Abstract
Objective
Non-variceal upper gastrointestinal bleeding (NVUGIB) in patients receiving oral anticoagulants (OACs) may be fatal; however, little is known about re-bleeding and all-cause mortality after successful hemostasis. We investigated the clinical characteristics and risk factors for re-bleeding and death after successful hemostasis.
Methods
Patients receiving OACs and diagnosed with NVUGIB between 2007 and 2021 were enrolled. All NVUGIB incidents were confirmed if definite bleeding in the upper gastrointestinal tract was detected via esophagogastroduodenoscopy.
Results
A total of 132 patients receiving OACs were diagnosed with NVUGIB. Males were the majority (72, 54.5%), and bleeding was detected mostly in the stomach (99, 75%) and was most often due to peptic ulcers (PU) (88, 66.7%). After successful hemostasis of index NVUGIB, 40 patients (30.3%) experienced re-bleeding. Among them, 15 (37.5%) died, and among those, 3 (2.3%) were related to re-bleeding. Multivariate analysis revealed that duodenal bleeding (odds ratio [OR]: 3.305; 95% confidence interval [CI]: 1.152-9.479, p = 0.026) and Charlson comorbidity index score (CCI) (OR: 1.22; 95% CI: 1.052–1.419, p = 0.009) were significant risk factors for re-bleeding. Index albumin levels (OR: 0.134; 95% CI: 0.035–0.506, p = 0.003), previous PU or upper gastrointestinal bleeding (UGIB) history (OR: 4.626; 95% CI: 1.375–15.567, p = 0.013), and CCI (OR: 1.293; 95% CI: 1.058–1.581, p = 0.012) were related all-cause mortality.
Conclusion
CCI and duodenal bleeding are risk factors for re-bleeding in patients with NVUGIB who were receiving OACs, while low index albumin levels and previous PU and UGIB history are associated with all-cause mortality.
KEY MESSAGES
While taking oral anticoagulants can offer various benefits, the risks of re-bleeding and all-cause mortality remain.
A Charlson comorbidity index of higher than 4.5 and duodenal bleeding occurring while receiving oral anticoagulants increase the risk of rebleeding.
Hypoalbuminemia <3.25 g/dL, history of peptic ulcer or upper gastrointestinal bleeding and Charlson comorbidity index were significant risk factors for all-cause mortality.
1. Introduction
As the elderly population increases, the prevalence of cardiovascular, cerebrovascular, and peripheral arterial diseases increases, resulting in an increased number of patients receiving oral anticoagulants (OACs) [Citation1]. OACs are associated with decreased all-cause mortality rates, fewer vascular disease incidents, and improved quality of life [Citation2–4]. Despite these beneficial effects, OAC use has been associated with a four-fold increased risk of gastrointestinal bleeding (GIB) [Citation5], which is significantly associated with increased all-cause mortality, increased need for surgery and transfusion, prolonged hospitalization, and increased overall healthcare costs in the older adults [Citation6,Citation7].
Endoscopic hemostasis and radiologic embolization are feasible and effective hemostatic methods for GIB, with endoscopic hemostasis being the most common treatment [Citation8,Citation9]. Despite the emergence of novel endoscopic hemostatic technologies, such as new mechanical hemoclips, noncontact pharmacological hemostatic agents, new thermal hemostatic devices, and medications for suppressed acid production in the last decade, upper GIB (UGIB) is associated with significant re-bleeding (15–20%) and all-cause mortality rates [Citation10]. Although scoring systems have been developed to predict re-bleeding, which increases all-cause mortality rates and medical costs, the causes of re-bleeding and the associated risk factors for all-cause mortality are still unclear [Citation11–13].
Oral anticoagulants come in two categories: warfarin, which has been traditionally used, and direct oral anticoagulants (DOACs), also known as non-vitamin K antagonist oral anticoagulants (NOACs), which are a newer orally administered class of anticoagulants. The mechanism of action of warfarin does not directly affect vitamin K; however, it rather inhibits the enzyme vitamin K epoxide reductase, which recycles oxidized vitamin K, resulting in an anti-coagulant effect. Moreover, warfarin not only inhibits vitamin K-dependent clotting factors II, VII, IX, and X, but also inhibits regulatory factors such as protein C, protein S, and protein Z [Citation14].
In contrast, DOACs induce anticoagulation through a different mechanism. According to their mechanism of anticoagulation action, DOACs are divided into two groups: direct inhibitors of thrombin (dabigatran) and direct inhibitors of activated factor X (FXa) (rivaroxaban, apixaban, edoxaban, and betrixaban). In terms of the start of the action, pharmacokinetic and pharmacodynamic properties, and a fixed daily dose that does not require routine coagulation monitoring, DOACs are superior compared with vitamin K antagonists.
