Abstract
Purpose
We conducted this umbrella review to review the current evidence on the relationship between COVID-19 and sexual health in both men and women.
Methods
We conducted searches in Pubmed, Embase, and the Cochrane dataset for meta-analyses that met our pre-set inclusion criteria. We included studies with detailed information investigating the link between COVID-19 and sexual health in men/women. We did not limit the language.
Results
The results of the included studies frequently relied on the Female Sexual Function Index to assess sexual health in women. For men, the International Index of Male Function and hospital diagnoses were commonly used to assess sexual health. Currently, there is conflicting evidence regarding the impact of COVID-19 on sexual health. However, since most studies were observational in nature, additional study designs are necessary to draw definitive conclusions across different contexts.
Conclusion
Our findings highlight the importance of sexual health among COVID-19 patients and people affected due to COVID-19. Further critical studies should investigate the mechanism underlying the association between COVID-19 and sexual health.
Keywords:
Introduction
The first spread of the severe acute respiratory syndrome-related coronavirus type 2 (SARS-CoV-2) occurred in China in late December 2019 [Citation1]. Out of all affected individuals, approximately 14% develop symptoms related to coronavirus Disease 2019 (COVID-19), while 5% experience severe discomfort and require intensive care [Citation2]. Although the main prognostic feature is recovery, some patients continue to experience various symptoms long after the acute infection [Citation3]. This condition has recently been named ‘post-COVID-19 condition’ by the World Health Organization (WHO) [Citation4]. It is characterized by a wide range of persistent or new-onset symptoms, which can be continuous or fluctuating, lasting for more than 2 months after a microbiologically confirmed or suspected SARS-CoV-2 infection, and cannot be explained by an alternative diagnosis.
During the pandemic, many people experienced changes in their lives, such as illness and lower income, which reduced their recreational activities emotionally and physically and affected their frequency of sexual activity [Citation5–8]. Sexual dysfunction refers to a diverse range of conditions which result in notable impairment of individual’s sexual response or their ability to derive pleasure from sexual experiences. These conditions include disorders related to low sexual desire, erectile dysfunction (ED), difficulties with orgasm and ejaculation, and painful experiences during genital-pelvic penetration [Citation9]. This occurrence is widespread among the general population and is linked to various physical and psychological issues [Citation10].
However, the association between sexual activity and COVID-19 remains unclear. Sexual activity can exacerbate SARS-CoV-2 spread [Citation11,Citation12]. As a result of implementing measures to prevent and control the COVID-19 pandemic, individuals may find themselves spending more time at home, which could lead to a higher occurrence of sexual activity. COVID-19, on the other hand, may affect sexual function and lead to decreased libido [Citation13]. In particular, many researchers have found differences in sexual desire between the sexes. For example, a study in the UK showed that men generally had higher levels of sexual desire compared to women, with women experiencing a significant decline in libido during the lockdown period [Citation14]. Similarly, another study discovered that around one-quarter of the participants reported a decrease in libido, with 18% of males and 8% of females reporting an increase [Citation15].
In an era of an aging population and declining fertility, research on COVID-19 and sexual activity is needed to help public health policies and policymakers provide effective plans to improve the quality of sexual activity. With the availability of diverse results, umbrella review is needed to provide a comprehensive evaluation of available information on this specific topic based on systematic reviews and meta-analyses [Citation16]. This umbrella review aimed to explore the link between COVID-19 and sexual function, aiming to summarize the present evidence from published studies on the impact of the COVID-19 on sexual activity and function.
Methods
Search methods and selection of the studies
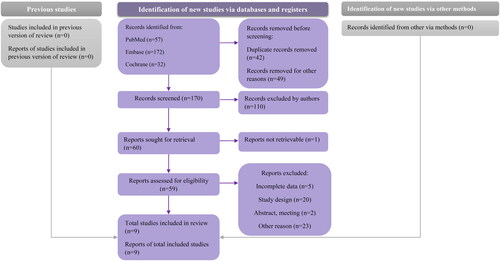
In order to conduct an umbrella review, it is necessary to establish predetermined goals and inclusion criteria, use a transparent and comprehensive search strategy, evaluate the methodology employed, and summarize the available evidence succinctly. In this umbrella review, we included (1) only meta-analyses that studied the link between COVID-19 and sexual health in both men and women; (2) We did not limit language; (3) We excluded studies that only included lesbian, gay, bisexual, and transgender (LGBT) participants since it was beyond our research topic. To be clear, according to the Population, Intervention, Comparator, Outcome (PICO) rule, the search strategy is listed in . Several databases were searched to perform a thorough analysis, including PubMed, Cochrane, and Embase, for articles published until March 18, 2023. We conducted an umbrella review that revealed the association between COVID-19 and sexual health in men and women in detailed results. This umbrella review was registered in the International Prospective Register of Systematic Reviews (ID: 411584).
Table 1. Searching strategy till 18 March 2023.Table Footnote*
Data extraction
The search and screening processes were independently conducted by two authors (W. Y. L. and Z. L. J.). After removing duplicate studies, the two authors started screening according to the eligibility criteria and research purpose. In addition, the analyses included in the evaluation were assessed using the I2 statistic, small-study effects, level of comparison, and random-effects summary. Furthermore, they collected data on the author, year, number of participants, patients’ diseases, outcome measurement tools, I2 statistics, and study types. Any disagreements were resolved through discussions involving a third senior author (T.H.T).
