Abstract
Background
Cerebrovascular disease is a significant cause of morbidity and mortality in Africa, and using neuroimaging techniques has improved the diagnosis and management of this disease. However, there is a lack of comprehensive reviews of the role and effectiveness of neuroimaging techniques in the African context.
Methods
We reviewed the literature to evaluate the role of neuroimaging in diagnosing and managing cerebrovascular disease in Africa. Our search included electronic databases such as PubMed, Scopus, and Google Scholar from 2000 to April 2023. We included peer-reviewed studies written in English that reported on the use of neuroimaging in diagnosing and managing cerebrovascular disease in African populations. We excluded non-peer-reviewed articles, letters, editorials, and studies unrelated to cerebrovascular disease, neuroimaging, or Africa. A total of 102 potential articles were identified; after applying our exclusion criteria and removing duplicated articles, 51 articles were reviewed.
Results
Our findings suggest that neuroimaging techniques such as CT, MRI, and Skull x-ray play a crucial role in diagnosing and managing cerebrovascular disease in Africa. CT and MRI were the most commonly used techniques, with CT being more widely available and less expensive than MRI. However, challenges to using neuroimaging in Africa include the high cost of equipment and maintenance, lack of trained personnel, and inadequate infrastructure. These challenges limit the widespread use of neuroimaging in diagnosing and managing cerebrovascular disease in Africa.
Conclusion
Neuroimaging techniques are essential for diagnosing and managing cerebrovascular disease in Africa, but challenges to their use must be addressed to improve healthcare outcomes. Our policy recommendations can help improve the availability and accessibility of neuroimaging services in Africa.
Introduction
Cerebrovascular disease, which includes stroke and other conditions that affect blood vessels in the brain, is a significant cause of morbidity and mortality worldwide [Citation1]. The burden of cerebrovascular disease is particularly high in Africa, with an annual incidence of 316 per 100,000 individuals, where it is a leading cause of disability-adjusted life years lost and mortality [Citation2]. Furthermore, cerebrovascular disease disproportionately affects younger populations in Africa compared to other parts of the world [Citation3]. Risk factors for cerebrovascular disease in Africa include hypertension, diabetes, tobacco use, dyslipidaemia, and a sedentary lifestyle [Citation2]. These risk factors are often compounded by inadequate access to healthcare services and limited resources for prevention and treatment.
In Africa, the impact of cerebrovascular disease extends beyond health outcomes to social and economic consequences. Stroke survivors often face significant disability, reducing the quality of life and increasing the burden on caregivers and the healthcare system. The economic impact of cerebrovascular disease in Africa is also significant, with direct and indirect costs that exceed the financial resources of individuals and governments [Citation4]. Despite the high burden of cerebrovascular disease in Africa, neuroimaging, which plays a critical role in diagnosis and management, is often limited in availability and quality [Citation5]. This limitation contributes to delays in diagnosis, suboptimal treatment, and poor patient outcomes.
The benefits of early diagnosis and effective management of cerebrovascular disease in Africa cannot be overstated. Not only does it improve patient outcomes, but it can also reduce healthcare costs, as patients with well-managed cerebrovascular disease are less likely to require costly hospitalizations or long-term care. Given the significant impact of cerebrovascular disease on individuals and society, healthcare providers in Africa must prioritize early diagnosis and effective management.
Neuroimaging plays a crucial role in diagnosing and managing cerebrovascular disease [Citation6]. Neuroimaging has become an essential tool for clinicians in identifying and treating cerebrovascular disease with its non-invasive, accurate, and detailed visualization of the brain’s structure and function. The use of neuroimaging in the diagnosis of the cerebrovascular disease has greatly improved the ability to identify and classify different types of strokes, such as ischaemic and haemorrhagic strokes, as well as their underlying causes. Computed Tomography (CT) and Magnetic Resonance Imaging (MRI) can identify the location and extent of brain tissue damage caused by a stroke, informing treatment decisions and improving outcomes. In addition to diagnosis, neuroimaging also plays a critical role in the ongoing management of cerebrovascular disease. Repeated imaging can help monitor disease progression, evaluate the effectiveness of treatment interventions, and detect potential complications. Neuroimaging techniques such as functional MRI (fMRI) and positron emission tomography (PET) can also provide insights into brain function and metabolism, aiding in identifying brain regions affected by cerebrovascular disease and helping guide treatment decisions. Despite the growing importance of neuroimaging in Africa, there still needs to be more adequate imaging resources, particularly in rural areas. This review examines the essential role of neuroimaging in diagnosing and managing cerebrovascular disease in Africa. While previous studies have examined the role of neuroimaging in diagnosing and managing cerebrovascular disease in various contexts, this review aims to address the specific challenges and opportunities unique to the African setting.
Methodology
We conducted a comprehensive review of the literature to critically evaluate the role of neuroimaging in diagnosing and managing cerebrovascular disease in Africa. A search of electronic databases, including PubMed, Scopus, and Google Scholar, was performed from 2000 inception to April 2023 using the following search terms: ‘cerebrovascular disease,’ ‘neuroimaging’, ‘Africa’, ‘computed tomography’, ‘magnetic resonance imaging’, ‘ultrasound’, and their relevant variations. To be included in the review, studies had to be written in English and published in peer-reviewed journals and reports on the use of neuroimaging in diagnosing and managing cerebrovascular disease in African populations. We excluded non-peer-reviewed articles, letters, editorials and studies unrelated to cerebrovascular disease, neuroimaging, or Africa. A total of 102 potential articles were identified; after applying our exclusion criteria and removing duplicated articles, 51 articles were reviewed.
The study selection process aimed to ensure reproducibility and minimize bias. Two independent reviewers screened the titles and abstracts of the identified articles for eligibility, and full-text articles were reviewed as necessary. Inter-rater agreement between the independent reviewers was assessed using a kappa statistic, which demonstrated substantial agreement (κ = 0.8). Data extraction was conducted using a standardized form, which included study design, sample size, neuroimaging technique used, main findings, and limitations. Any discrepancies were resolved through consensus.
The Cochrane Risk of Bias tool for randomized controlled trials and the Newcastle-Ottawa Scale for observational studies were used to assess the quality of the included studies. Data were synthesized narratively, focusing on the role of neuroimaging in diagnosing and managing cerebrovascular disease in Africa.
Limitations of this review include the potential for publication bias as studies not published in English were excluded and heterogeneity in the study designs, patient populations, and neuroimaging techniques used in the included studies. Despite these limitations, this review provides a comprehensive overview of the current literature on the role of neuroimaging in cerebrovascular disease in Africa. It highlights the need for further research and healthcare infrastructure improvements.
Neuroimaging techniques for cerebrovascular disease
In the African context, a range of neuroimaging techniques are used to diagnose and manage cerebrovascular disease. See . These techniques have proved to be invaluable in the early detection of cerebrovascular disease and the assessment of its severity. The selection of the appropriate imaging modality depends on various factors, including the suspected pathology, the age and clinical status of the patient, and the availability of resources [Citation7].
Table 1. Comparison of neuroimaging modalities for cerebrovascular disease management in Africa.
Computed tomography (CT)
Neuroimaging, particularly computed tomography (CT), plays an essential role in diagnosing and managing cerebrovascular disease in Africa. CT is a widely available and relatively affordable imaging technique that produces comprehensive cross-sectional brain images using X-rays and computer technology [Citation8]. The utilization of CT in diagnosing and managing cerebrovascular disease is particularly critical in Africa, where access to other imaging modalities, such as magnetic resonance imaging (MRI), is limited. Furthermore, CT imaging can be conducted quickly and efficiently, which is crucial in managing cerebrovascular disease, where time is of the essence.