In previous studies, DOACs were shown to be associated with a lower overall risk of GIB than warfarin [Citation15–17]. Regarding the GIB risk among the different DOACs, both dabigatran and rivaroxaban were associated with a higher risk of GIB (OR: 1.58 and 1.48, respectively), while apixaban and edoxaban showed no association. However, in other studies comparing DOACs as a group to warfarin, the risk of major GIB was similar. When conducting dose-based comparisons, standard doses of dabigatran, rivaroxaban, and edoxaban showed higher major GIB risk compared to warfarin, while standard-dose apixaban exhibited a lower major GIB risk compared to other standard-dose DOACs [Citation18]. However, as no direct head-to-head comparisons of GIB risk among various NOACs were conducted in randomized controlled trials, it is challenging to determine which drug has the lowest GIB risk [Citation19]. Furthermore, little is understood about the re-bleeding and all-cause mortality associated with non-variceal UGIB (NVUGIB) that occurs during receiving OAC therapy. This study has aimed to evaluate the clinical outcomes and potential predictive factors associated with re-bleeding and all-cause mortality in patients who experience NVUGIB while receiving OACs.
2. Materials and methods
2.1. Patients and data collection
This retrospective observational study included patients aged >18 years who required hospitalization or visited the emergency room because of UGIB episodes between January 2000 and December 2021. We enrolled 132 patients diagnosed with NVUGIB, all of whom were receiving OACs, such as warfarin, apixaban, edoxaban, dabigatran, and rivaroxaban when the index NVUGIB episodes occurred. For each OAC, warfarin dosage was adjusted based on INR values monitored during follow-up period. On the other hand, NOACs were initiated with dose reductions determined by factors such as kidney function, body weight, and age. For Apixaban, if the patient’s Creatinine clearance (CrCl) is >50 mL/min, the dosage is 5 mg twice daily. However, for patients aged ≥ 80 years, body weight ≤60kg, or serum creatinine ≥1.5 mg/dL, the dosage is reduced to 2.5 mg twice daily [Citation20]. Rivaroxaban was administered at a dose of 20 mg once daily if the patient’s CrCl was >50 mL/day. However, for patients with CrCl ≤50 mL/min, the dosage is reduced to 15 mg once daily. Edoxaban was administered at a dose of 60 mg once a day if the patient’s body weight was >60 kg. If the body weight is ≤60kg, the dosage is reduced to 30 mg once daily. When CrCl is >50 mL/min, the dosage remains at 60 mg once daily, and for patients with CrCl ranging from 15 to 50 mL/min, the dosage is reduced to 30 mg once daily [Citation21]. Regarding Dabigatran, for patients with CrCl >30 mL/min, 150 mg was administered twice a day (BID). However, for patients with CrCl ranging from 15 to 30 mL/min, the dosage is reduced to 75 mg BID [Citation22]. We performed Esophagogastroduodenoscopy (EGD) to confirm NVUGIB in all enrolled patients and excluded those with lower GI or variceal bleeding.
We collected patient data by reviewing medical records and gathering information on demographic data; medication history, including aspirin and non-steroidal anti-inflammatory drugs (NSAIDs); hemodynamic status; laboratory test results; and endoscopic findings, including the location of bleeding and hemostasis, the need for radiologic intervention, and the Helicobacter pylori (H.pylori) infection. Based on these data, the re-bleeding and all-cause mortality rates were determined. Moreover, drug management during hospitalization was recorded in terms of interruptions, resumption, time of interruption, and drug use at discharge.
At the time of admission, all enrolled patients started receiving antisecretory treatment with high doses of proton pump inhibitors (PPIs), that is, 80 mg IV bolus, followed by 8 mg/h continuous infusion for 72 h in all cases that required endoscopic hemostatic treatment, according to the relevant guidelines [Citation23]. All index NVUGIB episodes were stabilized by endoscopic hemostasis, radiological embolization, or pharmacological treatment. All clinical information was recorded in a database using Microsoft Office Excel (Redmond, Washington, USA, 2016).
2.2. Definitions
NVUGIB was defined as a ≥ 2 g/dL decrease in hemoglobin with the presence of at least one of the following symptoms or signs: hematemesis, melena, hematochezia, endoscopic active bleeding, or bleeding stigmata (signs of recent bleeding shown via endoscopy), and when an EGD reveals a non-variceal bleeding source in the UGI tract proximal to the ligament of Treitz [Citation24].
Recurrent bleeding following initial hemostasis is commonly defined as recurrent hematemesis or bloody nasogastric aspirate after index endoscopy; recurrent tachycardia or hypotension after achieving hemodynamic stability; melena and/or hematochezia following the normalization of stool color, or a decrease in hemoglobin level by ≥2 g/dL after achieving a stable value [Citation25,Citation26]. Moreover, all recurrent NVUGIB incidents were confirmed via an endoscopic examination. All-cause mortality refers to death identified in inpatient settings.
2.3. Statistical analysis
Continuous parameters were presented as mean ± standard deviation and discrete parameters were presented as numbers and percentages (%). Group comparisons were performed using independent sample t-tests for continuous data and Pearson’s chi-squared tests for categorical data. A multivariate logistic regression analysis was used to test the eligible prognostic factors for re-bleeding and all-cause mortality. Results were considered statistically significant at p-values < 0.05. Statistical analyses were performed using IBM SPSS Statistics 22.0 (Armonk, New York, USA).