Methodological quality
We further analyzed the quality of this umbrella review based on the A MeaSurement Tool to Assess
Systematic Reviews (AMSTAR-2) guidelines, which systematically grade evidence-based medical papers [Citation17,Citation18]. However, as a high score may overlook some significant limitations, no overall score was given [Citation19]. The AMSTAR-2 method is considered a reliable and accurate approach for assessing the quality of systematic reviews and meta-analyses that involve both interventional and observational research [Citation20]. In contrast to commonly used tools like risk of bias in non-randomized studies of intervention, AMSTAR-2 assesses the process of selecting study designs for inclusion, reasons for excluding studies, sources of primary study funding, and any conflicts of interest among the reviewers [Citation21]. AMSTAR-2 is often employed in umbrella reviews [Citation22].
Epidemiological credibility
High epidemiological credibility refers to having the strongest available evidence and no indication of significant variation or prejudice [Citation23]. In addition, we characterized the studies included in our analysis as follows [Citation24]:
‘Persuasive’ – statistically significant per the random-effects model with a p-value of < 0.000001, > 1000 cases, low heterogeneity among the selected studies (I2 < 50%), 95% CI (excluding the null value), and no evidence of small-study effects or significant bias.
‘Highly recommended’ – statistically significant with a p of < 0.000001, > 1000 cases, with most studies indicating a significant effect.
‘Recommended’ – > 1000 cases and significant effects at a p of < 0.001.
‘Weak evidence’ – nominally significant associations (p < 0.05).
‘Poor evidence’ – obtained from samples with < 1000 cases.
This umbrella review offered a broad perspective to evaluate existing literature and examine convergent and divergent research findings. Clinicians can benefit from umbrella reviews by gaining quick access to a comprehensive overview of a particular topic, facilitating evidence-based practice; therefore, we needed to show the quality of the included studies reasonably. Additionally, we assessed the presence of small-study effects, which is a phenomenon where smaller studies tend to show more significant treatment outcomes compared to larger studies [Citation25]. This phenomenon is commonly resulted due to publication bias, as indicated by an Egger’s test p-value of < 0.10 [Citation22,Citation26]. The random-effects model is an approach in the meta-analysis that accounts for variability that cannot be attributed to chance. Although the fixed-effects model may have less control over factors that may affect outcomes, it typically results in less variability [Citation27]. Nonetheless, the pooled estimates derived from the fixed-effects model were considered more dependable than those obtained from the random-effects model [Citation24].
Results
Study characteristics
After excluding duplicates, meta-analyses were included to check eligibility further, and we finally included nine meta-analyses. The screening process is illustrated in . All nine studies were published in 2022. We have presented characteristics of the included studies in , the average age is above 18 years in all included studies. Three of these studies examined the effects of COVID-19 on male sexual function [Citation28–30], three of them only examined female sexual function [Citation31–33], and the rest of the included studies included both men and women [Citation34–36]. Based on the criteria and characteristics of each study, we classified them into several groups. The study by Zhang et al. [Citation28], Mourikis et al. [Citation34], Hessami et al. [Citation31], and Pérez-López et al. [Citation33] could be classified as ‘recommend’. The remaining five studies were classified as having ‘weak evidence’ [Citation29,Citation30,Citation32,Citation35,Citation36]. In the review, only three studies used Egger’s test to detect the possibility of selection bias; therefore, we could not compare small-study effects.
Table 2. Characteristics of included studies.
Outcome analysis
Zhang et al. included eight studies and found that COVID-19 was associated with a higher risk of ED [Citation28]. Mourikis et al. included 11 studies and found that COVID-19 had an impact on female sexual function; in contrast, no significant effect was observed among males [Citation34]. Hessami et al. also found a significant decrease in sexual function among females [Citation31]. Masoudi et al. found that COVID-19-related restrictions were associated with lower sexual activity and decreased sexual functions [Citation35]. Dashti et al. included five studies and found that female sexual function did not change significantly before and after the COVID-19 pandemic [Citation32]. Klepinowski et al. observed significantly lower sperm volume, sperm concentration, and total sperm in male ejaculation [Citation29]. Delcea et al. observed a significant reduction in sexual activity due to COVID-19 [Citation36]. Perez-Lopez et al. concluded that there was a significant reduction in sexual function in females owing to COVID-19 [Citation33]. Pizzol et al. found that the prevalence of ED was higher in healthcare workers than in non-healthcare workers [Citation30].
Outcome measurement
The outcome measurements varied among the included meta-analyses. Zhang et al. measured ED using the International Coding of Diseases (ICD-10) and International Index of Erectile Function (IIEF-5) [Citation28]. In a study by Mourikis et al. the Female Sexual Function Index (FSFI), IIEF-5, and structured questionnaires were used to quantify outcome diagnoses [Citation34]. In the studies by Hessami et al. [Citation31], Dashti et al. [Citation32] and Pérez-López et al. [Citation33], the total FSFI score with subtypes including desire, arousal, lubrication, orgasm, pain, and satisfaction was used to measure changes in sexual function. Masoudi et al. used the FSFI and IIEF-5 to study sexual function changes in females and males [Citation35]. Klepinowski et al. used medical analyses to measure sperm quality and sexual function [Citation29]. Delcea et al. mainly used questionnaires to measure changes in sexual behavior and sexual function [Citation36]. Pizzol et al. did not specify how they measured the ED among healthcare workers [Citation30]. Despite using various instruments, most of the included studies provided detailed descriptions of the measurement tools.