In Africa, four- and eight-slice CT scanners are the least expensive and efficient models [Citation8]. However, larger studies benefit from the superior speed of 16-slice CT scanners compared to 4- and 8-slice models [Citation9]. Radiology departments with a steady patient flow are often more likely to have 32- and 40-slice CT scanners, which offer even faster examination times [Citation10]. These scanners are more expensive than those with fewer slices. The use of CT without intravenous contrast is typically appropriate for evaluating various conditions related to cerebrovascular disease, including suspected ischaemic stroke, transient ischaemic attack, elevated intracranial pressure, head trauma, and surgical emergencies [Citation11]. Contrast-enhanced CT can detect anomalous contrast enhancement, including brain metastases, meningiomas, abscesses, and lymphomas [Citation12]. However, each mode has advantages and limitations, and the choice of mode depends on the patient’s condition and the diagnostic question that needs to be answered. Non-contrast CT is the most commonly used mode of CT for the brain, while contrast-enhanced CT involves injecting a contrast dye into the patient’s vein. In Nigeria, a CT scanner census conducted in 2018 revealed that there were 183 CT scanners installed in the country, with 57.4% owned by private investors and 42.6% owned by the federal and state governments [Citation13]. However, the distribution of scanners across the country is uneven, with the South having more scanners than the North and FCT. Notably, three states in northern Nigeria have yet to have a single scanner. While CT scanning has greatly improved the diagnosis and management of cerebrovascular disease in Africa, the availability and accessibility of CT scanners remain challenging in many regions [Citation14]. Addressing this issue and ensuring the safe and effective use of CT scanning in Africa is crucial for improving the outcomes of patients with cerebrovascular disease.
CT is the imaging modality of choice in the acute phase of cerebrovascular disease. It provides rapid and accurate detection of intracranial hemorrhage, which is crucial for selecting patients for thrombolytic therapy. Moreover, CT can distinguish between ischaemic and haemorrhagic stroke, which is essential for therapeutic decisions [Citation15]. Furthermore, CT has been shown to have higher sensitivity in detecting early ischaemic stroke changes than MRI. In a study comparing the two imaging modalities, CT showed a sensitivity of 53% in detecting early ischaemic changes within 3 h of symptom onset, while MRI showed a sensitivity of only 29% [Citation16]. This indicates that CT may be more able to detect early ischaemic changes in the acute phase of stroke, allowing for faster diagnosis and management. Another advantage of CT in stroke management is its rapid acquisition time, which allows for quicker diagnosis and faster initiation of treatment. CT can provide images in as little as 5-10 s, while MRI may take several minutes [Citation17]. This is especially important in the acute phase of stroke, where time is of the essence in achieving optimal outcomes.
While CT scanning remains a valuable diagnostic tool in neuroimaging, it has some limitations. One of the main limitations is the exposure of patients to ionizing radiation. While modern CT scanners have significantly reduced radiation doses compared to older models, the potential long-term risks of radiation exposure cannot be ignored [Citation18]. Additionally, CT scans may not be as sensitive as other neuroimaging techniques, such as MRI, for detecting certain brain abnormalities [Citation19]. CT also has limitations in assessing cerebral perfusion and metabolic activity, which can be important in evaluating cerebrovascular disease [Citation20]. Also, CT scanning is not able to provide functional or metabolic information about the brain, which may be crucial in the diagnosis and management of certain neurological conditions [Citation21]. Despite these limitations, CT scanning remains a valuable tool in diagnosing and managing cerebrovascular disease in Africa, particularly in resource-limited settings where access to more advanced imaging modalities such as MRI may be limited.
Magnetic resonance imaging (MRI)
The advent of MRI in the early 1980s marked a significant milestone in non-invasive imaging technology. MRI employs non-ionizing electromagnetic radiation to produce cross-sectional images of internal structures [Citation22]. This technique has been hailed for its ability to reveal detailed information about tissues’ biochemical and structural properties, making it an essential tool in neurological research and diagnosis. Initially, MRI was considered primarily as an anatomical imaging technique. However, MRI and functional imaging fields have begun to merge over the years, with researchers developing new methods to capture useful information and spatially focused spectra.
MRI harnesses the magnetic properties of protons, specifically hydrogen atoms in the body, to create a magnetic field when exposed to an external magnetic field. The body then receives a radiofrequency pulse that excites the protons. After the pulse stops, the protons return to their initial state, releasing absorbed energy as a radiofrequency signal, which a receiver coil picks up. The MRI machine then uses this signal to generate brain images, reflecting various tissue properties depending on the MRI sequences used. These include diffusion-weighted imaging, T1-weighted imaging, and T2-weighted imaging, each with unique advantages. T2-weighted images, for example, are sensitive to changes in tissue water content, such as edema and inflammation, while T1-weighted images are useful for distinguishing between normal and aberrant brain structures. On the other hand, diffusion-weighted imaging can reveal critical details about the white matter microstructure, as it is sensitive to the diffusion of water molecules in tissue.
In recent years, significant advancements in MRI technology have transformed our understanding of brain function and structure. One such development is the use of echo planar imaging (EPI), which enables rapid ‘snap-shot imaging’ in both functional MRI (fMRI) and diffusion imaging. EPI is highly sensitive to blood susceptibility contrast processes, making it a widely used pulse sequence for human brain diffusion imaging [Citation23]. Researchers have attempted to enhance the speed of EPI by reducing T2 signal decay, echo train duration length, and image artefacts. Nevertheless, these techniques have not reduced the prolonged initial sequence time required to record the blood oxygen level-dependent (BOLD) or diffusion fMRI contrast. Despite these challenges, the latest MRI technologies have enabled researchers to achieve unprecedented spatial and temporal resolution levels, resulting in precise and comprehensive mapping of the brain’s structure and function. Ultra-high field MRI, which uses magnetic field strengths above 7 Tesla, has allowed for unparalleled visualization of brain structures. In addition, cutting-edge diffusion MRI methods such as diffusion tensor imaging (DTI) have offered novel insights into the microstructure and connectivity of white matter. By employing machine learning techniques to analyze MRI data, researchers have detected patterns that would be difficult or impossible to identify using conventional statistical methods. This approach has been applied to various neurological conditions, including predicting disease progression, identifying biomarkers, and grouping patients based on imaging characteristics.
Despite these remarkable achievements, access to MRI facilities remains limited in Africa. In a study by Ogbole et al. 84 MRI facilities were identified that provided care to 372,551,411 people in the West African sub-region, with Nigeria accounting for over half of the available units [Citation24]. Of these, 25% had low field strengths. In Ghana, the 14 MRI units were distributed relatively evenly between the private (57%) and public (43%) sectors, and the country had the highest number of MRI units per million of the population. Nevertheless, ongoing efforts to improve access to MRI in these regions are underway, and the continued development of MRI technology holds great promise for advancing our understanding of the brain.
MRI has a superior soft tissue contrast resolution, allowing for better differentiation between healthy and diseased tissues [Citation25]. Additionally, it has a higher sensitivity and specificity for detecting acute ischaemic stroke, particularly in the hyperacute phase, when time is of the essence [Citation26]. MRI can also provide additional information on the extent and severity of the ischaemic injury, including the size and location of the infarcted area and the presence of haemorrhage [Citation26]. However, MRI has limitations, particularly in the African context. MRI equipment’s high cost and technical requirements make it less accessible than other imaging modalities, such as CT [Citation27]. Additionally, the need for more trained personnel and adequate infrastructure in many African countries can hinder the widespread adoption of MRI for cerebrovascular disease diagnosis and management. Despite these limitations, the advantages of MRI make it a valuable tool in the management of cerebrovascular disease, particularly in the early detection and accurate diagnosis of stroke. Moreover, with ongoing efforts to improve MRI accessibility and the development of more cost-effective and portable MRI equipment, the potential for MRI to contribute to reducing cerebrovascular disease burden in Africa is promising.
Skull X-ray
X-ray imaging in evaluating cerebrovascular disease in Africa has been limited due to the low sensitivity and specificity in detecting such conditions [Citation28]. However, x-ray imaging remains valuable in resource-limited settings where advanced radiographic techniques are not readily available. In some cases, x-ray imaging can provide useful information in evaluating cerebrovascular disease. For instance, it can be used to detect calcifications in the cerebral arteries, which may indicate atherosclerosis [Citation29]. Additionally, x-ray imaging can detect fractures or dislocations of the skull and spine, which may be the result of trauma-induced cerebrovascular injury [Citation30]. While plain skull X-ray has a lower diagnostic potential than CT and MRI, its use remains valuable in resource-constrained settings. Nevertheless, in light of the limitations of plain skull X-rays, they should be supplemented with CT scans and other imaging modalities, especially in developing countries, to improve the accuracy of diagnosis and patient management.
