting factors. Pharmacol Ther. 1995;65(1):1–18. doi:10.1016/0163-7258(94)00047-7.
- Liu S-H, Lai Y-L, Chen B-L, et al. Ultrasound enhances the expression of brain-derived neurotrophic factor in astrocyte through activation of TrkB-Akt and calcium-CaMK signaling pathways. Cereb Cortex. 2017;27(6):3152–3160.
- Zhang Y, Ren L, Liu K, et al. Transcranial ultrasound stimulation of the human motor cortex. iScience. 2021;24(12):103429. doi:10.1016/j.isci.2021.103429.
- Dallapiazza RF, Timbie KF, Holmberg S, et al. Noninvasive neuromodulation and thalamic mapping with low-intensity focused ultrasound. J Neurosurg. 2018;128(3):875–884. doi:10.3171/2016.11.JNS16976.
- Calabresi P, Picconi B, Tozzi A, et al. Direct and indirect pathways of basal ganglia: a critical reappraisal. Nat Neurosci. 2014;17(8):1022–1030. doi:10.1038/nn.3743.
- Marogianni C, Sokratous M, Dardiotis E, et al. Neurodegeneration and Inflammation-An interesting interplay in Parkinson’s disease. Int J Mol Sci. 2020;21(22):8421. doi: 10.3390/ijms21228421.
- Guo T, Li H, Lv Y, et al. Pulsed transcranial ultrasound stimulation immediately after the ischemic brain injury is neuroprotective. IEEE Trans Biomed Eng. 2015;62(10):2352–2357. doi:10.1109/TBME.2015.2427339.
- Altland OD, Dalecki D, Suchkova VN, et al. Low-intensity ultrasound increases endothelial cell nitric oxide synthase activity and nitric oxide synthesis. J Thromb Haemost. 2004;2(4):637–643. doi:10.1111/j.1538-7836.2004.00655.x.
- Block ML, Wu X, Pei Z, et al. Nanometer size diesel exhaust particles are selectively toxic to dopaminergic neurons: the role of microglia, phagocytosis, and NADPH oxidase. FASEB J. 2004;18(13):1618–1620. doi:10.1096/fj.04-1945fje.
- Chen Y, Zhu G, Liu D, et al. Subthalamic nucleus deep brain stimulation suppresses neuroinflammation by fractalkine pathway in Parkinson’s disease rat model. Brain Behav Immun. 2020;90:16–25. doi:10.1016/j.bbi.2020.07.035.
- Kovacs ZI, Kim S, Jikaria N, et al. Disrupting the blood-brain barrier by focused ultrasound induces sterile inflammation. Proc Natl Acad Sci U S A. 2017;114(1):E75–E84.
- Shin WH, Lee D-Y, Park KW, et al. Microglia expressing interleukin-13 undergo cell death and contribute to neuronal survival in vivo. Glia. 2004;46(2):142–152. doi:10.1002/glia.10357.
- Strle K, Zhou JH, Shen WH, et al. Interleukin-10 in the brain. Crit Rev Immunol. 2001;21(5):23. doi:10.1615/CritRevImmunol.v21.i5.20.
- Yang M-S, Ji K-A, Jeon S-B, et al. Interleukin-13 enhances cyclooxygenase-2 expression in activated rat brain microglia: implications for death of activated microglia. J Immunol. 2006;177(2):1323–1329. doi:10.4049/jimmunol.177.2.1323.
- Suzumura A, Takeuchi H, Zhang G, et al. Roles of glia-derived cytokines on neuronal degeneration and regeneration. Ann N Y Acad Sci. 2006;1088(1):219–229. doi:10.1196/annals.1366.012.
- Bobola MS, Chen L, Ezeokeke CK, et al. Transcranial focused ultrasound, pulsed at 40 Hz, activates microglia acutely and reduces Aβ load chronically, as demonstrated in vivo. Brain Stimul. 2020;13(4):1014–1023. doi:10.1016/j.brs.2020.03.016.
- Javitch JA, D’Amato RJ, Strittmatter SM, et al. Parkinsonism-inducing neurotoxin, N-methyl-4-phenyl-1,2,3,6 -tetrahydropyridine: uptake of the metabolite N-methyl-4-phenylpyridine by dopamine neurons explains selective toxicity. Proc Natl Acad Sci U S A. 1985;82(7):2173–2177. doi:10.1073/pnas.82.7.2173.