Publication bias
Zhang et al. used Begg’s (p = 0.71) and Egger’s (p = 0.006) tests to measure publication bias, which indicated the existence of publication bias, and they excluded five studies using the trim-fill method, after which the results did not change much [Citation28]. Mourikis et al. used funnel plots to visualize publication bias, and asymmetry indicated the possibility of publication bias [Citation34]. Hessami et al. revealed no significant publication bias based on Egger’s test (p = 0.722) or Begg’s test (p = 0.851) [Citation31]. Masoudi et al. [Citation35], Pérez-López et al. [Citation33], Pizzol et al. [Citation30] did not assess publication bias because the number of studies included was < 10. Dashti et al. observed publication bias when examining lubrication and pain; however, they did not detect publication bias in desire, arousal, orgasm, satisfaction, or total score.[Citation32] Klepinowski et al. did not assess the publication bias in their analysis [Citation29]. Delcea et al. did not observe a significant publication bias based on funnel plots or the results of the trim-and-fill analysis [Citation36].
Residual confounding
Since all the meta-analyses included only observational studies, confounding and reverse causality were possible, and we could not conclude a causal relationship between COVID-19 and sexual function changes. Due to confounding and selection biases, we admit that the estimations of COVID-19 and sexual function may be biased.
Discussion
The studies included in the present review generally found that COVID-19 was significantly associated with sexual function in females; in contrast, the conclusions of the studies on relevant indicators of sexual function (including sperm volume, sperm concentration, and total sperm) in males were inconsistent. Therefore, it was important to explore the associations between these obscure results. This review summarizes the literature on COVID-19 and its impact on sexual function and provides evidence for treating sexual dysfunction.
Physiological effect
COVID-19 can cause respiratory diseases, such as coughing, difficulty in breathing, and hypoxemia [Citation37,Citation38]. A meta-analysis summarizing lung involvement in discharged patients with COVID-19 found a prevalence of lung function impairment > 40% 3 months after discharge [Citation39]. A possible mechanism is the inflammatory response induced by COVID-19, specifically an increase in interleukin 6 [Citation40]. Internal dysfunction of the pulmonary circulation capillaries may also induce lung injury, leading to ground-glass shadows and pulmonary fibrosis [Citation39]. Low oxygen saturation caused by pulmonary fibrosis may also affect sexual health [Citation41]. Nitric oxide, which allows proper erectile function, may have a negative effect due to insufficient oxygen intake [Citation42]. Additionally, lack of oxygen intake may cause the patient to become less mentally active and have less energy to engage in sexual activity with a partner.
A study involving more than 80,000 individuals showed that COVID-19 is an independent risk factor for cardiovascular health [Citation43]. Viruses can invade angiotensin-converting enzyme 2 and transmembrane serine protease 2 expressed by endothelial cells, leading to diffuse endodermatitis and affecting cardiovascular microcirculatory function [Citation44–47]. Studies have shown that COVID-19 can cause acute vascular events and pulmonary embolisms [Citation48,Citation49]. SARS-CoV-2 may also cause other diseases through poor immune responses, such as abnormal coagulation function, viral myocarditis, and myocardial ischemia [Citation50–52]. These affect motor function in young people, resulting in arrhythmias and postural tachycardia syndromes due to autonomic nervous system involvement, particularly common in young people [Citation53–55]. These cardiovascular effects can affect sexual health [Citation56]. These effects are independent of anxiety and depression as risk factors for sexual dysfunction [Citation57,Citation58]. Sex is a mild form of physical exercise, and myocarditis and arrhythmias can make intercourse uncomfortable and dangerous [Citation59]. As a result, sweating and shortness of breath will likely occur during sex [Citation60,Citation61]. The clinical drugs used by patients with COVID-19, such as-β-blockers and diuretics, may cause temporary abnormal effects on sexual function [Citation62,Citation63].
COVID-19 may also be damaging to the endocrine system. ACE2, which viruses attack, is highly expressed in the hypothalamus, pituitary, thyroid, and testicular interstitial cells [Citation64–66]. When ACE2 expression is impaired in hypothalamic, pituitary, and thyroid cells, thyroid dysfunction may occur, affecting sexual intercourse [Citation67]. Some studies have reported examples of thyrotoxicosis following SARS-CoV-2 [Citation68–70]. Impaired pituitary function may result in secondary endothelial dysfunction, clotting disorders, and increased blood flow due to underlying inflammation [Citation71]. COVID-19 can also cause type 1 diabetes in susceptible individuals [Citation72]. The accelerated development of type 2 diabetes may occur after an autoimmune response to beta cells, pancreatic inflammation, islet remodeling, and progressive beta cell dysfunction [Citation73]. Testicular damage can lead to hypogonadism, affecting the immune and reproductive health and impairing male fertility [Citation74–76]. An endocrine imbalance is a risk factor for sexual dysfunction [Citation77–80]. Studies have shown that reduced testosterone levels in patients with COVID-19 may lead to hypogonadism and decreased sexual responsiveness [Citation75,Citation76,Citation81]. Diabetes is also an independent risk factor for sexual dysfunction as it affects cardiovascular function and can lead to weight gain [Citation82]. Decreased physical fitness may lead to other diseases, such as depression and insulin resistance, leading to decreased gonadotropin function [Citation83].