Others
In recent years, significant advancements in medical imaging technologies have expanded our ability to diagnose and treat neurological disorders. While many imaging modalities are available worldwide, some emerging techniques have yet to gain widespread adoption in certain regions, such as Africa. Positron emission tomography (PET) is a highly sensitive imaging technique that can detect changes in brain metabolism and blood flow and has shown promise in diagnosing and monitoring various neurological conditions [Citation31]. However, its use still needs to be improved due to the high cost of equipment, radiotracers, and technical expertise required to operate and interpret results. Similarly, magnetic resonance spectroscopy (MRS) offers a non-invasive way to measure brain metabolites, which can provide insights into neurodegenerative disorders [Citation32]. However, MRS is only widely available in some African countries, mainly due to the cost of specialized equipment and lack of trained personnel.
Neuroimaging findings in cerebrovascular disease
Neuroimaging techniques offer comprehensive insight into cerebrovascular lesions’ location, size, and characteristics and play a crucial role in the decision-making process for the most appropriate management strategy [Citation33]. CT can identify various abnormalities associated with cerebrovascular disease, including haemorrhage, ischaemia, oedema, and infarction. Haemorrhage is a common CT finding in cerebrovascular disease and can be classified as intracerebral haemorrhage (ICH) or subarachnoid haemorrhage (SAH) [Citation34]. ICH appears as a hyperdense lesion on non-contrast CT scans, while SAH appears as a hyperdense signal in the basal cisterns and sulci [Citation34]. CT imaging can also detect early ischaemic changes such as loss of grey-white matter differentiation and obscuration of the lentiform nucleus [Citation35]. CT perfusion and CT angiography can further evaluate blood flow and identify tissue areas at risk of infarction. Cerebral edema is another important CT finding in cerebrovascular disease, particularly in large hemispheric infarction. This can lead to midline shift, herniation, and brainstem compression, important prognostic factors. CT can also identify infarction in the acute and subacute stages, with acute infarcts appearing as hypodense lesions and subacute infarcts appearing as a hypodense core surrounded by a hyperdense rim, known as the ‘isodense sign.’ Approximately 30% of patients with anterior circulation large vessel occlusion (LVO) may show early ischaemic changes on CT within the first 3 h of symptom onset, and 60% may show these changes within the first 6 h [Citation35].
The Alberta Stroke Program Early CT Score (ASPECTS) is a semi-quantitative score that sums up the extent of early ischaemic changes in the middle cerebral artery (MCA) territory on non-contrast CT [Citation36]. ASPECTS is a useful tool for assessing the extent of early ischaemic changes and can be used to guide management decisions. In clinical trials of endovascular treatment (EVT) for acute ischaemic stroke, most studies published in 2015 required a minimum ASPECTS of 6 or greater for inclusion [Citation37]. However, the MR CLEAN trial in the Netherlands did not use this inclusion criterion [Citation38]. The ASPECTS score can help identify patients most likely to benefit from EVT and guide the selection of appropriate treatment strategies.
One of the most common MRI findings in cerebrovascular disease is ischaemic injury, which can be seen as restricted diffusion in diffusion-weighted imaging (DWI) and as corresponding hyperintensity on apparent diffusion coefficient (ADC) maps [Citation39]. DWI can detect ischaemic changes within minutes of symptom onset, allowing for early diagnosis and treatment [Citation39]. MRI is also highly sensitive in detecting haemorrhagic lesions, including intracerebral hemorrhage (ICH), subarachnoid hemorrhage (SAH), and microbleeds [Citation40]. Susceptibility-weighted imaging (SWI) can detect even small hemorrhages, and gradient-recalled echo (GRE) imaging is particularly useful in detecting microbleeds. In addition to ischaemic and haemorrhagic lesions, MRI can identify other cerebrovascular abnormalities, such as cerebral aneurysms and vascular malformations [Citation41]. Magnetic resonance angiography (MRA) can provide detailed images of the cerebral vasculature, allowing for the detection of aneurysms, arteriovenous malformations (AVMs), and other vascular lesions [Citation41]. MRI is also useful in evaluating cerebral perfusion and metabolism. Perfusion-weighted imaging (PWI) can identify areas of hypoperfusion, while magnetic resonance spectroscopy (MRS) can provide information on the metabolic status of the brain tissue [Citation41].
Although both CT and MRI can detect hyperacute hemorrhage, susceptibility-weighted imaging (SWI) on MRI is equally accurate and more sensitive to detect subtle haemorrhagic changes within the ischaemic lesion. In addition, the susceptibility vessel sign on MRI, analogous to the hyperdense vessel sign on CT, can identify acute intra-arterial thrombus, which is associated with a better response to stent retriever thrombectomy than contact aspiration [Citation42]. The absence of these signs may indicate a fibrin-predominant thrombus [Citation42]. MRI may also predict underlying intracranial atherosclerotic disease in large vessel occlusion (LVO), as shown by smaller infarct volume and scattered or border zone infarcts. Furthermore, conventional MRI can demonstrate intravascular thrombus, evidenced by the absence of flow void on T2-weighted imaging, vascular hyperintensity on fluid-attenuated inversion recovery (FLAIR), and hypointense vascular sign on gradient-recalled echo (GRE) sequence.
Role of neuroimaging in cerebrovascular disease management
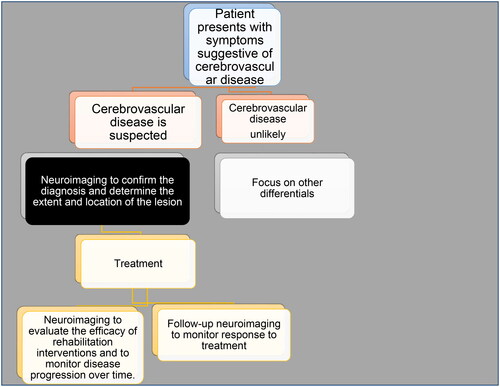
In managing acute stroke, time is of the essence as a ‘golden hour’ is available for making prompt diagnostic and therapeutic decisions to minimize complications [Citation43]. Symptoms such as weakness or numbness, visual disturbances, sensory changes, altered consciousness, dizziness, dysphagia, headache, and speech difficulties commonly indicate stroke [Citation44]. A swift and accurate diagnosis is vital, as delays can lead to irreversible brain tissue damage. To facilitate prompt diagnosis, stroke assessment scales such as the Face Arm Speech Time (FAST), Cincinnati Prehospital Stroke Scale (CPSS), Los Angeles Prehospital Stroke Scale (LAPSS), and Melbourne Ambulance Stroke Scale (MASS) have been developed [Citation45]. Severity grading scores such as the Los Angeles Motor Scale (LAMS), Kurashiki Prehospital Stroke Scale (KPSS), and National Institutes of Health Stroke Scale (NIHSS) are also available [Citation46]. However, the ultimate diagnosis is primarily determined by imaging, and choosing the most appropriate imaging technique can result in an early and potentially life-saving diagnosis of acute stroke. See .
One of the most important applications of neuroimaging in cerebrovascular disease diagnosis is differentiating between ischaemic and haemorrhagic strokes [Citation33]. CT is the preferred imaging modality for the initial evaluation of stroke patients because it can quickly and accurately identify the presence of hemorrhage [Citation34]. Haemorrhagic strokes are associated with a worse prognosis and require different treatment strategies than ischaemic strokes, which are caused by a lack of blood flow to the brain [Citation47]. In addition to distinguishing between ischaemic and haemorrhagic strokes, neuroimaging can also help identify the location and extent of the stroke, which is crucial in selecting appropriate treatment strategies. For example, CT angiography can identify large-vessel occlusions (LVOs), which can help determine if the patient is a candidate for endovascular therapy. Moreover, neuroimaging can identify other causes of stroke, such as cerebral venous thrombosis or arteriovenous malformations, which may require different treatment strategies than traditional ischaemic or haemorrhagic strokes. Furthermore, neuroimaging can be important in identifying risk factors for future strokes. For example, identifying white matter hyperintensities on MRI may indicate small vessel disease, which is associated with an increased risk of recurrent stroke [Citation48].
In Africa, where there is a high burden of stroke and limited healthcare resources, neuroimaging offers a noninvasive and cost-effective method for diagnosing and managing cerebrovascular disease. However, access to neuroimaging facilities in Africa remains a major challenge, especially in rural and low-income areas. This has led to delayed diagnosis and treatment, which can worsen outcomes for patients with cerebrovascular disease. Therefore, there is a need for increased investment in neuroimaging facilities and training of healthcare professionals to improve the diagnosis and management of cerebrovascular disease in Africa. Additionally, research is needed to understand better the unique patterns of cerebrovascular disease in Africa, which can inform the development of targeted and effective management strategies. Accurate and timely diagnosis of cerebrovascular disease is crucial for selecting appropriate treatment strategies and improving patient outcomes.