3. Results
3.1. Patients and baseline characteristics
A total of 132 patients (72 males & 60 females, mean age: 73.0 ± 10.1 years) were enrolled in this study. Warfarin (n = 89; 67.4%), apixaban (n = 19; 14.4%), rivaroxaban (n = 16; 12.1%), edoxaban (n = 5; 3.8%), and dabigatran (n = 19; 14.4%) were the OAC types being used. After the index NVUGIB episodes, OACs were discontinued in 40 patients and continued in the remaining 92 based on the judgment of the prescribing physicians. Moreover, 59 patients (46.1%) were receiving other drugs such as antiplatelet agents, NSAIDs, or steroids. Among the 59 patients, 48 patients (36.4%) were receiving anti-platelet drugs in combination with OAC. Specifically, 32 patients (24.2%) were receiving aspirin, 28 patients (21.2%) were receiving clopidogrel, and 13 patients (9.8%) were receiving both aspirin and clopidogrel together (DAPT). Additionally, 1 patient (0.8%) was receiving triflusal, another type of anti-platelet drug. In the re-bleeding group, 14 patients (35%) were concurrently receiving anti-platelet drugs and OAC. In the non-rebleeding group, 34 patients (37%) were receiving both anti-platelet drugs and OAC together. However, no significant difference was observed between the two groups (p = 0.830) (). Regarding the mortality group, 10 patients (35.7%) were concurrently receiving anti-platelet drugs and OAC. In the non-mortality group, 38 patients (36.5%) were receiving both anti-platelet and OAC. There was no significant difference between the two groups (p = 0.936) (Supplementary Table 1). The most common symptom was melena (n = 83; 62.9%), followed by anemia (n = 24; 18.2%), hematemesis (n = 16; 12.1%), and hematochezia (n = 9; 6.8%). More than half of the patients experienced atrial fibrillation (82; 63.6%) or hypertension (71; 53.8%). Previous UGIB history was confirmed in 25 patients (18.9%).
Table 1. Baseline characteristics of initial NVUGIB.
Males were predominant in the non-re-bleeding group compared with the re-bleeding group (56 [60.9%] vs. 16 [40%], respectively, p = 0.027). Other variables such as the OAC type, comorbidities, combinations of other drugs, symptoms or signs, and previous UGIB history were not significantly different between the two groups (). Of the 132 patients, 28 (21.2%) passed away during the follow-up period. Significant differences in underlying malignancy (24% vs. 57.1%, p = 0.001), underlying cerebrovascular attack (26.9% vs. 3.6%, p = 0.008), and previous UGIB history (14.4% vs. 35.7%, p = 0.011) were observed between the all-cause mortality group and the non-all-cause mortality group, respectively. The other variables were not significantly different between the two groups (Supplementary Table 1).
3.2. Clinical characteristics and outcomes
Index bleeding most commonly occurred in the stomach (75%), followed by the duodenum (17.4%) and esophagus (7.6%). The most common cause of bleeding was peptic ulcers (PU) (n = 88; 66.7%), followed by UGI malignancy (n = 24; 18.2%), Mallory-Weiss tear (n = 5; 3.8%), angiodysplasia (n = 5; 3.8%), anastomotic bleeding (n = 5; 3.8%), and others such as erosion and pancreatic pseudoaneurysm (n = 5; 3.8%). OAC inhibitors were administered after hospitalization, especially after initial laboratory tests. Vitamin K was administered to patients receiving warfarin. However, in the case of patients taking NOACs, we could not administer them because our hospital did not have inhibitors.
After successful hemostasis was achieved, we analyzed the clinical course of the enrolled patients. The median time of follow-up was 51.9 months (Interquartile Range: 14.42-128.02), and a total of 40 patients (30.3%) experienced re-bleeding. Of these 40 patients, two patients (5%) demonstrated four re-bleeding episodes, five (12.5%) demonstrated two re-bleeding episodes, and 33 (82.5%) demonstrated one re-bleeding episode. The mean number of re-bleeding episodes was 1.27 ± 0.113. The mean duration from the occurrence of index bleeding to the occurrence of re-bleeding was 24.7 ± 32.2 months.
The mean Charlson comorbidity index (CCI) score was 6.1 ± 4.9, and it was significantly higher in the re-bleeding group than in the non-re-bleeding group (5.4 ± 2.4 vs. 7.8 ± 7.9, p = 0.009). Other prediction scores, such as the Rockall Score (RS) and Glasgow-Blatchford Score (GBS), did not differ between both groups ().
Table 2. Clinical characteristics of initial NVUGIB.
Most bleeding episodes were stabilized by administering pharmacological therapy; however, 37 patients (28%) required endoscopic hemostasis, and one (0.8%) required radiological embolization. The endoscopic hemostatic methods being used included electrocautery probe in 28 patients (21.2%), clip placement in 5 (3.8%), endoscopic band ligation in 2 (1.5%), a combination of epinephrine injection and electrocoagulation in 1 (0.8%), and argon plasma coagulation in 1 (0.8%).
Significant differences in UGI malignancy (13.5% vs. 35.7%, p = 0.007), albumin levels (3.30 ± 0.38 vs. 3.02 ± 0.48, p = 0.003), and OACs resumption (74.8% vs. 53.6%, p = 0.03) were observed in the two groups stratified by all-cause mortality (Supplementary Table 2).