Psychological effect
Several different outcomes may have resulted from prolonged isolation. First, isolation inevitably decreases the frequency of intercourse among non-cohabiting couples [Citation84]. However, no clear evidence exists that long-term isolation directly affects sexual health, primarily through depression and anxiety. According to statistics, the prevalence of depression and post-traumatic stress disorder within 3 months after COVID-19 discharge is not low, both of which are > 15% [Citation85,Citation86]. Therefore, it is considered a possible risk factor for sexual dysfunction [Citation87–89]. Second, the anxiety experienced by cohabiting couples who lose their jobs or spend too much time at home due to isolation may lead to less sexual communication between partners. Third, couples in good economic conditions can enjoy a cohabiting life without working for a long time; however, there is no study in the literature to prove this. In addition, studies have shown that cognitive deficits and sleep disturbances caused by COVID-19 lead to chronic fatigue, which may be associated with decreased sexual function [Citation90–92].
Focusing on people who have COVID-19 or have recovered from COVID-19 may help reduce anxiety, thereby reducing its impact on sexual function. A Chinese study comparing patients with COVID-19 with healthy individuals found that the prevalence of ED in males was significantly higher at the first follow-up; however, it improved over time [Citation93]. Other studies have found that COVID-19 may cause a loss of smell and taste, which could negatively affect sexual behavior because sensory sensations are very important during sex [Citation37,Citation38,Citation94,Citation95].
Gender gaps
Our study found that females had worse sexual satisfaction and functioning than males. For young females, sexual dysfunction may be less common; however, more likely to be the result of anxiety. Females are more likely to be anxious about important issues than males. Chronic stress associated with the COVID-19 pandemic can negatively affect females’ sex life [Citation96]. In addition, studies have shown that persistent dyspnea caused by COVID-19 is more common in females, leading to a marked decline in sexual activity among females [Citation97]. Studies have shown that male sex is a risk factor for sexual dysfunction at the pandemic’s beginning [Citation98]. However, during a prolonged pandemic, all family members are at home due to restrictions and lockdowns, which increases the burden on females in their families and working lives. This might have had a wider impact on their sex lives [Citation99]. In addition, studies have shown that the incidence of sexual partner violence against females during the COVID-19 pandemic was 26.6%, with psychological violence accounting for the highest proportion (13.3%), followed by physical violence (8.3%) and sexual violence (5.3%) [Citation100].This indicates that females are a more vulnerable group in intimate relationships, contributing to low sexual satisfaction in females [Citation101].
Strengths and limitations
This study aimed to investigate the impact of COVID-19 on sexual function systematically. Most included studies used objective tools to measure COVID-19 and sexual function with reliable results and high clinical value in real-world scenarios. Given that COVID-19 is the largest global pandemic in recent years, we believe this study is of great public interest.
Nevertheless, there were some limitations in this study. Firstly, due to the varying results and effect sizes in the studies included, it was not possible to calculate pooled results. Secondly, most of the included studies were retrospective and observational studies could not conclude causality. Thus, it is necessary to conduct other study designs to assess the causal effects of COVID-19 on sexual function. Thirdly, statistical heterogeneity was present due to differences in measurement tools, sample sizes, and other factors related to sexual dysfunction. Therefore, caution should be exercised when interpreting the results. Fourthly, future research should focus on summarizing the mechanisms through which COVID-19 impacts sexual health. Additionally, while our study only explored the link between COVID-19 and sexual function, we acknowledge that other conditions, such as reproductive ability, may also be associated with sexual function. Lastly, there was a lack of high-quality meta-analyses examining the relationship between COVID-19 and sexual dysfunction. Thus, it is crucial to conduct further high-quality meta-analyses that encompass all factors influencing sexual function, including sexual organ health.
Conclusion
This review provided an in-depth examination of the impact of COVID-19 on sexual function, expanding the clinical scope of this disease. This study also provided insight into the exact mechanisms of COVID-19 on sexual function and psychosexual disorders, understanding the natural history of the disease, developing the best methods for timely treatment, and minimizing the harmful effects of COVID-19 on sexual life. Regular follow-up visits with patients or recovered patients are strongly recommended to screen them for their physical and mental health and provide psychological support to help heal their mood changes and enhance their sexual capacity.
Author contributions
W-YL, ZL-J, C-CW and T-HT conducted the study and drafted the manuscript. W-YL and ZL-J participated in the design of the study and performed data synthesis. C-CW and T-HT conceived the study and participated in its design and coordination. All of the authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1–11. doi:10.1056/NEJMoa2001316.
- Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–793. doi:10.1001/jama.2020.12839.
- Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601–615. doi:10.1038/s41591-021-01283-z.