With the availability of advanced imaging techniques, clinicians can better identify the underlying cause and location of the stroke, which can inform the selection of appropriate treatment strategies. For example, endovascular therapy has been shown to improve outcomes in patients with large vessel occlusion (LVO), but this treatment is only effective within a limited time window [Citation49]. Accurate identification of LVO through neuroimaging can help ensure that eligible patients receive timely and appropriate treatment. Similarly, identifying cerebral venous thrombosis or arteriovenous malformations through neuroimaging may require different treatment strategies than traditional ischaemic or haemorrhagic strokes. Neuroimaging also plays a critical role in monitoring the response to treatment. Follow-up imaging can assess treatment efficacy and identify potential complications, such as cerebral edema or hemorrhage. In patients with ischaemic stroke, follow-up imaging can evaluate the extent of infarction and identify any salvageable brain tissue, which can help guide decisions about further intervention. In patients undergoing endovascular therapy, imaging can be used to evaluate the procedure’s success and identify any residual occlusion. In addition to guiding treatment decisions and monitoring response, neuroimaging can assess for complications such as cerebral edema or hemorrhage. These complications can be life-threatening and require immediate intervention. Early identification through neuroimaging can help clinicians intervene before the complication becomes severe.
One key application of neuroimaging in prognosis is the prediction of long-term outcomes based on imaging findings. For example, the extent of brain tissue damage seen on MRI or CT scans can provide insight into the patient’s cognitive and functional abilities and their risk of developing dementia. Studies have shown that patients with larger areas of brain damage on neuroimaging are more likely to experience cognitive decline and functional impairment in the long term [Citation50, Citation51]. Neuroimaging also plays a critical role in assessing the risk of recurrent stroke. Imaging can identify patients with large vessel occlusion (LVO) who may be at high risk of recurrent stroke and benefit from more aggressive treatment. In addition, imaging can identify patients with other stroke subtypes, such as cerebral venous thrombosis or arteriovenous malformations, who may have different risk profiles and require different treatment strategies to prevent a recurrence.
Challenges and opportunities in neuroimaging in Africa
The limitations in healthcare infrastructure and their impact on the implementation and accessibility of neuroimaging technologies in Africa are significant. See .
Table 2. Challenges and policy recommendations for neuroimaging in cerebrovascular disease management in Africa.
A. Limitations in Healthcare Infrastructure
In many African countries, the implementation of neuroimaging technologies is hindered by significant limitations in healthcare infrastructure. The primary challenges revolve around needing more specialized imaging facilities and qualified personnel, particularly in rural and low-income areas. The limited availability of these facilities poses substantial obstacles to achieving timely and accurate diagnoses of cerebrovascular disease, thereby impacting patient outcomes.
One of the key issues is the concentration of specialized imaging facilities in urban areas, leading to a pronounced geographic disparity in access to neuroimaging services. Patients residing in rural and remote areas often need help in reaching these services. The necessity of seeking nearby facilities forces them to undertake long journeys, which may entail considerable time, effort, and financial resources. These geographic barriers contribute to delays in diagnosis and subsequent treatment initiation, resulting in negative patient outcomes, including increased morbidity and mortality rates. The lack of specialized imaging facilities in remote areas further compounds the challenge, leaving a significant portion of the population needing access to essential diagnostic tools. Consequently, patients may receive suboptimal care due to the inability to obtain accurate and timely diagnoses, leading to delayed or inappropriate treatment plans.
This limitation in healthcare infrastructure restricts the availability of neuroimaging services and exacerbates the existing healthcare disparities between urban and rural areas. The concentration of resources in urban centers perpetuates inequities in healthcare access, leaving vulnerable populations in underserved regions with limited options for neuroimaging diagnoses. The inability to access timely and accurate neuroimaging services contributes to adverse health outcomes, including prolonged illness, increased disability, and preventable deaths.
B. Need for Specialized Imaging Facilities and Qualified Personnel
The need for specialized imaging facilities and qualified personnel presents a significant challenge in implementing neuroimaging technologies in many African countries. This challenge stems from the need for a well-trained workforce proficient in neuroimaging techniques, which hampers the provision of high-quality diagnostic services. A primary consequence of the shortage of specialized personnel is the long wait times for imaging procedures. In African countries where the availability of healthcare resources is already limited, the need for qualified personnel exacerbates the strain on the healthcare system. As a result, patients requiring neuroimaging studies often experience delays in accessing these essential diagnostic services. Prolonged waiting periods can harm patient outcomes, as timely diagnosis and subsequent treatment initiation are crucial in managing cerebrovascular diseases.
Furthermore, the accuracy and interpretation of neuroimaging results are often compromised due to the inadequate expertise available. Radiologists play a pivotal role in accurately interpreting neuroimaging studies, as they possess the knowledge and skills to identify and analyze subtle abnormalities in brain images. Their expertise is crucial in guiding treatment decisions and ensuring optimal patient care. However, the shortage of radiologists in many African countries poses a significant challenge to accurate and timely result interpretation. This shortage can lead to potential issues such as misdiagnosis, delayed diagnosis, or even underutilization of neuroimaging studies, impacting patient outcomes and overall healthcare quality.
The need for more qualified personnel in neuroimaging can be attributed to various factors. Firstly, the limited availability of educational programs specifically tailored to neuroimaging contributes to the inadequate training of healthcare professionals in this specialized field. Many African countries need more comprehensive educational curricula and dedicated training opportunities focused on neuroimaging. This scarcity restricts the number of professionals with specialized knowledge and skills in neuroimaging techniques.
Moreover, the need for more experienced trainers and mentors in neuroimaging further exacerbates the challenge. The limited number of professionals with significant expertise in neuroimaging techniques makes it difficult to establish robust training programs and mentorship opportunities. The need for more guidance and mentorship for aspiring neuroimagers hampers the development of a skilled workforce, perpetuating the shortage of qualified personnel in neuroimaging.
The shortage of specialized imaging facilities and qualified personnel impacts the availability of neuroimaging services and contributes to healthcare disparities. Rural and underserved areas, in particular, lack access to specialized facilities and personnel. Due to geographic barriers and limited resources, patients in these regions need help accessing neuroimaging services. As a result, they are disproportionately affected by delayed diagnoses, reduced treatment options, and compromised patient outcomes.
C. High Cost of Neuroimaging Equipment and Maintenance
The high cost of neuroimaging equipment and its maintenance is another substantial obstacle to the widespread implementation of these technologies in many healthcare institutions across Africa. The upfront costs of acquiring imaging equipment, such as CT scanners and MRI machines, can be exorbitant and beyond the financial means of resource-constrained facilities. Neuroimaging equipment, especially advanced modalities like MRI, requires significant capital investment. The prices of these machines can range from hundreds of thousands to millions of dollars. For many healthcare institutions in Africa, allocating substantial funding for purchasing neuroimaging equipment becomes a major challenge. The limited financial resources often lead to prioritization of other urgent healthcare needs, resulting in a need for more availability of neuroimaging technologies. In addition to the initial acquisition costs, ongoing maintenance and repairs add to the financial burden. Neuroimaging equipment requires regular servicing, calibration, and technical support to ensure accurate and reliable imaging results. These maintenance expenses can be considerable and require dedicated budgets to sustain the proper functioning of the equipment over its lifespan. Without sufficient resources allocated for maintenance, the equipment deteriorates in performance and lifespan, further hindering access to high-quality neuroimaging services.
Limited availability and high costs create a substantial barrier to accessing African neuroimaging technologies. This situation affects the diagnosis and the management of cerebrovascular disease. Neuroimaging is essential for monitoring disease progression, evaluating treatment effectiveness, and detecting potential complications. Patients experience suboptimal care and outcomes without access to these critical technologies.
D. Dearth of Knowledge and Capacity Building
The dearth of knowledge and the urgent need for capacity building in neuroimaging present significant challenges within the African healthcare context. In an era of rapid advancement in neuroimaging technologies, healthcare professionals must continuously update their skills and knowledge to provide optimal patient care. However, professionals in Africa often face numerous obstacles in acquiring the necessary expertise and knowledge in neuroimaging techniques, which hampers their ability to keep pace with the latest developments in the field. A primary contributing factor to this knowledge gap is the limited availability of training programs and educational resources specifically tailored to neuroimaging within the African region. Many healthcare institutions across Africa lack comprehensive educational curricula and dedicated training opportunities in this specialized field. The scarcity of such programs restricts access to formal education and hinders healthcare providers’ ability to acquire the necessary knowledge and skills in neuroimaging. As a result, professionals may struggle to keep up with the evolving field and the latest advancements in neuroimaging technologies.