We also analyzed for H. pylori infection. To confirm the presence of H. pylori infection, we performed Giemsa stain or rapid urease test. H. pylori infection was confirmed in 78 of 132 patients (59.1%), of which 30 (38.5%) tested positive for H. pylori. Eradication therapy was administered in 18 (60%) of positive patients. Among the patients who received eradication therapy, eradication failed in 1 patient (5.6%) and was successful in 14 patients (77.8%). The results for the remaining 3 patients (16.6%) could not be confirmed by follow-up loss.
3.3. Re-bleeding and the associated predictive factors
Among the 132 patients receiving OACs, re-bleeding occurred in 40 (30.3%). Univariate analysis showed that female (p = 0.029), duodenal bleeding (p = 0.049), malignancy (p = 0.024), CCI scores (p = 0.016), and clipping method (p = 0.042) were significantly associated with re-bleeding. However, a multivariate analysis showed that duodenal bleeding (odds ratio [OR]: 3.305; 95% confidence interval [CI]: 1.152–9.479, p = 0.026), and CCI scores (OR: 1.22; 95% CI: 1.052–1.1419, p = 0.009) were significantly relevant factors (). If multivariable analysis is performed including both comorbidities and CCI, a multicollinearity issue may occur between them. Therefore, we checked for collinearity issues related to CCI and its included comorbidities. The analysis showed that all Variance Inflation Factor (VIF) values were below 10, indicating that there was no multicollinearity issue (Supplementary Table 3).
Table 3. Regression analysis for re-bleeding.
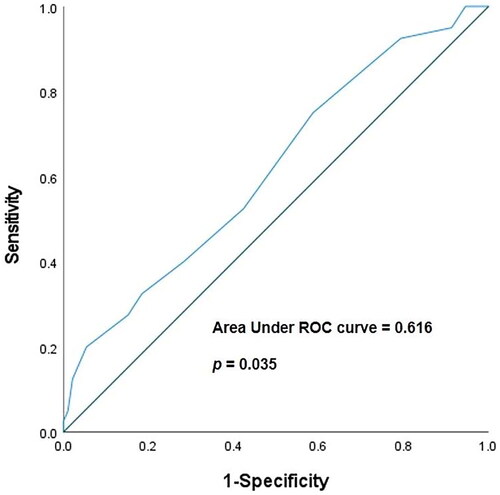
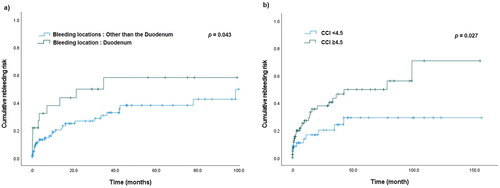
Using Youden’s J statistical method, we calculated the cut-off value, sensitivity, and specificity of the predicted CCI score for re-bleeding risk. The area under the receiver operating characteristics curve (AUROC) was 0.616 (95% CI: 0.512–0.719, p = 0.035). The cut-off value for CCI was 4.5, and the sensitivity and specificity were 75.0% and 41.3%, respectively (Shown in ). Moreover, a Kaplan–Meier analysis revealed that duodenal bleeding and CCI scores higher than 4.5 were associated with significantly higher re-bleeding risk (p = 0.043 & p = 0.027, respectively) (Shown in ).
3.4. All-cause mortality and the associated predictive factors
The all-cause mortality rate during the follow-up period was 21.2% (28 patients), 37.5% (15 patients) in the re-bleeding group, and 14.1% (13 patients) in the non-re-bleeding group (p < 0.001). Three patients (7.5%) in the re-bleeding group experienced bleeding-related deaths, and only one patient (1%) in the non-re-bleeding group experienced bleeding-related death. Most patients died from non-bleeding-related causes.
The results of the univariate and multivariate analyses are presented in , and the multivariate analysis showed that the index albumin levels (OR: 0.134; 95% CI: 0.035–0.506, p = 0.003), a previous PU or UGIB History (OR: 4.626; 95% CI: 1.375–15.567, p = 0.013), and CCI scores (OR: 1.293; 95% CI: 1.058–1.581, p = 0.012) were significantly associated with all-cause mortality. We have checked for multicollinearity issues (Supplementary Table 3).
Table 4. Regression Analysis for all-cause mortality.
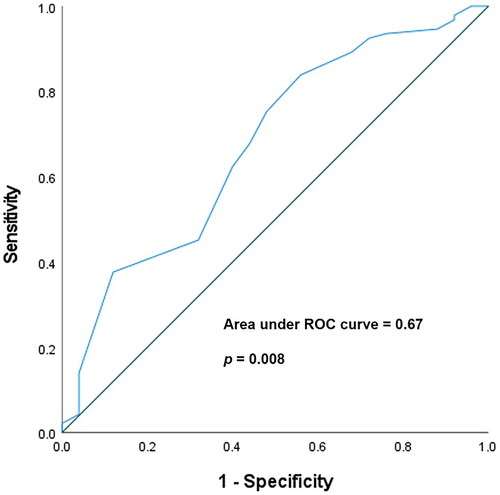
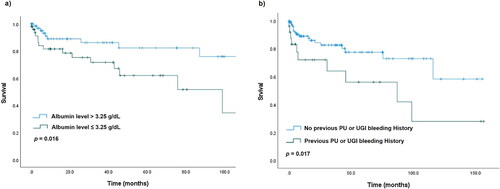
Using Youden’s J statistical method, we calculated the cut-off value, sensitivity, and specificity of the predicted albumin levels for all-cause mortality. The AUROC was 0.67 (95% CI: 0.553–0.795, p = 0.008). The cut-off value for albumin levels was 3.25 g/dL, and the sensitivity and specificity were 62.4% and 60.0%, respectively (Shown in ). The Kaplan–Meier analysis showed that low index albumin levels (≤3.25 g/dL) and a previous PU or UGIB history were significantly associated with higher all-cause mortality (p = 0.016 & p = 0.017, respectively) (Shown in ).