- Li J, Zhang D-D, Wang C-Q, et al. Protective effects of low-intensity pulsed ultrasound on aluminum overload-induced cerebral damage through epigenetic regulation of brain-derived neurotrophic factor expression. Biosci Rep. 2019;39(1):BSR20181185. doi: 10.1042/BSR20181185.
- Surmeier DJ, Obeso JA, Halliday GM. Selective neuronal vulnerability in Parkinson disease. Nat Rev Neurosci. 2017;18(2):101–113. doi:10.1038/nrn.2016.178.
- Choi W-S, Kruse SE, Palmiter RD, et al. Mitochondrial complex I inhibition is not required for dopaminergic neuron death induced by rotenone, MPP+, or paraquat. Proc Natl Acad Sci U S A. 2008;105(39):15136–15141. doi:10.1073/pnas.0807581105.
- Cassarino DS, Parks JK, Parker WD, et al. The parkinsonian neurotoxin MPP + opens the mitochondrial permeability transition pore and releases cytochrome c in isolated mitochondria via an oxidative mechanism. Biochim Biophys Acta. 1999;1453(1):49–62. doi:10.1016/s0925-4439(98)00083-0.
- Chang C-J, Hsu S-H, Lin F-T, et al. Low-intensity-ultrasound-accelerated nerve regeneration using cell-seeded poly(D, L-lactic acid-co-glycolic acid) conduits: an in vivo and in vitro study. J Biomed Mater Res B Appl Biomater. 2005;75(1):99–107. doi:10.1002/jbm.b.30269.
- Jiang W, Wang Y, Tang J, et al. Low-intensity pulsed ultrasound treatment improved the rate of autograft peripheral nerve regeneration in rat. Sci Rep. 2016;6(1):22773. doi:10.1038/srep22773.
- Weiwei W, Li L, Wei W, editors. et al. Effects of ultrasound on behavior and dopamine content in striatum of Parkinson’s disease model mouse. Proceedings of the 2017 International Conference on Material Science, Energy and Environmental Engineering (MSEEE 2017); 2017. Atlantis Press.
- Hu Y, Zhong W, Wan JMF, et al. Ultrasound can modulate neuronal development: impact on neurite growth and cell body morphology. Ultrasound Med Biol. 2013;39(5):915–925. doi:10.1016/j.ultrasmedbio.2012.12.003.
- Crisci AR, Ferreira AL. Low-intensity pulsed ultrasound accelerates the regeneration of the sciatic nerve after neurotomy in rats. Ultrasound Med Biol. 2002;28(10):1335–1341. doi:10.1016/s0301-5629(02)00576-8.
- Zhang H, Lin X, Wan H, et al. Effect of low-intensity pulsed ultrasound on the expression of neurotrophin-3 and brain-derived neurotrophic factor in cultured Schwann cells. Microsurgery. 2009;29(6):479–485. doi:10.1002/micr.20644.
- Tsuang Y-H, Liao L-W, Chao Y-H, et al. Effects of low intensity pulsed ultrasound on rat Schwann cells metabolism. Artif Organs. 2011;35(4):373–383. doi:10.1111/j.1525-1594.2010.01086.x.
- Kusuyama J, Bandow K, Shamoto M, et al. Low intensity pulsed ultrasound (LIPUS) influences the multilineage differentiation of mesenchymal stem and progenitor cell lines through ROCK-Cot/Tpl2-MEK-ERK signaling pathway. J Biol Chem. 2014;289(15):10330–10344. doi:10.1074/jbc.M113.546382.
- Lv Y, Zhao P, Chen G, et al. Effects of low-intensity pulsed ultrasound on cell viability, proliferation and neural differentiation of induced pluripotent stem cells-derived neural crest stem cells. Biotechnol Lett. 2013;35(12):2201–2212. doi:10.1007/s10529-013-1313-4.
- Ling L, Wei T, He L, et al. Low-intensity pulsed ultrasound activates ERK1/2 and PI3K-Akt signalling pathways and promotes the proliferation of human amnion-derived mesenchymal stem cells. Cell Prolif. 2017;50(6):e12383. doi: 10.1111/cpr.12383.
- Budhiraja G, Sahu N, Subramanian A. Low-intensity ultrasound upregulates the expression of cyclin-D1 and promotes cellular proliferation in human mesenchymal stem cells. Biotechnol J. 2018;13(4):e1700382. doi:10.1002/biot.201700382.