Furthermore, the dissemination of knowledge regarding the latest developments in neuroimaging within the African context is often inadequate. The accessibility and availability of up-to-date educational resources, research publications, and guidelines may be limited, making it challenging for healthcare providers to stay informed about the latest techniques, protocols, and best practices in neuroimaging. This lack of access to timely information impedes their ability to provide the most effective and advanced care to patients.
Moreover, the scarcity of qualified trainers and experts in neuroimaging further compounds the challenge. The limited number of professionals with significant experience and expertise in neuroimaging techniques makes it exceedingly difficult to establish robust training programs and mentorship opportunities. The need for proper guidance and mentorship for aspiring neuroimagers significantly hampers the growth of expertise in the field. The lack of experienced trainers and mentors in neuroimaging prevents the effective transfer of knowledge and skills to the next generation of healthcare professionals.
To effectively address the challenges surrounding neuroimaging in Africa, it is crucial to prioritize efforts to increase access to affordable neuroimaging technologies. This can be achieved through various strategies, including investing in cost-effective neuroimaging technologies, exploring innovative imaging solutions, and adapting and optimizing existing neuroimaging technologies for the African context.
A. Increasing Access to Affordable Neuroimaging Technologies
One approach to increase access to affordable neuroimaging technologies is to invest in cost-effective alternatives to traditional high-cost equipment. This involves identifying and implementing imaging solutions that maintain diagnostic accuracy while being more accessible to resource-constrained settings. For example, portable or handheld devices, which are more affordable and easier to transport, can be utilized for certain neuroimaging applications. These devices can provide basic imaging capabilities and serve as a practical option in areas with limited access to specialized imaging facilities.
In addition to exploring cost-effective alternatives, there is a need to develop innovative imaging solutions specifically tailored to the African context. This requires research and development efforts focused on creating affordable, reliable, and well-suited technologies for the unique challenges and resource limitations in African healthcare settings. For instance, developing low-cost, robust imaging devices specifically designed to withstand environmental conditions prevalent in Africa, such as extreme temperatures and power fluctuations, can greatly enhance access to neuroimaging technologies.
Adapting and optimizing existing neuroimaging technologies for the African context is another important strategy to increase accessibility. This involves tailoring the implementation and operation of established technologies to suit the local needs and resource constraints in African healthcare settings. For example, optimizing imaging protocols to minimize scanning times can help address the issue of long waiting periods for imaging procedures, improving patient access to timely diagnostic services. Additionally, considering the specific diseases and conditions prevalent in Africa when developing imaging techniques can ensure the technology is well-suited for accurate diagnosis and effective management.
Collaboration between academia, industry, and healthcare institutions is critical in implementing these strategies. Partnerships between researchers, engineers, and healthcare professionals can facilitate development and implementation of cost-effective and context-specific neuroimaging technologies. Academic institutions can contribute by researching low-cost imaging solutions, while industry partners can provide expertise in technology development, manufacturing, and distribution. Collaboration with healthcare institutions ensures that the technologies are effectively integrated into the existing healthcare infrastructure and workflows, enabling widespread access and utilization.
Efforts to increase access to affordable neuroimaging technologies should involve engaging with regulatory bodies and policymakers to streamline processes and reduce barriers. Advocacy for supportive policies, such as import regulations and tax exemptions for medical equipment, can help reduce the financial burden of acquiring and maintaining neuroimaging technologies. Additionally, initiatives to strengthen local manufacturing capabilities and promote technology transfer can contribute to the long-term sustainability of affordable neuroimaging solutions.
B. Reducing Costs Associated with Neuroimaging
Reducing costs associated with neuroimaging is essential to improve access and affordability of these critical diagnostic services in Africa. Efforts to minimize expenses related to equipment maintenance, repairs, and upgrades, as well as exploring collaborations with manufacturers or third-party service providers, and implementing effective equipment management protocols, can contribute to achieving this objective.
One strategy to reduce costs is to minimize equipment maintenance, repairs, and upgrades expenses. Neuroimaging equipment requires regular servicing, calibration, and technical support to ensure accurate and reliable imaging results. However, these maintenance activities can often be costly, especially when relying on original equipment manufacturers (OEMs) or authorized service providers. Collaborating with local maintenance technicians or third-party service providers who offer competitive pricing can help reduce these expenses without compromising the quality of service. Establishing long-term service contracts or maintenance agreements with these providers can ensure regular equipment upkeep while minimizing costs.
Collaborating with equipment manufacturers or suppliers is another avenue to reduce costs. Healthcare institutions can negotiate favorable pricing or financing options by working closely with manufacturers when acquiring new neuroimaging equipment. This may include bulk purchases, discounted rates, or leasing arrangements that distribute the costs over time. Additionally, engaging with manufacturers in research and development partnerships can lead to the co-development of cost-effective solutions or adaptations of existing technologies specifically tailored to the African context. Such collaborations can help reduce the upfront costs associated with neuroimaging equipment and make it more affordable for healthcare institutions.
Implementing effective equipment management protocols is crucial for optimizing the lifespan and performance of neuroimaging equipment, thus reducing long-term costs. This involves establishing preventive maintenance schedules, implementing quality control measures, and training biomedical engineering staff to ensure proper equipment handling and usage. Adhering to manufacturer-recommended maintenance practices and implementing regular performance checks can help identify potential issues early on and prevent costly breakdowns or downtime. Furthermore, adopting efficient inventory management systems to track spare parts and consumables can help optimize procurement processes and minimize associated costs.
In addition to reducing costs related to equipment, healthcare institutions should also explore opportunities for cost-sharing and resource pooling. Collaborative initiatives among healthcare facilities within a region or consortium can enable joint procurement, sharing of maintenance resources, and knowledge exchange. This can help leverage economies of scale and reduce individual costs for each participating institution. Furthermore, partnerships with government agencies, nonprofit organizations, and philanthropic entities can provide access to funding or grants to reduce costs associated with neuroimaging equipment and services.
Effective cost-reduction strategies should also consider the long-term sustainability of neuroimaging services. This includes planning for equipment replacement cycles, budgeting for ongoing maintenance and repair expenses, and ensuring proper training and capacity building for biomedical engineering staff. Adopting a lifecycle approach to equipment management can help healthcare institutions anticipate and prepare for future costs, ensuring the continued availability and affordability of neuroimaging services.
C. Exploring Alternative Service Delivery Models
Exploring alternative service delivery models is crucial to improving access to Africa’s neuroimaging services. Implementing telemedicine, establishing robust telemedicine infrastructure, and training healthcare professionals in telemedicine are key strategies to bridge the gap between urban and rural areas and expand access to neuroimaging services.
One important alternative service delivery model is the implementation of telemedicine, which involves using telecommunications technology to provide remote healthcare services. Telemedicine can be a valuable tool for bridging the geographical divide and bringing neuroimaging services to underserved rural areas. Through telemedicine, patients in remote locations can have their imaging scans performed locally and have the results transmitted to expert radiologists in urban centres for interpretation. This eliminates the need for patients to travel long distances for imaging services, reducing both the cost and time burden. Moreover, telemedicine enables timely access to specialist expertise, facilitating accurate diagnosis and appropriate treatment planning.
To effectively implement telemedicine, establishing a robust telemedicine infrastructure is essential. This includes developing reliable and secure communication networks that transmit large imaging data files and ensure patient privacy. Adequate internet connectivity, high-speed data transmission, and encryption protocols are critical components of telemedicine infrastructure. Collaborating with telecommunication companies and investing in technology infrastructure can support establishing a robust telemedicine network. Additionally, integrating telemedicine platforms into healthcare information systems can enhance seamless data sharing and streamline teleconsultation.
Training healthcare professionals in telemedicine is another crucial aspect of exploring alternative service delivery models. Healthcare providers need the necessary skills and knowledge to utilize telemedicine technologies and perform remote consultations effectively. Training programs can educate healthcare professionals on telemedicine workflows, equipment operation, data security, and communication protocols. This ensures that healthcare providers are competent in using telemedicine platforms and can confidently diagnose and interpret neuroimaging results remotely. Continuous professional development programs can also keep healthcare professionals up to date with the latest advancements in telemedicine technology and practices.