- WHO. A clinical case definition of post COVID-19 condition by a Delphi consensus. Geneva: World Health Organization; 2021.
- Sepúlveda-Loyola W, Rodríguez-Sánchez I, Pérez-Rodríguez P, et al. Impact of social isolation due to COVID-19 on health in older people: mental and physical effects and recommendations. J Nutr Health Aging. 2020;24(9):938–947. doi:10.1007/s12603-020-1500-7.
- Biagioli V, Albanesi B, Belloni S, et al. Living with cancer in the COVID-19 pandemic: an italian survey on self-isolation at home. Eur J Cancer Care (Engl). 2021;30(2):e13385. doi:10.1111/ecc.13385.
- Grossman ES, Hoffman YSG, Palgi Y, et al. COVID-19 related loneliness and sleep problems in older adults: worries and resilience as potential moderators. Pers Individ Dif. 2021;168:110371. doi:10.1016/j.paid.2020.110371.
- Ferreira-Filho ES, de Melo NR, Sorpreso ICE, et al. Contraception and reproductive planning during the COVID-19 pandemic. Expert Rev Clin Pharmacol. 2020;13(6):615–622. doi:10.1080/17512433.2020.1782738.
- Battle DE. Diagnostic and statistical manual of mental disorders (DSM). Codas. 2013;25(2):191–192.
- Rosen RC. Prevalence and risk factors of sexual dysfunction in men and women. Curr Psychiatry Rep. 2000;2(3):189–195. doi:10.1007/s11920-996-0006-2.
- Cabello F, Sánchez F, Farré JM, et al. Consensus on recommendations for safe sexual activity during the COVID-19 coronavirus pandemic. J Clin Med. 2020;9(7):2297.
- Patrì A, Gallo L, Guarino M, et al. Sexual transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): a new possible route of infection? J Am Acad Dermatol. 2020;82(6):e227. doi:10.1016/j.jaad.2020.03.098.
- Panzeri M, Ferrucci R, Cozza A, et al. Changes in sexuality and quality of couple relationship during the COVID-19 lockdown. Front Psychol. 2020;11:565823. doi:10.3389/fpsyg.2020.565823.
- Wignall L, Portch E, McCormack M, et al. Changes in sexual desire and behaviors among UK young adults during social lockdown due to COVID-19. J Sex Res. 2021;58(8):976–985. doi:10.1080/00224499.2021.1897067.
- Luetke M, Hensel D, Herbenick D, et al. Romantic relationship conflict due to the COVID-19 pandemic and changes in intimate and sexual behaviors in a nationally representative sample of American adults. J Sex Marital Ther. 2020;46(8):747–762. doi:10.1080/0092623X.2020.1810185.
- Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. Chichester, West Sussex, Hoboken: The Cochrane Collaboration and John Wiley & Sons Ltd; 2008.
- Poole R, Kennedy OJ, Roderick P, et al. Coffee consumption and health: umbrella review of meta-analyses of multiple health outcomes. BMJ. 2017;359:j5024. doi:10.1136/bmj.j5024.
- Shea BJ, Hamel C, Wells GA, et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol. 2009;62(10):1013–1020. doi:10.1016/j.jclinepi.2008.10.009.
- Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi:10.1136/bmj.j4008.
- Pieper D, Mathes T, Eikermann M. Can AMSTAR also be applied to systematic reviews of non-randomized studies? BMC Res Notes. 2014;7(1):609. doi:10.1186/1756-0500-7-609.
- Swierz MJ, Storman D, Zajac J, et al. Similarities, reliability and gaps in assessing the quality of conduct of systematic reviews using AMSTAR-2 and ROBIS: systematic survey of nutrition reviews. BMC Med Res Methodol. 2021;21(1):261. doi:10.1186/s12874-021-01457-w.
- Tsai C-Y, Jiesisibieke ZL, Tung T-H. Association between dry eye disease and depression: an umbrella review. Front Public Health. 2022;10:910608. doi:10.3389/fpubh.2022.910608.
- Bellou V, Belbasis L, Tzoulaki I, et al. Risk factors for type 2 diabetes mellitus: an exposure-wide umbrella review of meta-analyses. PLoS One. 2018;13(3):e0194127. doi:10.1371/journal.pone.0194127.
- Gu D-T, Tung T-H, Jiesisibieke ZL, et al. Safety of cinnamon: an umbrella review of meta-analyses and systematic reviews of randomized clinical trials. Front Pharmacol. 2021;12:790901. doi:10.3389/fphar.2021.790901.
- Schwarzer G, Carpenter JR, Rucker G, et al. Small-study effects in meta-analysis. In: Schwarzer G, Carpenter JR, Rücker G, editors. Meta-analysis. Cham: Springer International Publishing; 2015. pp. 107–141.
- Bellou V, Belbasis L, Tzoulaki I, et al. Environmental risk factors and parkinson’s disease: an umbrella review of meta-analyses. Parkinsonism Relat Disord. 2016;23:1–9. doi:10.1016/j.parkreldis.2015.12.008.
- Basagaña X, Pedersen M, Barrera-Gómez J, et al. Analysis of multicentre epidemiological studies: contrasting fixed or random effects modelling and meta-analysis. Int J Epidemiol. 2021;50(1):355–355. doi:10.1093/ije/dyy117.