- Rich MC, Sherwood J, Bartley AF, et al. Focused ultrasound blood brain barrier opening mediated delivery of MRI-visible albumin nanoclusters to the rat brain for localized drug delivery with temporal control. J Control Release. 2020;324:172–180. doi:10.1016/j.jconrel.2020.04.054.
- Xhima K, Nabbouh F, Hynynen K, et al. Noninvasive delivery of an α-synuclein gene silencing vector with magnetic resonance-guided focused ultrasound. Mov Disord. 2018;33(10):1567–1579. doi:10.1002/mds.101.
- Kroll RA, Neuwelt EA. Outwitting the blood-brain barrier for therapeutic purposes: osmotic opening and other means. Neurosurgery. 1998;42(5):1083. doi:10.1097/00006123-199805000-00082.
- Baseri B, Choi JJ, Deffieux T, et al. Activation of signaling pathways following localized delivery of systemically administered neurotrophic factors across the blood-brain barrier using focused ultrasound and microbubbles. Phys Med Biol. 2012;57(7):N65–N81. doi:10.1088/0031-9155/57/7/N65.
- Samiotaki G, Acosta C, Wang S, et al. Enhanced delivery and bioactivity of the neurturin neurotrophic factor through focused ultrasound—mediated blood—brain barrier opening in vivo. J Cereb Blood Flow Metab. 2015;35(4):611–622. 1doi:10.1038/jcbfm.2014.236.
- Lu B, Nagappan G, Guan X, et al. BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat Rev Neurosci. 2013;14(6):401–416. doi:10.1038/nrn3505.
- Wang F, Shi Y, Lu L, et al. Targeted delivery of GDNF through the blood-brain barrier by MRI-guided focused ultrasound. PLoS One. 2012;7(12):e52925. doi:10.1371/journal.pone.0052925.
- Ji R, Smith M, Niimi Y, et al. Focused ultrasound enhanced intranasal delivery of brain derived neurotrophic factor produces neurorestorative effects in a Parkinson’s disease mouse model. Sci Rep. 2019;9(1):19402. doi:10.1038/s41598-019-55294-5.
- Yan Y, Chen Y, Liu Z, et al. Brain delivery of curcumin through low-intensity ultrasound-induced blood-brain barrier opening via lipid-PLGA nanobubbles. Int J Nanomedicine. 2021;16:7433–7447. doi:10.2147/IJN.S327737.
- Niu J, Xie J, Guo K, et al. Efficient treatment of Parkinson’s disease using ultrasonography-guided rhFGF20 proteoliposomes. Drug Deliv. 2018;25(1):1560–1569. doi:10.1080/10717544.2018.1482972.
- Todd N, Zhang Y, Power C, et al. Modulation of brain function by targeted delivery of GABA through the disrupted blood-brain barrier. Neuroimage. 2019;189:267–275. doi:10.1016/j.neuroimage.2019.01.037.
- Burgess A, Ayala-Grosso CA, Ganguly M, et al. Targeted delivery of neural stem cells to the brain using MRI-guided focused ultrasound to disrupt the blood-brain barrier. PLoS One. 2011;6(11):e27877. doi:10.1371/journal.pone.0027877.
- Kordower JH, Emborg ME, Bloch J, et al. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson’s disease. Science. 2000;290(5492):767–773. doi:10.1126/science.290.5492.767.
- Huang Q, Deng J, Wang F, et al. Targeted gene delivery to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Exp Neurol. 2012;233(1):350–356. doi:10.1016/j.expneurol.2011.10.027.
- Fan C-H, Ting C-Y, Lin C-Y, et al. Noninvasive, targeted, and non-viral ultrasound-mediated GDNF-plasmid delivery for treatment of Parkinson’s disease. Sci Rep. 2016;6(1):19579. doi:10.1038/srep19579.
- Ye D, Sultan D, Zhang X, et al. Focused ultrasound-enabled delivery of radiolabeled nanoclusters to the pons. J Control Release. 2018;283:143–150. doi:10.1016/j.jconrel.2018.05.039.
- Long L, Cai X, Guo R, et al. Treatment of Parkinson’s disease in rats by Nrf2 transfection using MRI-guided focused ultrasound delivery of nanomicrobubbles. Biochem Biophys Res Commun. 2017;482(1):75–80. doi:10.1016/j.bbrc.2016.10.141.