Moreover, establishing guidelines and standards for telemedicine practices is vital to ensure quality and safety. Developing protocols for patient selection, data management, and communication procedures can promote standardized telemedicine practices across healthcare institutions. This helps maintain the integrity and accuracy of neuroimaging services provided through telemedicine, instilling confidence in both healthcare providers and patients.
Collaboration between healthcare institutions, government agencies, and technology providers is crucial for the successful implementation of telemedicine. Public-private partnerships can support the investment and development of telemedicine infrastructure, ensuring its availability in both urban and rural areas. Government policies and regulations can promote the integration of telemedicine into the healthcare system and provide reimbursement mechanisms to incentivize healthcare providers to offer telemedicine services. Moreover, fostering collaboration with technology companies can lead to the development of user-friendly telemedicine platforms tailored to the African context, considering factors such as low-bandwidth connectivity and mobile device compatibility.
D. Capacity Building for Healthcare Providers
Capacity building for healthcare providers is essential to enhance their skills and knowledge in neuroimaging, thereby improving the diagnosis and treatment of cerebrovascular diseases. To achieve this, offering comprehensive training programs and continuous professional development, fostering collaborations between radiologists and other healthcare professionals, and enhancing knowledge sharing and multidisciplinary approaches to neuroimaging interpretation is crucial.
One key aspect of capacity building is the provision of comprehensive training programs for healthcare providers. These programs should cover various aspects of neuroimaging, including imaging techniques, image interpretation, quality assurance, and safety protocols. Training should be tailored to the specific needs of healthcare professionals, such as radiologists, technicians, and clinicians, and consider the African context. Offering theoretical knowledge and hands-on practical training is important to ensure a comprehensive understanding of neuroimaging principles and techniques. Continuous professional development opportunities should also be provided to keep healthcare providers updated with the latest neuroimaging technology and practices advancements.
Fostering collaborations between radiologists and other healthcare professionals is another important element of capacity building. Neuroimaging interpretation often requires a multidisciplinary approach involving collaboration between radiologists, neurologists, neurosurgeons, and other specialists. Creating forums and platforms for knowledge exchange and collaboration can enhance the understanding of neuroimaging findings and facilitate more comprehensive patient management. Collaborative case discussions, multidisciplinary rounds, and joint research projects can promote a holistic approach to neuroimaging interpretation and improve patient outcomes.
Enhancing knowledge sharing is crucial for capacity building in neuroimaging. Creating platforms for knowledge dissemination, such as conferences, workshops, and online resources, can facilitate sharing of expertise, best practices, and research findings. These platforms should focus on neuroimaging in Africa, addressing specific challenges and highlighting innovative approaches. Collaborations with international organizations, academic institutions, and professional societies can provide access to a broader network of experts and resources, fostering a culture of continuous learning and improvement in neuroimaging practices.
Moreover, promoting a multidisciplinary approach to neuroimaging interpretation can enhance the effectiveness and efficiency of service delivery. A more comprehensive understanding of neuroimaging findings can be achieved by encouraging collaboration and knowledge sharing among healthcare professionals from different disciplines. For example, radiologists can work closely with neurologists to correlate imaging findings with clinical symptoms and provide more accurate and context-specific diagnoses. This multidisciplinary approach can help ensure that neuroimaging results are interpreted in the broader clinical context, leading to more targeted and effective treatment plans.
It is important to allocate adequate resources and funding for training programs, knowledge-sharing platforms, and research collaborations to support capacity-building efforts. Governments, healthcare institutions, and international organizations should prioritize investments in capacity-building initiatives, recognizing its significant impact on improving African neuroimaging services. Funding agencies can provide grants and scholarships to support healthcare professionals’ participation in training programs and research activities. Additionally, partnerships with academic institutions and international experts can facilitate knowledge transfer and mentorship programs, further enhancing capacity-building efforts.
E. Infrastructure Development and Public-Private Partnerships
Infrastructure development and public-private partnerships are vital in expanding access to neuroimaging services in Africa. By building and renovating facilities, collaborating with the public and private sectors to improve infrastructure, and creating partnerships to support neuroimaging initiatives, healthcare systems can enhance the availability and accessibility of neuroimaging technologies.
One critical aspect of infrastructure development is building and renovating facilities to accommodate neuroimaging equipment and services. This involves identifying the needs and demands of the local population, assessing existing infrastructure gaps, and strategically planning the location and design of new healthcare facilities. Building dedicated imaging centers or incorporating neuroimaging departments within existing healthcare facilities can provide a centralized hub for neuroimaging services. Additionally, renovating existing facilities to meet the technical requirements of neuroimaging equipment, such as CT scanners and MRI machines, ensures these technologies’ safe and efficient operation.
Collaborating with the public and private sectors is essential to improve infrastructure for neuroimaging services. Public sector involvement, such as government agencies and ministries of health, can provide the necessary regulatory frameworks, policies, and funding to support infrastructure development. Governments can allocate resources for building new facilities, upgrading existing ones, and investing in essential equipment. Public-private partnerships can also be established, leveraging the expertise and resources of private companies to support infrastructure development. Collaboration with private sector entities, such as healthcare technology companies and equipment manufacturers, can help ensure the availability of state-of-the-art neuroimaging technologies and support maintenance and servicing requirements.
Creating partnerships is crucial to supporting neuroimaging initiatives and addressing infrastructure challenges. Collaboration with international organizations, academic institutions, non-governmental organizations (NGOs), and philanthropic foundations can provide additional resources and expertise to support infrastructure development. These partnerships can offer technical assistance, funding opportunities, and knowledge transfer to strengthen healthcare systems’ capacity for neuroimaging services. For example, international organizations may provide guidance on best practices in infrastructure planning and offer grants for infrastructure development projects. NGOs and foundations may contribute funding or equipment donations to support the establishment or renovation of neuroimaging facilities.
Engaging local communities and stakeholders is also essential in infrastructure development. Involving community leaders, patient advocacy groups, and healthcare professionals in the planning and decision-making processes ensures that infrastructure development aligns with the needs and cultural sensitivities of the local population. This participatory approach fosters a sense of ownership and collaboration, facilitating neuroimaging infrastructure’s successful implementation and sustainability.
Additionally, promoting research and evaluation of infrastructure development initiatives is crucial to assess their impact and identify areas for improvement. Conducting studies on the effectiveness and efficiency of newly established neuroimaging facilities, evaluating patient outcomes, and assessing the cost-effectiveness of infrastructure investments can provide valuable insights for future planning and resource allocation.
F. Existing Initiatives and Collaborations
Existing initiatives and collaborations are playing a significant role in improving access to neuroimaging technologies in Africa. Efforts such as the Consortium for Advancement of MRI Education and Research in Africa (CAMERA) and RAD-AID are making strides. These initiatives focus on supporting sustainable MRI training and mentorship programs and utilizing data-driven approaches for planning and implementing neuroimaging projects.
The Consortium for Advancement of MRI Education and Research in Africa (CAMERA) is a notable initiative sponsored by the Chan Zuckerberg Initiative. CAMERA consists of African MRI experts and global partners committed to establishing a sustainable MRI training and mentorship program in African countries. The program aims to provide adequate training for emerging African MRI scientists and technologists. It involves the establishment of an African Chapter of the International Society of Magnetic Resonance in Medicine (ISMRM), which fosters collaboration and knowledge exchange among professionals. Additionally, CAMERA focuses on building a mentorship program for early-career African MRI scientists, developing an online MRI resource platform, and providing hands-on training courses for MRI engineers. These initiatives enhance the capacity and expertise in MRI technology within the African healthcare context.
RAD-AID is another impactful initiative focused on improving access to neuroimaging technologies in underserved communities, including those in Africa. RAD-AID is an international charitable organization that employs a cyclic iteration of data gathering, analysis, planning, self-correction, and new data collection to evaluate, plan, and carry out projects. RAD-AID tailors radiology projects to specific requirements through its data-driven approach, considering the local context and needs. The organization implements various interventions, such as setting up training sessions, establishing workstations, installing equipment, publishing research, and creating new technology. By working collaboratively with local healthcare providers, RAD-AID aims to strengthen capacity, promote sustainable practices, and improve the quality of radiology services, including neuroimaging, in underserved areas of Africa.