- Zhang J, Shi W, Zou M, et al. Prevalence and risk factors of erectile dysfunction in COVID-19 patients: a systematic review and meta-analysis. J Endocrinol Invest. 2023;46(4):795–804. doi:10.1007/s40618-022-01945-w.
- Klepinowski T, Klepinowska M, Sagan L, et al. Does SARS-CoV-2 affect human semen? A systematic review and meta-analysis. Arch Sex Behav. 2023;52(2):669–677. doi:10.1007/s10508-022-02520-3.
- Pizzol D, Shin JI, Trott M, et al. Social environmental impact of COVID-19 and erectile dysfunction: an explorative review. J Endocrinol Invest. 2022;45(3):483–487. doi:10.1007/s40618-021-01679-1.
- Hessami K, Sayegh N, Abdolmaleki AS, et al. Women’s sexual function before and during COVID‐19 pandemic: a systematic review and meta‐analysis. J Obstet Gynaecol Res. 2022;48(9):2285–2295. doi:10.1111/jog.15337.
- Dashti S, Bolghanabadi N, Ghavami V, et al. The impact of COVID-19 on female sexual function: a systematic review and meta-analysis. J Sex Marital Ther. 2022;48(5):520–531. doi:10.1080/0092623X.2021.2006842.
- Pérez-López FR, López-Baena MT, Pérez-Roncero G, et al. Female sexual function before and during the severe acute respiratory syndrome coronavirus-2 pandemic: a systematic review and meta-analysis of longitudinal studies. Gynecol Endocrinol. 2022;38(8):632–638. doi:10.1080/09513590.2022.2082404.
- Mourikis I, Kokka I, Koumantarou-Malisiova E, et al. Exploring the adult sexual wellbeing and behavior during the COVID-19 pandemic. A systematic review and meta-analysis. Front Psychiatry. 2022;13:949077. doi:10.3389/fpsyt.2022.949077.
- Masoudi M, Maasoumi R, Bragazzi NL. Effects of the COVID-19 pandemic on sexual functioning and activity: a systematic review and meta-analysis. BMC Public Health. 2022;22(1):189. doi:10.1186/s12889-021-12390-4.
- Delcea C, Chirilă V-I, Săuchea A-M. Effects of COVID-19 on sexual life–a meta-analysis. Sexologies. 2021;30(1):e49–e54. doi:10.1016/j.sexol.2020.12.001.
- Mandal S, Barnett J, Brill SE, et al. ‘Long-COVID’: a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax. 2021;76(4):396–398. doi:10.1136/thoraxjnl-2020-215818.
- Halpin SJ, McIvor C, Whyatt G, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J Med Virol. 2021;93(2):1013–1022. doi:10.1002/jmv.26368.
- So M, Kabata H, Fukunaga K, et al. Radiological and functional lung sequelae of COVID-19: a systematic review and meta-analysis. BMC Pulm Med. 2021;21(1):97. doi:10.1186/s12890-021-01463-0.
- Moodley YP, Scaffidi AK, Misso NL, et al. Fibroblasts isolated from normal lungs and those with idiopathic pulmonary fibrosis differ in interleukin-6/gp130-mediated cell signaling and proliferation. Am J Pathol. 2003;163(1):345–354. doi:10.1016/S0002-9440(10)63658-9.
- Graney BA, Wamboldt FS, Baird S, et al. Looking ahead and behind at supplemental oxygen: a qualitative study of patients with pulmonary fibrosis. Heart Lung. 2017;46(5):387–393. doi:10.1016/j.hrtlng.2017.07.001.
- Verratti V, Di Giulio C, Berardinelli F, et al. The role of hypoxia in erectile dysfunction mechanisms. Int J Impot Res. 2007;19(5):496–500. doi:10.1038/sj.ijir.3901560.
- Katsoularis I, Fonseca-Rodríguez O, Farrington P, et al. Risk of acute myocardial infarction and ischaemic stroke following COVID-19 in Sweden: a self-controlled case series and matched cohort study. Lancet. 2021;398(10300):599–607. doi:10.1016/S0140-6736(21)00896-5.
- Liu PP, Blet A, Smyth D, et al. The science underlying COVID-19: implications for the cardiovascular system. Circulation. 2020;142(1):68–78. doi:10.1161/CIRCULATIONAHA.120.047549.
- Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi:10.1016/S0140-6736(20)30937-5.
- Sardu C, Gambardella J, Morelli MB, et al. Hypertension, thrombosis, kidney failure, and diabetes: is COVID-19 an endothelial disease? A comprehensive evaluation of clinical and basic evidence. J Clin Med. 2020;9(5):1417.
- Jung F, Krüger-Genge A, Franke RP, et al. COVID-19 and the endothelium. Clin Hemorheol Microcirc. 2020;75(1):7–11. doi:10.3233/CH-209007.
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi:10.1016/S0140-6736(20)30566-3.
- Pons S, Fodil S, Azoulay E, et al. The vascular endothelium: the cornerstone of organ dysfunction in severe SARS-CoV-2 infection. Crit Care. 2020;24(1):353. doi:10.1186/s13054-020-03062-7.