- Mead BP, Mastorakos P, Suk JS, et al. Targeted gene transfer to the brain via the delivery of brain-penetrating DNA nanoparticles with focused ultrasound. J Control Release. 2016;223:109–117. doi:10.1016/j.jconrel.2015.12.034.
- Mead BP, Kim N, Miller GW, et al. Novel focused ultrasound gene therapy approach noninvasively restores dopaminergic neuron function in a rat Parkinson’s disease model. Nano Lett. 2017;17(6):3533–3542. doi:10.1021/acs.nanolett.7b00616.
- Hu K, Chen X, Chen W, et al. Neuroprotective effect of gold nanoparticles composites in Parkinson’s disease model. Nanomedicine. 2018;14(4):1123–1136. doi:10.1016/j.nano.2018.01.020.
- Zhao Y-Z, Jin R-R, Yang W, et al. Using gelatin nanoparticle mediated intranasal delivery of neuropeptide substance P to enhance neuro-recovery in hemiparkinsonian rats. PLoS One. 2016;11(2):e0148848. doi:10.1371/journal.pone.0148848.
- Huang R, Ma H, Guo Y, et al. Angiopep-conjugated nanoparticles for targeted long-term gene therapy of Parkinson’s disease. Pharm Res. 2013;30(10):2549–2559. doi:10.1007/s11095-013-1005-8.
- You L, Wang J, Liu T, et al. Targeted brain delivery of rabies virus glycoprotein 29-modified deferoxamine-loaded nanoparticles reverses functional deficits in parkinsonian mice. ACS Nano. 2018;12(5):4123–4139. doi:10.1021/acsnano.7b08172.
- Chen Y, Sun J, Lu Y, et al. Complexes containing cationic and anionic pH-sensitive liposomes: comparative study of factors influencing plasmid DNA gene delivery to tumors. Int J Nanomedicine. 2013;8:1573–1593.
- Lentacker I, Wang N, Vandenbroucke RE, et al. Ultrasound exposure of lipoplex loaded microbubbles facilitates direct cytoplasmic entry of the lipoplexes. Mol Pharm. 2009;6(2):457–467. doi:10.1021/mp800154s.
- Yue P, Miao W, Gao L, et al. Ultrasound-triggered effects of the microbubbles coupled to GDNF plasmid-loaded PEGylated liposomes in a rat model of Parkinson’s disease. Front Neurosci. 2018;12:222. doi:10.3389/fnins.2018.00222.
- Lin C-Y, Hsieh H-Y, Chen C-M, et al. Non-invasive, neuron-specific gene therapy by focused ultrasound-induced blood-brain barrier opening in Parkinson’s disease mouse model. J Control Release. 2016;235:72–81. doi:10.1016/j.jconrel.2016.05.052.
- Lin C-Y, Lin Y-C, Huang C-Y, et al. Ultrasound-responsive neurotrophic factor-loaded microbubble- liposome complex: preclinical investigation for Parkinson’s disease treatment. J Control Release. 2020;321:519–528. doi:10.1016/j.jconrel.2020.02.044.
- Bartus RT, Johnson EM. Clinical tests of neurotrophic factors for human neurodegenerative diseases, part 1: where have we been and what have we learned? Neurobiol Dis. 2017;97(Pt B):156–168. doi:10.1016/j.nbd.2016.03.027.
- Thévenot E, Jordão JF, O’Reilly MA, et al. Targeted delivery of self-complementary adeno-associated virus serotype 9 to the brain, using magnetic resonance imaging-guided focused ultrasound. Hum Gene Ther. 2012;23(11):1144–1155. doi:10.1089/hum.2012.013.
- Alonso A, Reinz E, Leuchs B, et al. Focal delivery of AAV2/1-transgenes into the rat brain by localized ultrasound-induced BBB opening. Mol Ther Nucleic Acids. 2013;2(2):e73. doi:10.1038/mtna.2012.64.
- Wang S, Olumolade OO, Sun T, et al. Noninvasive, neuron-specific gene therapy can be facilitated by focused ultrasound and recombinant adeno-associated virus. Gene Ther. 2015;22(1):104–110. doi:10.1038/gt.2014.91.