These initiatives highlight the importance of sustainable training and mentorship programs, which are critical for developing a skilled workforce in neuroimaging. By providing comprehensive training and mentorship opportunities, emerging healthcare professionals can gain the necessary expertise and knowledge to operate neuroimaging technologies effectively. This capacity-building approach ensures the continuity of high-quality neuroimaging services in Africa.
Moreover, utilizing data-driven approaches for planning and implementing neuroimaging projects is crucial for maximizing the impact and effectiveness of these initiatives. By collecting and analyzing data on healthcare infrastructure, disease burden, and resource availability, stakeholders can make informed decisions regarding the allocation of resources and implementation strategies. Data-driven approaches help identify areas of high need, optimize the distribution of neuroimaging technologies, and assess the impact of interventions. This approach also enables ongoing evaluation and adjustment of initiatives to ensure their sustainability and effectiveness.
Furthermore, collaboration among stakeholders, including governments, healthcare institutions, academic institutions, international organizations, and local communities, is vital for the success of initiatives aiming to improve access to neuroimaging technologies. Partnerships foster knowledge sharing, resource mobilization, and collective problem-solving. They provide opportunities for technical assistance, funding support, and capacity building. Collaborations also enable sharing of best practices and lessons learned, contributing to developing sustainable and context-specific approaches to neuroimaging in Africa.
Continued support and investment in initiatives like CAMERA and RAD-AID are crucial to advance access to neuroimaging technologies. These initiatives are models for sustainable training programs, mentorship, and data-driven implementation approaches. By leveraging existing collaborations and fostering new partnerships, stakeholders can collectively work toward expanding access to neuroimaging technologies, ultimately improving the diagnosis and treatment of neurological conditions in Africa.
Conclusion
Neuroimaging plays a critical role in diagnosing and managing cerebrovascular disease in Africa. The region faces a significant burden of cerebrovascular disease, characterized by high morbidity and mortality rates. Neuroimaging techniques, such as CT and MRI, provide valuable insights into the structure and function of the brain, enabling the identification and classification of different types of strokes and their underlying causes. Additionally, neuroimaging facilitates disease monitoring, treatment evaluation, and the detection of potential complications. However, the effective utilization of neuroimaging in Africa is impeded by various challenges. Limited healthcare infrastructure, including a shortage of specialized imaging facilities and trained personnel, hampers the timely and accurate diagnosis of cerebrovascular disease. Moreover, geographic disparities exist, with rural and remote areas experiencing limited access to neuroimaging services. Financial constraints associated with the high costs of neuroimaging equipment and maintenance further contribute to the barriers faced by resource-constrained healthcare institutions. To address these challenges, concerted efforts are required to increase access to affordable neuroimaging technologies and reduce associated costs. This necessitates investments in cost-effective equipment, exploration of innovative imaging solutions, and the adaptation and optimization of neuroimaging technologies to suit the African context. Measures to minimize expenses related to equipment maintenance, repairs, and upgrades, along with implementing effective equipment management protocols, are also essential in reducing financial burdens. Exploring alternative service delivery models, such as telemedicine, holds promise in bridging the gap between urban and rural areas and expanding access to neuroimaging services. Robust telemedicine infrastructure and comprehensive training for healthcare professionals in telemedicine are integral to the successful implementation of this approach. Capacity building for healthcare providers constitutes another crucial component. The provision of comprehensive training programs and the promotion of collaborations among radiologists and other healthcare professionals foster knowledge sharing and multidisciplinary approaches to neuroimaging interpretation. Infrastructure development and public-private partnerships are instrumental in improving access to neuroimaging services. Building and renovating facilities, collaborative efforts with the public and private sectors, and establishing partnerships contribute to addressing healthcare infrastructure limitations and enhancing neuroimaging technologies’ availability. Furthermore, existing initiatives and collaborations, exemplified by CAMERA and RAD-AID, demonstrate successful endeavors in improving access to neuroimaging technologies in Africa. Supporting sustainable training and mentorship programs and using data-driven approaches for planning and implementing neuroimaging projects are pivotal in driving progress in this domain.
Authors’ contributions
NA and DO conceived the idea for the review and conducted the literature search. Prior to submission, two independent reviewers were invited to vet the articles included in this review for relevance and accuracy, and their feedback was incorporated into the final version of the manuscript. NA and MA extracted and analyzed the data. MA and ME provided critical feedback on the manuscript. NA drafted the initial manuscript. All authors contributed to the interpretation of the findings, revised the manuscript critically, and approved the final version. NA is the corresponding author and takes full responsibility for the integrity of the work as a whole, from inception to publication.
Author’s statement
All author meet the four criteria.
Disclosure statement
All author declares no conflict of interest.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the reviews.
Additional information
Funding
References
- Khaku AS, Tadi P. 2022. Cerebrovascular disease. StatPearls Publishing.
- Akinyemi RO, Ovbiagele B, Adeniji OA, et al. Stroke in Africa: profile, progress, prospects and priorities. Nat Rev Neurol. 2021;17(10):1–17. doi: 10.1038/s41582-021-00542-4.
- Akinyemi RO, Owolabi MO, Ihara M, et al. Stroke, cerebrovascular diseases and vascular cognitive impairment in africa. Brain Res Bull. 2019;145:97–108. doi: 10.1016/j.brainresbull.2018.05.018.
- Keates AK, Mocumbi AO, Ntsekhe M, et al. Cardiovascular disease in africa: epidemiological profile and challenges. Nat Rev Cardiol. 2017;14(5):273–293. doi: 10.1038/nrcardio.2017.19.
- Paradise MB, Shepherd CE, Wen W, et al. Neuroimaging and neuropathology indices of cerebrovascular disease burden: a systematic review. Neurology. 2018;91(7):310–320. doi: 10.1212/WNL.0000000000005997.
- Juttukonda MR, Donahue MJ. Neuroimaging of vascular reserve in patients with cerebrovascular diseases. Neuroimage. 2019;187:192–208. doi: 10.1016/j.neuroimage.2017.10.015.
- Cirillo L, Rustici A, Toni F, et al. Vessel wall MRI: clinical implementation in cerebrovascular disorders-technical aspects. Radiol Med. 2022;127(6):645–651. doi: 10.1007/s11547-022-01484-7.
- Fouche PE, Jenkins LS, Vermeulen A. Appropriateness of computed tomography and magnetic resonance imaging scans in a rural regional hospital in South Africa: a 6-year follow-up study. S Afr Med J. 2020;111(1):46–51. doi: 10.7196/SAMJ.2020.v111i1.14860.
- Mather R. Multislice CT: 64 slices and beyond. Radiol Manag. 2005;27(3):46–52.
- Flohr T, Ohnesorge B, Schaller S. Heutiger Stand und zukünftige Entwicklungen in der Mehrschichtcomputertomographie [Current status and future potentials of multislice computed tomography]. Radiologe. 2004;44(2):113–120. doi: 10.1007/s00117-003-1011-7.
- Huynh K, Baghdanian AH, Baghdanian AA, et al. Updated guidelines for intravenous contrast use for CT and MRI. Emerg Radiol. 2020;27(2):115–126. doi: 10.1007/s10140-020-01751-y.
- Smirniotopoulos JG, Murphy FM, Rushing EJ, et al. Patterns of contrast enhancement in the brain and meninges. Radiographics. 2007;27(2):525–551. doi: 10.1148/rg.272065155.
- Adejoh T, Onwujekwe EC, Abba M, et al. Computed tomography scanner census and adult head dose in Nigeria. Egyptian JRadiol Nucl Med. 2018;49(1):66–70. doi: 10.1016/j.ejrnm.2017.09.001.
- Muhogora WE, Rehani MM. 2009. Patient doses in CT and radiography in Africa. In: Dössel, O., Schlegel, W.C. (editors) World congress on medical physics and biomedical engineering, september 7 - 12, 2009, Munich, Germany. IFMBE proceedings., vol 25/3. Berlin, Heidelberg: Springer. doi: 10.1007/978-3-642-03902-7_153.
- Potter CA, Vagal AS, Goyal M, et al. CT for treatment selection in acute ischemic stroke: a code stroke primer. Radiographics. 2019;39(6):1717–1738. doi: 10.1148/rg.2019190142.
- Althaus K, Dreyhaupt J, Hyrenbach S, et al. MRI as a first-line imaging modality in acute ischemic stroke: a sustainable concept. Ther Adv Neurol Disord. 2021;14:17562864211030363. doi: 10.1177/17562864211030363.