4. Discussion
We found that a high CCI score and duodenal bleeding were significant risk factors for re-bleeding after successful hemostasis in patients who experienced NVUGIB while receiving OACs. In addition, a low-index albumin level and a previous PU or UGIB history were significantly associated with all-cause mortality. The strength of our study was that all NVUGIB incidents were confirmed by an endoscopic examination, and it did not rely solely on laboratory findings such as anemia or patient symptoms such as melena or hematemesis. Furthermore, all patients with index NVUGIB were successfully treated with medication, endoscopic procedures (performed by experts), or radiological interventions. The GI tract has a rich blood supply; however, the integrity of its mucosa is frequently disrupted [Citation27,Citation28], leading to more susceptibility to bleeding caused by endogenous intra-luminal factors such as acid and digestive enzymes [Citation29]. Especially in the duodenum, bleeding and re-bleeding frequently occur not only because of these factors but also because of anatomical factors. The gastroduodenal artery, originating from the common hepatic artery, is located posterosuperior to the first part of the duodenum. Because of this vascular distribution, an erosion of the artery or its branches can increase the risk of massive bleeding and re-bleeding in the duodenum [Citation30]. In our study, these anatomical and physiological features might have contributed to the frequent re-bleeding episodes in patients with duodenal bleeding.
Several scoring systems have been developed to assess the risk of re-bleeding and all-cause mortality following index NVUGIB using clinical and endoscopic variables [Citation31–33]. In a previous study, the GBS was found to be the best scoring system for predicting re-bleeding or all-cause mortality in NVUGIB compared with the RS and AIMS65 [Citation34]. However, these scoring systems do not consider the effects of anticoagulant use and may not be useful for predicting re-bleeding or all-cause mortality in patients receiving OACs. Therefore, our study showed results that differ from those of previous studies. Among GBS, CCI, and RS, CCI was found to be a statistically significant predictor of re-bleeding and all-cause mortality risk compared with the GBS and RS.
The CCI assesses the burden of a patient’s comorbidities or other health conditions by weighting various complications according to their association with the one-year risk of death [Citation35]. The higher the score, the greater the burden of comorbidity and the higher the predicted all-cause mortality risk. The CCI does not specifically focus on GIB; however, it focuses on concurrent conditions that can contribute to GIB, such as liver diseases, chronic kidney diseases, and malignancies. Based on these findings, CCI may be associated with re-bleeding and all-cause mortality. In our study, the mean CCI score in the re-bleeding group was higher than that in the non-re-bleeding group (p = 0.009). However, to further clarify the relationship between high CCI scores and re-bleeding or all-cause mortality, further studies with larger numbers of patients are required.
Previous studies have recommended the use of gastroprotective agents, such as PPIs or H2 receptor antagonists (H2RAs) combined with OACs to reduce the risk of bleeding. Gastric acid suppression plays an important role in reducing the risk of acute bleeding or re-bleeding in patients with NVUGIB. This is supported by the theory that an elevated intragastric pH promotes clot formation [Citation36]. However, whether this reduces the risk of re-bleeding while receiving OACs remains unclear. In our study, most patients (103/122; 84.4%) received gastroprotective agents, including PPIs or H2RAs after the occurrence of NVUGIB; however, we could not find any statistically significant impact of PPIs or H2RAs on the prevention of re-bleeding (data not presented). This most likely happened because of the small sample size. However, compared with PPIs, recently developed potassium-competitive acid blockers, such as vonoprazan and tegoprazan, have been developed to improve gastroesophageal reflux disease symptoms and eradicate H. pylori infection, raise the pH holding time ratio [Citation37–39]. In a study that compared vonoprazan and lansoprazole for the treatment of PUs, an acid-related disease, it was confirmed that both had similar tolerability profiles; vonoprazan was not less effective in treating gastric ulcer and duodenal ulcer [Citation40]. Furthermore, in a previous study conducted in Japan, the non-inferiority of vonoprozan to PPI in the treatment of UGIB that occurred for 6 months in patients with ischemic heart disease receiving two or more antithrombotic agents was confirmed [Citation41]. This may help reduce the risk of re-bleeding in patients with NVUGIB receiving OACs by maintaining an increased intragastric pH for a longer period. However, further studies are required to clarify this issue.
Tung et al. confirmed that similar to RS, hypoalbuminemia is a marker of in-hospital all-cause mortality [Citation42]. Additionally, in a previous prospective study, albumin level was identified as an indicator of re-bleeding in patients with PU bleeding [Citation43]. However, these studies either did not take into consideration the use of anticoagulant agents or only partially represented the use of anticoagulant agents (8.1%) in a small sample size. In our study, the Kaplan-Meier graph showed that patients with an albumin level of <3.25 g/dL had higher all-cause mortality rates. These findings suggested that hypoalbuminemia is a significant risk factor for all-cause mortality in patients receiving OACs. This may be because albumin plays an important role in maintaining normal physiological processes and can be associated with malnutrition or other comorbidities. If hypoalbuminemia is detected, physicians should be aware of the associated all-cause mortality in patients receiving OACs and hospitalized for NVUGIB.