- Trinh D, Nash J, Goertz D, et al. Microbubble drug conjugate and focused ultrasound blood brain barrier delivery of AAV-2 SIRT-3. Drug Deliv. 2022;29(1):1176–1183. doi:10.1080/10717544.2022.2035855.
- Kantor B, McCown T, Leone P, et al. Chapter two - clinical applications involving CNS gene transfer. In: Friedmann T, Dunlap JC, Goodwin SF, editors. Advances in genetics. Vol. 87. Academic Press; 2014. p. 71–124. doi: 10.1016/B978-0-12-800149-3.00002-0.
- Quadri SA, Waqas M, Khan I, et al. High-intensity focused ultrasound: past, present, and future in neurosurgery. Neurosurg Focus. 2018;44(2):E16. doi:10.3171/2017.11.FOCUS17610.
- Kim H, Taghados SJ, Fischer K, et al. Noninvasive transcranial stimulation of rat abducens nerve by focused ultrasound. Ultrasound Med Biol. 2012;38(9):1568–1575. doi:10.1016/j.ultrasmedbio.2012.04.023.
- Darrow DP, O’Brien P, Richner TJ, et al. Reversible neuroinhibition by focused ultrasound is mediated by a thermal mechanism. Brain Stimul. 2019;12(6):1439–1447. doi:10.1016/j.brs.2019.07.015.
- Hamill OP, Martinac B. Molecular basis of mechanotransduction in living cells. Physiol Rev. 2001;81(2):685–740. doi:10.1152/physrev.2001.81.2.685.
- Tyler WJ. The mechanobiology of brain function. Nat Rev Neurosci. 2012;13(12):867–878. doi:10.1038/nrn3383.
- Ibsen S, Tong A, Schutt C, et al. Sonogenetics is a non-invasive approach to activating neurons in Caenorhabditis elegans. Nat Commun. 2015;6(1):8264. doi:10.1038/ncomms9264.
- Tyler WJ, Tufail Y, Finsterwald M, et al. Remote excitation of neuronal circuits using low-intensity, low-frequency ultrasound. PLoS One. 2008;3(10):e3511. doi:10.1371/journal.pone.0003511.
- Kubanek J, Shi J, Marsh J, et al. Ultrasound modulates ion channel currents. Sci Rep. 2016;6(1):24170. doi:10.1038/srep24170.
- Boland LM, Drzewiecki MM. Polyunsaturated fatty acid modulation of voltage-gated ion channels. Cell Biochem Biophys. 2008;52(2):59–84. doi:10.1007/s12013-008-9027-2.
- Rinaldi PC, Jones JP, Reines F, et al. Modification by focused ultrasound pulses of electrically evoked responses from an in vitro hippocampal preparation. Brain Res. 1991;558(1):36–42. doi:10.1016/0006-8993(91)90711-4.
- Mihran RT, Barnes FS, Wachtel H. Temporally-specific modification of myelinated axon excitability in vitro following a single ultrasound pulse. Ultrasound Med Biol. 1990;16(3):297–309. doi:10.1016/0301-5629(90)90008-z.
- Arnadóttir J, Chalfie M. Eukaryotic mechanosensitive channels. Annu Rev Biophys. 2010;39(1):111–137. doi:10.1146/annurev.biophys.37.032807.125836.
- Siechen S, Yang S, Chiba A, et al. Mechanical tension contributes to clustering of neurotransmitter vesicles at presynaptic terminals. Proc Natl Acad Sci U S A. 2009;106(31):12611–12616. doi:10.1073/pnas.0901867106.
- Chen K-T, Wei K-C, Liu H-L. Theranostic strategy of focused ultrasound induced Blood-Brain barrier opening for CNS disease treatment. Front Pharmacol. 2019;10:86. doi:10.3389/fphar.2019.00086.
- Plaksin M, Kimmel E, Shoham S. Cell-type-selective effects of intramembrane cavitation as a unifying theoretical framework for ultrasonic neuromodulation. eNeuro. 2016;3(3):ENEURO.0136-15.2016. doi:10.1523/ENEURO.0136-15.2016.
- Wall PD, Fry WJ, Stephens R, et al. Changes produced in the central nervous system by ultrasound. Science. 1951;114(2974):686–687. doi:10.1126/science.114.2974.686.
- Liu H-L, Fan C-H, Ting C-Y, et al. Combining microbubbles and ultrasound for drug delivery to brain tumors: current progress and overview. Theranostics. 2014;4(4):432–444. doi:10.7150/thno.8074.