- Ogbole GI, Owolabi MO, Ogun O, et al. Time of presentation of stroke patients for CT imaging in a Nigerian tertiary hospital. Ann Ib Postgrad Med. 2015;13(1):23–28.
- Howard SA, Rosenthal MH, Qin L, et al. Quantifying decreased radiation exposure from modern CT scan technology and surveillance programs of germ cell tumors. Am J Clin Oncol. 2018;41(10):949–952. doi: 10.1097/COC.0000000000000399.
- Douglas DB, Muldermans JL, Wintermark M. Neuroimaging of brain trauma. Curr Opin Neurol. 2018;31(4):362–370. doi: 10.1097/WCO.0000000000000567.
- Byrne D, Walsh JP, Sugrue G, et al. CT imaging of acute ischemic stroke [formula: see text]. Can Assoc Radiol J. 2020;71(3):266–280. doi: 10.1177/0846537120902068.
- Wang X, Zhang L, Sun W, et al. Changes of metabolites in acute ischemic stroke and its subtypes. Front Neurosci. 2020;14:580929. doi: 10.3389/fnins.2020.580929.
- Viard A, Eustache F, Segobin S. History of magnetic resonance imaging: a trip down memory lane. Neuroscience. 2021;474:3–13. doi: 10.1016/j.neuroscience.2021.06.038.
- Poustchi-Amin M, Mirowitz SA, Brown JJ, et al. Principles and applications of echo-planar imaging: a review for the general radiologist. Radiographics. 2001;21(3):767–779. doi: 10.1148/radiographics.21.3.g01ma23767.
- Ogbole GI, Adeyomoye AO, Badu-Peprah A, et al. Survey of magnetic resonance imaging availability in West Africa. Pan Afr Med J. 2018;30:240. doi: 10.11604/pamj.2018.30.240.14000.
- Sammet S. Magnetic resonance safety. Abdom Radiol. 2016;41(3):444–451. doi: 10.1007/s00261-016-0680-4.
- Adebayo OD, Culpan G. Diagnostic accuracy of computed tomography perfusion in the prediction of haemorrhagic transformation and patient outcome in acute ischaemic stroke: a systematic review and meta-analysis. Eur Stroke J. 2020;5(1):4–16. doi: 10.1177/2396987319883461.
- Kawooya MG, Kisembo HN, Remedios D, et al. An Africa point of view on quality and safety in imaging. Insights Imaging. 2022;13(1):58. doi: 10.1186/s13244-022-01203-w.
- Urimubenshi G, Cadilhac DA, Kagwiza JN, et al. Stroke care in africa: a systematic review of the literature. Int J Stroke. 2018;13(8):797–805. doi: 10.1177/1747493018772747.
- Baatiema L, Otim M, Mnatzaganian G, et al. Towards best practice in acute stroke care in Ghana: a survey of hospital services. BMC Health Serv Res. 2017;17(1):108. doi: 10.1186/s12913-017-2061-2.
- Baatiema L, de-Graft Aikins A, Sav A, et al. Barriers to evidence-based acute stroke care in Ghana: a qualitative study on the perspectives of stroke care professionals. BMJ Open. 2017;7(4):e015385. doi: 10.1136/bmjopen-2016-015385.
- Lameka K, Farwell MD, Ichise M. Positron emission tomography. Handb Clin Neurol. 2016;135:209–227. doi: 10.1016/B978-0-444-53485-9.00011-8.
- Hall H, Cuellar-Baena S, Dahlberg C, et al. Magnetic resonance spectroscopic methods for the assessment of metabolic functions in the diseased brain. Curr Top Behav Neurosci. 2012;11:169–198. doi: 10.1007/7854_2011_166.
- Carnevale L, Lembo G. Innovative MRI techniques in neuroimaging approaches for cerebrovascular diseases and vascular cognitive impairment. Int J Mol Sci. 2019;20(11):2656. doi: 10.3390/ijms20112656.
- Ferro JM, Infante J. Cerebrovascular manifestations in hematological diseases: an update. J Neurol. 2021;268(9):3480–3492. doi: 10.1007/s00415-021-10441-9.
- Wang C, Yin Z, Zhang X, et al. Clinical significance of hyperdense lesions on non-enhanced brain CT obtained immediately after arterial revascularization in acute ischemic stroke patients. Comput Math Methods Med. 2021;2021:1562502. doi: 10.1155/2021/1562502.
- Pop NO, Tit DM, Diaconu CC, et al. The Alberta stroke program early CT score (ASPECTS): a predictor of mortality in acute ischemic stroke. Exp Ther Med. 2021;22(6):1371. doi: 10.3892/etm.2021.10805.
- Prakkamakul S, Yoo AJ. ASPECTS CT in acute ischemia: review of current data. Top Magn Reson Imaging. 2017;26(3):103–112. doi: 10.1097/RMR.0000000000000122.
- Fransen PS, Beumer D, Berkhemer OA, et al. MR CLEAN, a multicenter randomized clinical trial of endovascular treatment for acute ischemic stroke in The Netherlands: study protocol for a randomized controlled trial. Trials. 2014;15(1):343. doi: 10.1186/1745-6215-15-343.
- Hsieh YZ, Luo YC, Pan C, et al. Cerebral small vessel disease biomarkers detection on MRI-sensor-based image and deep learning. Sensors. 2019;19(11):2573. doi: 10.3390/s19112573.
- Elmegiri M, Koivunen RJ, Tatlisumak T, et al. MRI characterization of non-traumatic intracerebral hemorrhage in young adults. Front Neurol. 2020;11:558680. doi: 10.3389/fneur.2020.558680.
- Haller S, Haacke EM, Thurnher MM, et al. Susceptibility-weighted imaging: technical essentials and clinical neurologic applications. Radiology. 2021;299(1):3–26. doi: 10.1148/radiol.2021203071.
- Bourcier R, Duchmann Z, Sgreccia A, et al. Diagnostic performances of the susceptibility vessel sign on MRI for the prediction of macroscopic thrombi features in acute ischemic stroke. J Stroke Cerebrovasc Dis. 2020;29(11):105245. doi: 10.1016/j.jstrokecerebrovasdis.2020.105245.
- Grøan M, Ospel J, Ajmi S, et al. Time-based decision making for reperfusion in acute ischemic stroke. Front Neurol. 2021;12:728012. doi: 10.3389/fneur.2021.728012.
- Murphy SJ, Werring DJ. Stroke: causes and clinical features. Medicine. 2020;48(9):561–566. ) doi: 10.1016/j.mpmed.2020.06.002.
- Zhelev Z, Walker G, Henschke N, et al. Prehospital stroke scales as screening tools for early identification of stroke and transient ischemic attack. Cochrane Database Syst Rev. 2019;4(4):CD011427. doi: 10.1002/14651858.CD011427.pub2.
- Llanes JN, Kidwell CS, Starkman S, et al. The los angeles motor scale (LAMS): a new measure to characterize stroke severity in the field. Prehosp Emerg Care. 2004;8(1):46–50. doi: 10.1080/312703002806.
- Unnithan AKA, M Das J, Mehta P. 2022). Hemorrhagic stroke. StatPearls Publishing.
- de Bresser J, Kuijf HJ, Zaanen K, ., et al. White matter hyperintensity shape and location feature analysis on brain MRI; proof of principle study in patients with diabetes. Sci Rep. 2018;8(1):1893. doi: 10.1038/s41598-018-20084-y.
- Hilditch CA, Nicholson P, Murad MH, et al. Endovascular management of acute stroke in the elderly: a systematic review and meta-analysis. AJNR Am J Neuroradiol. 2018;39(5):887–891. doi: 10.3174/ajnr.A5598.
- Manca R, De Marco M, Ince PG, et al. Heterogeneity in regional damage detected by neuroimaging and neuropathological studies in older adults with COVID-19: a cognitive-neuroscience systematic review to inform the long-term impact of the virus on neurocognitive trajectories. Front Aging Neurosci. 2021;13:646908. doi: 10.3389/fnagi.2021.646908.
- Madonna D, Delvecchio G, Soares JC, et al. Structural and functional neuroimaging studies in generalized anxiety disorder: a systematic review. Braz J Psychiatry. 2019;41(4):336–362. doi: 10.1590/1516-4446-2018-0108.