Sung et al. found that non-bleeding-related factors were the most common cause of death in patients with NVUGIB due to PU bleeding [Citation44]. Additionally, in a systematic review, Lau et al. confirmed that comorbidities are risk factors for all-cause mortality after PU bleeding. Factors associated with all-cause mortality include underlying causes, such as malignancy, cardiovascular disease, liver cirrhosis, renal insufficiency, or advanced age [Citation45]. In our study, most non-bleeding-related mortalities were attributed to malignancies (12/28; 42.9%), followed by cardiovascular diseases (5/28; 17.9%). The association between a history of PU or UGIB and all-cause mortality, observed in our study, was likely because of underlying comorbidities. However, additional large-scale studies are required to further clarify this.
5. Limitations
This study had several limitations. First, the number of participants was small as the study was conducted at a single tertiary hospital. Therefore, the low re-bleeding rate in this cohort might be a type-II statistical error. In addition, the small sample size might have led to unexpected results that are difficult to explain, such as stroke being an independent protective factor. A larger number of participants should be included to validate the results. Second, our results were retrospective. Although we conducted a multivariate analysis to reduce potential bias, it was challenging to completely control for confounding factors. However, it was difficult to conduct a prospective randomized study. Therefore, demonstrating an association between re-bleeding and all-cause mortality using various factors does not prove causality. Third, we could not measure the plasma level of OAC, known as bleeding risk, in all patients receiving OAC. When receiving warfarin, the association with plasma levels and re-bleeding risk could be assessed by measuring the INR. However, in the case of receiving DOAC, our hospital does not have a method to measure the plasma level of DOACs. As a result, we were unable to determine the association between DOAC plasma levels and bleeding risk.
6. Conclusions
In conclusion, our study provides new insights into re-bleeding and all-cause mortality after successful hemostatic procedures for NVUGIB during receiving OACs. CCI higher than 4.5 and duodenal bleeding were associated with re-bleeding, and hypoalbuminemia of < 3.25 g/dL and a history of PU or UGIB were significant risk factors for all-cause mortality. These indicators are convenient and valuable since they can be easily assessed in a clinical setting. A thorough identification of risk factors for re-bleeding and all-cause mortality will result in improved patient outcomes.
Author contributions
Won Shik Kim, Seung Han Kim, and Moon Kyung Joo designed the study. Won Shik Kim, Seung Han Kim, Moon Kyung Joo, Jong-Jae Park, and Beom Jae Lee performed the endoscopic procedures. Won Shik Kim and Moon Kyung Joo collected and analyzed the data and drafted the manuscript. Moon Kyung Joo reviewed and approved the final version of the manuscript. Beom Jae Lee, Jong-Jae Park, and Hoon Jai Chun provided advice on the study design and manuscript writing. Won Shik Kim and Seung Han Kim created the figures and tables. All the authors have read and agreed to the published version of this manuscript.
Ethical statement
The study was conducted in accordance with the ethical principles of the Declaration of Helsinki. As this was a retrospective study involving the collection of existing data and records, the requirement for informed consent was waived. This study was reviewed and approved by [the Institutional Review Board of Korea University Guro Hospital], approval number [2020GR0027].
Supplemental Materials
Download MS Word (33.6 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding authors.
Additional information
Funding
References
- Yang HJ. Management of peptic ulcer bleeding in patients taking aspirin or anticoagulant. Korean J Gastroenterol. 2020;76(5):1–13. doi: 10.4166/kjg.2020.138.
- Ezekowitz MD, Bridgers SL, James KE, et al. Warfarin in the prevention of stroke associated with nonrheumatic atrial fibrillation. N Engl J Med. 1992;327(20):1406–1412. doi: 10.1056/NEJM199211123272002.
- Das AK, Willcoxson PD, Corrado OJ, et al. The impact of long-term warfarin on the quality of life of elderly people with atrial fibrillation. Age Ageing. 2007;36(1):95–97. doi: 10.1093/ageing/afl062.
- Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Kardiol Pol. 2016;74(12):1359–1469. doi: 10.5603/KP.2016.0172.
- Lanas Á, Carrera-Lasfuentes P, Arguedas Y, et al. Risk of upper and lower gastrointestinal bleeding in patients taking nonsteroidal anti-inflammatory drugs, antiplatelet agents, or anticoagulants. Clin Gastroenterol Hepatol. 2015;13(5):906–912. e2. doi: 10.1016/j.cgh.2014.11.007.
- Diener H-C. Preventing major gastrointestinal bleeding in elderly patients. Lancet. 2017;390(10093):435–437. doi: 10.1016/S0140-6736(17)31507-6.
- Li L, Geraghty OC, Mehta Z, et al. Age-specific risks, severity, time course, and outcome of bleeding on long-term antiplatelet treatment after vascular events: a population-based cohort study. Lancet. 2017;390(10093):490–499. doi: 10.1016/S0140-6736(17)30770-5.