- Kushner J, Kim D, So PTC, et al. Dual-channel two-photon microscopy study of transdermal transport in skin treated with low-frequency ultrasound and a chemical enhancer. J Invest Dermatol. 2007;127(12):2832–2846. doi:10.1038/sj.jid.5700908.
- Chen H, Kreider W, Brayman AA, et al. Blood vessel deformations on microsecond time scales by ultrasonic cavitation. Phys Rev Lett. 2011;106(3):034301. doi:10.1103/PhysRevLett.106.034301.
- Hu S, Zhang X, Unger M, et al. Focused ultrasound-induced cavitation sensitizes cancer cells to radiation therapy and hyperthermia. Cells. 2020;9(12):2595. doi:10.3390/cells9122595.
- Xia C-y, Zhang Z, Xue Y-X, et al. Mechanisms of the increase in the permeability of the blood-tumor barrier obtained by combining low-frequency ultrasound irradiation with small-dose bradykinin. J Neurooncol. 2009;94(1):41–50. doi:10.1007/s11060-009-9812-9.
- Deng J, Huang Q, Wang F, et al. The role of caveolin-1 in blood-brain barrier disruption induced by focused ultrasound combined with microbubbles. J Mol Neurosci. 2012;46(3):677–687. doi:10.1007/s12031-011-9629-9.
- Davies PF, Dewey CF, Bussolari SR, et al. Influence of hemodynamic forces on vascular endothelial function. In vitro studies of shear stress and pinocytosis in bovine aortic cells. J Clin Invest. 1984;73(4):1121–1129. doi:10.1172/JCI111298.
- Apodaca G. Modulation of membrane traffic by mechanical stimuli. Am J Physiol Renal Physiol. 2002;282(2):F179–F190. doi:10.1152/ajprenal.2002.282.2.F179.
- van Wamel A, Kooiman K, Harteveld M, et al. Vibrating microbubbles poking individual cells: drug transfer into cells via sonoporation. J Control Release. 2006;112(2):149–155. doi:10.1016/j.jconrel.2006.02.007.
- Sheikov N, McDannold N, Sharma S, et al. Effect of focused ultrasound applied with an ultrasound contrast agent on the tight junctional integrity of the brain microvascular endothelium. Ultrasound Med Biol. 2008;34(7):1093–1104. doi:10.1016/j.ultrasmedbio.2007.12.015.
- Park J, Zhang Y, Vykhodtseva N, et al. The kinetics of blood brain barrier permeability and targeted doxorubicin delivery into brain induced by focused ultrasound. J Control Release. 2012;162(1):134–142. doi:10.1016/j.jconrel.2012.06.012.
- Rousou C, de Maar J, Qiu B, et al. The effect of microbubble-assisted ultrasound on molecular permeability across cell barriers. Pharmaceutics. 2022;14(3):494. doi:10.3390/pharmaceutics14030494.
- O’Reilly MA, Hynynen K. Ultrasound enhanced drug delivery to the brain and Central nervous system. Int J Hyperthermia. 2012;28(4):386–396. doi:10.3109/02656736.2012.666709.
- Balestrino R, Schapira AHV. Parkinson disease. Eur J Neurol. 2020;27(1):27–42. doi:10.1111/ene.14108.
- Meng Y, Voisin MR, Suppiah S, et al. Is there a role for MR-guided focused ultrasound in Parkinson’s disease? Mov Disord. 2018;33(4):575–579. doi:10.1002/mds.27308.
- Truong T-T, Chiu W-T, Lai Y-S, et al. Ca signaling-mediated low-intensity pulsed ultrasound-induced proliferation and activation of motor neuron cells. Ultrasonics. 2022;124:106739. doi:10.1016/j.ultras.2022.106739.
- Jiang Y, Lee HJ, Lan L, et al. Optoacoustic brain stimulation at submillimeter spatial precision. Nat Commun. 2020;11(1):881. doi:10.1038/s41467-020-14706-1.
- Kong C, Park SH, Shin J, et al. Factors associated with energy efficiency of focused ultrasound through the skull: a study of 3D-printed skull phantoms and its comparison with clinical experiences. Front Bioeng Biotechnol. 2021;9:783048. doi:10.3389/fbioe.2021.783048.