- Jose RJ, Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. 2020;8(6):e46–e47. doi:10.1016/S2213-2600(20)30216-2.
- Paterson I, Ramanathan K, Aurora R, et al. Long COVID-19: a primer for cardiovascular health professionals, on behalf of the CCS rapid response team. Can J Cardiol. 2021;37(8):1260–1262. doi:10.1016/j.cjca.2021.05.011.
- Lindner D, Fitzek A, Bräuninger H, et al. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol. 2020;5(11):1281–1285. doi:10.1001/jamacardio.2020.3551.
- Shoenfeld Y, Ryabkova VA, Scheibenbogen C, et al. Complex syndromes of chronic pain, fatigue and cognitive impairment linked to autoimmune dysautonomia and small fiber neuropathy. Clin Immunol. 2020;214:108384. doi:10.1016/j.clim.2020.108384.
- Goldstein DS. The possible association between COVID-19 and postural tachycardia syndrome. Heart Rhythm. 2021;18(4):508–509. doi:10.1016/j.hrthm.2020.12.007.
- Buoite Stella A, Furlanis G, Frezza NA, et al. Autonomic dysfunction in post-COVID patients with and witfhout neurological symptoms: a prospective multidomain observational study. J Neurol. 2022;269(2):587–596. doi:10.1007/s00415-021-10735-y.
- Colonnello E, Limoncin E, Ciocca G, et al. The lost penis syndrome: a new clinical entity in sexual medicine. Sex Med Rev. 2022;10(1):113–129. doi:10.1016/j.sxmr.2021.08.001.
- Sansone A, Mollaioli D, Ciocca G, et al. “Mask up to keep it up”: preliminary evidence of the association between erectile dysfunction and COVID-19. Andrology. 2021;9(4):1053–1059. doi:10.1111/andr.13003.
- Sansone A, Jannini EA. COVID-19 and erectile dysfunction: endothelial dysfunction and Beyond. World J Mens Health. 2021;39(4):820–821. doi:10.5534/wjmh.210081.
- Hellerstein HK, Friedman EH. Sexual activity and the postcoronary patient. Arch Intern Med. 1970;125(6):987–999. doi:10.1001/archinte.1970.00310060065006.
- Grossman VG, McGowan BA. Postural orthostatic tachycardia syndrome. Am J Nurs. 2008;108(8):58–60. doi:10.1097/01.NAJ.0000330266.83852.96.
- Benrud-Larson LM, Dewar MS, Sandroni P, et al. Quality of life in patients with postural tachycardia syndrome. Mayo Clin Proc. 2002;77(6):531–537. doi:10.4065/77.6.531.
- Benrud-Larson LM, Sandroni P, Haythornthwaite JA, et al. Correlates of functional disability in patients with postural tachycardia syndrome: preliminary cross-sectional findings. Health Psychol. 2003;22(6):643–648. doi:10.1037/0278-6133.22.6.643.
- Yafi FA, Jenkins L, Albersen M, et al. Erectile dysfunction. Nat Rev Dis Primers. 2016;2(1):16003. doi:10.1038/nrdp.2016.3.
- Hamming I, Timens W, Bulthuis MLC, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637. doi:10.1002/path.1570.
- Cariou B, Hadjadj S, Wargny M, et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia. 2020;63(8):1500–1515. doi:10.1007/s00125-020-05180-x.
- Apicella M, Campopiano MC, Mantuano M, et al. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020;8(9):782–792. doi:10.1016/S2213-8587(20)30238-2.
- Pizzocaro A, Colombo P, Vena W, et al. Outcome of Sars-COV-2-related thyrotoxicosis in survivors of covid-19: a prospective study. Endocrine. 2021;73(2):255–260. doi:10.1007/s12020-021-02758-2.
- Muller I, Cannavaro D, Dazzi D, et al. SARS-CoV-2-related atypical thyroiditis. Lancet Diabetes Endocrinol. 2020;8(9):739–741. doi:10.1016/S2213-8587(20)30266-7.
- Mattar SAM, Koh SJQ, Rama Chandran S, et al. Subacute thyroiditis associated with COVID-19. BMJ Case Rep. 2020;13(8):e237336. doi:10.1136/bcr-2020-237336.
- Ippolito S, Dentali F, Tanda ML. SARS-CoV-2: a potential trigger for subacute thyroiditis? Insights from a case report. J Endocrinol Invest. 2020;43(8):1171–1172. doi:10.1007/s40618-020-01312-7.
- Chigr F, Merzouki M, Najimi M. Autonomic brain centers and pathophysiology of COVID-19. ACS Chem Neurosci. 2020;11(11):1520–1522. doi:10.1021/acschemneuro.0c00265.
- Marchand L, Pecquet M, Luyton C. Type 1 diabetes onset triggered by COVID-19. Acta Diabetol. 2020;57(10):1265–1266. doi:10.1007/s00592-020-01570-0.
- Maddaloni E, Buzzetti R. Covid-19 and diabetes mellitus: unveiling the interaction of two pandemics. Diabetes Metab Res Rev. 2020;36(7):e33213321.