- Radaelli F, Dentali F, Repici A, et al. Management of anticoagulation in patients with acute gastrointestinal bleeding. Dig Liver Dis. 2015;47(8):621–627. doi: 10.1016/j.dld.2015.03.029.
- Barkun AN, Almadi M, Kuipers EJ, et al. Management of nonvariceal upper gastrointestinal bleeding: guideline recommendations from the international consensus group. Ann Intern Med. 2019;171(11):805–822. doi: 10.7326/M19-1795.
- Laine L, Peterson WL. Bleeding peptic ulcer. N Engl J Med. 1994;331(11):717–727. doi: 10.1056/NEJM199409153311107.
- Wolf AT, Wasan SK, Saltzman JR. Impact of anticoagulation on rebleeding following endoscopic therapy for nonvariceal upper gastrointestinal hemorrhage. Am J Gastroenterol. 2007;102(2):290–296. doi: 10.1111/j.1572-0241.2006.00969.x.
- Travis AC, Wasan SK, Saltzman JR. Model to predict rebleeding following endoscopic therapy for non‐variceal upper gastrointestinal hemorrhage. J Gastroenterol Hepatol. 2008;23(10):1505–1510. doi: 10.1111/j.1440-1746.2008.05594.x.
- Hong MJ, Lee S-Y, Kim JH, et al. Rebleeding after initial endoscopic hemostasis in peptic ulcer disease. J Korean Med Sci. 2014;29(10):1411–1415. doi: 10.3346/jkms.2014.29.10.1411.
- Rost S, Fregin A, Ivaskevicius V, et al. Mutations in VKORC1 cause warfarin resistance and multiple coagulation factor deficiency type 2. Nature. 2004;427(6974):537–541. doi: 10.1038/nature02214.
- Kwon S, Lee S-R, Choi E-K, et al. Non–vitamin K antagonist oral anticoagulants in patients with atrial fibrillation and prior gastrointestinal bleeding. Stroke. 2021;52(2):511–520. doi: 10.1161/STROKEAHA.120.030761.
- Lee H-J, Kim H-K, Kim B-S, et al. Risk of upper gastrointestinal bleeding in patients on oral anticoagulant and proton pump inhibitor co-therapy. PLoS One. 2021;16(6):e0253310. doi: 10.1371/journal.pone.0253310.
- Pae JY, Kim ES, Kim SK, et al. Gastrointestinal bleeding risk of non-vitamin K antagonist oral anticoagulants versus warfarin in general and after polypectomy: a population-based study with propensity score matching analysis. Intest Res. 2022;20(4):482–494. doi: 10.5217/ir.2021.00161.
- Radadiya D, Devani K, Brahmbhatt B, et al. Major gastrointestinal bleeding risk with direct oral anticoagulants: does type and dose matter?–a systematic review and network meta-analysis. Eur J Gastroenterol Hepatol. 2021;33(1S Suppl 1):e50–e8. doi: 10.1097/MEG.0000000000002035.
- Cannon CP, Kohli P. Danger ahead: watch out for indirect comparisons. J Am Coll Cardiol. 2012;60(8):747–748. doi: 10.1016/j.jacc.2012.05.012.
- Hawkins NM, Er L, Sandhu RK, et al. Validity of different dose reduction criteria for apixaban. Am Heart J. 2021;238:12–15. doi: 10.1016/j.ahj.2021.03.004.
- Bounameaux H, Camm AJ. Edoxaban: an update on the new oral direct factor Xa inhibitor. Drugs. 2014;74(11):1209–1231. doi: 10.1007/s40265-014-0261-1.
- Gong IY, Kim RB. Importance of pharmacokinetic profile and variability as determinants of dose and response to dabigatran, rivaroxaban, and apixaban. Can J Cardiol. 2013;29(7 Suppl):S24–S33. doi: 10.1016/j.cjca.2013.04.002.
- Gralnek IM, Stanley AJ, Morris AJ, et al. Endoscopic diagnosis and management of nonvariceal upper gastrointestinal hemorrhage (NVUGIH): european society of gastrointestinal endoscopy (ESGE) guideline–update 2021. Endoscopy. 2021;53(3):300–332. doi: 10.1055/a-1369-5274.
- Wilkins T, Khan N, Nabh A, et al. Diagnosis and management of upper gastrointestinal bleeding. Am Fam Physician. 2012;85(5):469–476.
- Sung JJ, Chiu PW, Chan FK, et al. Asia-Pacific working group consensus on non-variceal upper gastrointestinal bleeding: an update 2018. Gut. 2018;67(10):1757–1768. doi: 10.1136/gutjnl-2018-316276.
- Oakland K. Changing epidemiology and etiology of upper and lower gastrointestinal bleeding. Best Pract Res Clin Gastroenterol. 2019;42-43:101610. doi: 10.1016/j.bpg.2019.04.003.
- Goodman SG, Wojdyla DM, Piccini JP, et al. Factors associated with major bleeding events: insights from the ROCKET AF trial (rivaroxaban once-daily oral direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation). J Am Coll Cardiol. 2014;63(9):891–900. doi: 10.1016/j.jacc.2013.11.013.
- Aisenberg J, Chatterjee-Murphy P, Friedman Flack K, et al. Gastrointestinal bleeding with edoxaban versus warfarin: results from the ENGAGE AF-TIMI 48 trial (effective anticoagulation with factor Xa next generation in atrial fibrillation–thrombolysis in myocardial infarction). Circ Cardiovasc Qual Outcomes. 2018;11(5):e003998. doi: 10.1161/CIRCOUTCOMES.117.003998.
- Desai J, Kolb JM, Weitz JI, et al. Gastrointestinal bleeding with the new oral anticoagulants–defining the issues and the management strategies. Thromb Haemost. 2013;110(2):205–212. doi: 10.1160/TH13-02-0150.
- Larssen L, Moger T, Atle Bjørnbeth B, et al. Transcatheter arterial embolization in the management of bleeding duodenal ulcers: a 5.5-year retrospective study of treatment and outcome. Scand J Gastroenterol. 2008;43(2):217–222. doi: 10.1080/00365520701676443.
- Rockall T, Logan R, Devlin H, et al. Risk assessment after acute upper gastrointestinal haemorrhage. Gut. 1996;38(3):316–321. doi: 10.1136/gut.38.3.316.
- Strate LL, Saltzman JR, Ookubo R, et al. Validation of a clinical prediction rule for severe acute lower intestinal bleeding. Am J Gastroenterol. 2005;100(8):1821–1827. doi: 10.1111/j.1572-0241.2005.41755.x.
- Aoki T, Nagata N, Shimbo T, et al. Development and validation of a risk scoring system for severe acute lower gastrointestinal bleeding. Clin Gastroenterol Hepatol. 2016;14(11):1562–1570.e2. doi: 10.1016/j.cgh.2016.05.042.
- Stanley AJ, Laine L, Dalton HR, et al. Comparison of risk scoring systems for patients presenting with upper gastrointestinal bleeding: international multicentre prospective study. BMJ. 2017;356:i6432. doi: 10.1136/bmj.i6432.
- Drosdowsky A, Gough K. The charlson comorbidity index: problems with use in epidemiological research. J Clin Epidemiol. 2022;148:174–177. doi: 10.1016/j.jclinepi.2022.03.022.
- Green FW, Jr, Kaplan MM, Curtis LE, et al. Effect of acid and pepsin on blood coagulation and platelet aggregation: a possible contributor to prolonged gastroduodenal mucosal hemorrhage. Gastroenterology. 1978;74(1):38–43. doi: 10.1016/0016-5085(78)90352-9.
- Sakurai Y, Mori Y, Okamoto H, et al. Acid‐inhibitory effects of vonoprazan 20 mg compared with esomeprazole 20 mg or rabeprazole 10 mg in healthy adult male subjects‐a randomised open‐label cross‐over study. Aliment Pharmacol Ther. 2015;42(6):719–730. doi: 10.1111/apt.13325.
- Kagami T, Sahara S, Ichikawa H, et al. Potent acid inhibition by vonoprazan in comparison with esomeprazole, with reference to CYP 2C19 genotype. Aliment Pharmacol Ther. 2016;43(10):1048–1059. doi: 10.1111/apt.13588.
- Takeuchi T, Furuta T, Fujiwara Y, et al. Randomised trial of acid inhibition by vonoprazan 10/20 mg once daily vs rabeprazole 10/20 mg twice daily in healthy Japanese volunteers (SAMURAI pH study). Aliment Pharmacol Ther. 2020;51(5):534–543. doi: 10.1111/apt.15641.
- Miwa H, Uedo N, Watari J, et al. Randomised clinical trial: efficacy and safety of vonoprazan vs. lansoprazole in patients with gastric or duodenal ulcers–results from two phase 3, non‐inferiority randomised controlled trials. Aliment Pharmacol Ther. 2017;45(2):240–252. doi: 10.1111/apt.13876.
- Tsujita K, Deguchi H, Uda A, et al. Upper gastrointestinal bleeding in Japanese patients with ischemic heart disease receiving vonoprazan or a proton pump inhibitor with multiple antithrombotic agents: a nationwide database study. J Cardiol. 2020;76(1):51–57. doi: 10.1016/j.jjcc.2020.02.012.
- Tung C-F, Chow W-K, Chang C-S, et al. The prevalence and significance of hypoalbuminemia in non-variceal upper gastrointestinal bleeding. Hepato-gastroenterology. 2007;54(76):1153–1156.
- Kim JS, Kim B-W, Park SM, et al. Factors associated with rebleeding in patients with peptic ulcer bleeding: analysis of the korean peptic ulcer bleeding (K-PUB) study. Gut Liver. 2018;12(3):271–277. doi: 10.5009/gnl17138.
- Sung JJ, Tsoi KK, Ma TK, et al. Causes of mortality in patients with peptic ulcer bleeding: a prospective cohort study of 10,428 cases. Am J Gastroenterol. 2010;105(1):84–89. doi: 10.1038/ajg.2009.507.
- Lau JY, Sung J, Hill C, et al. Systematic review of the epidemiology of complicated peptic ulcer disease: incidence, recurrence, risk factors and mortality. Digestion. 2011;84(2):102–113. doi: 10.1159/000323958.