- Yang M, Chen S, Huang B, et al. Pathological findings in the testes of COVID-19 patients: clinical implications. Eur Urol Focus. 2020;6(5):1124–1129. doi:10.1016/j.euf.2020.05.009.
- Rastrelli G, Di Stasi V, Inglese F, et al. Low testosterone levels predict clinical adverse outcomes in SARS-CoV-2 pneumonia patients. Andrology. 2021;9(1):88–98. doi:10.1111/andr.12821.
- Isidori AM, Buvat J, Corona G, et al. A critical analysis of the role of testosterone in erectile function: from pathophysiology to treatment-a systematic review. Eur Urol. 2014;65(1):99–112. doi:10.1016/j.eururo.2013.08.048.
- Sansone A, Romanelli F, Gianfrilli D, et al. Endocrine evaluation of erectile dysfunction. Endocrine. 2014;46(3):423–430. doi:10.1007/s12020-014-0254-6.
- Sansone A, Romanelli F, Jannini EA, et al. Hormonal correlations of premature ejaculation. Endocrine. 2015;49(2):333–338. doi:10.1007/s12020-014-0520-7.
- Carosa E, Sansone A, Jannini EA. MANAGEMENT OF ENDOCRINE DISEASE: female sexual dysfunction for the endocrinologist. Eur J Endocrinol. 2020;182(6):R101. doi:10.1530/EJE-19-0903.
- Sansone A, Aversa A, Corona G, et al. Management of premature ejaculation: a clinical guideline from the Italian society of andrology and sexual medicine (SIAMS). J Endocrinol Invest. 2021;44(5):1103–1118. doi:10.1007/s40618-020-01458-4.
- Dhindsa S, Zhang N, McPhaul MJ, et al. Association of circulating sex hormones with inflammation and disease severity in patients with COVID-19. JAMA Netw Open. 2021;4(5):e2111398. doi:10.1001/jamanetworkopen.2021.11398.
- Bahar A, Elyasi F, Moosazadeh M, et al. Sexual dysfunction in men with type II diabetes. Caspian J Intern Med. 2020;11(3):295–303.
- Sansone A, Mollaioli D, Ciocca G, et al. Sexual dysfunction in men and women with diabetes: a reflection of their complications? Curr Diabetes Rev. 2022;18(1):e030821192147.
- Jacob L, Smith L, Butler L, et al. Challenges in the practice of sexual medicine in the time of COVID-19 in the United Kingdom. J Sex Med. 2020;17(7):1229–1236. doi:10.1016/j.jsxm.2020.05.001.
- Vassalini P, Serra R, Tarsitani L, et al. Depressive symptoms among individuals hospitalized with COVID-19: three-month follow-up. Brain Sci. 2021;11(9):1175.
- Tarsitani L, Vassalini P, Koukopoulos A, et al. Post-traumatic stress disorder among COVID-19 survivors at 3-month follow-up after hospital discharge. J Gen Intern Med. 2021;36(6):1702–1707. doi:10.1007/s11606-021-06731-7.
- Naidu SB, Shah AJ, Saigal A, et al. The high mental health burden of “long COVID” and its association with on-going physical and respiratory symptoms in all adults discharged from hospital. Eur Respir J. 2021;57(6):2004364. doi:10.1183/13993003.04364-2020.
- Corona G, Lee DM, Forti G, et al. Age-related changes in general and sexual health in Middle-aged and older men: results from the European Male Ageing Study (EMAS). J Sex Med. 2010;7(4 Pt 1):1362–1380. doi:10.1111/j.1743-6109.2009.01601.x.
- Jern P, Gunst A, Sandnabba K, et al. Are early and current erectile problems associated with anxiety and depression in young men? A retrospective self-report study. J Sex Marital Ther. 2012;38(4):349–364. doi:10.1080/0092623X.2012.665818.
- Momtaz YA, Hamid TA, Ibrahim R. The impact of mild cognitive impairment on sexual activity. Am J Alzheimers Dis Other Demen. 2013;28(8):759–762. doi:10.1177/1533317513504612.
- Freak-Poli R, Licher S, Ryan J, et al. Cognitive impairment, sexual activity and physical tenderness in community-dwelling older adults: a cross-sectional exploration. Gerontology. 2018;64(6):589–602. doi:10.1159/000490560.
- Cho JW, Duffy JF. Sleep, sleep disorders, and sexual dysfunction. World J Mens Health. 2019;37(3):261–275. doi:10.5534/wjmh.180045.
- Hu B, Ruan Y, Liu K, et al. A mid-to-long term comprehensive evaluation of psychological distress and erectile function in COVID-19 recovered patients. J Sex Med. 2021;18(11):1863–1871. doi:10.1016/j.jsxm.2021.08.010.
- Bertolo R, Cipriani C, Bove P. Anosmia and ageusia: a piece of the puzzle in the etiology of COVID-19-related transitory erectile dysfunction. J Endocrinol Invest. 2021;44(5):1123–1124. doi:10.1007/s40618-021-01516-5.
- Viveiros A, Rasmuson J, Vu J, et al. Sex differences in COVID-19: candidate pathways, genetics of ACE2, and sex hormones. Am J Physiol Heart Circ Physiol. 2021;320(1):H296–h304. doi:10.1152/ajpheart.00755.2020.
- Yuksel B, Ozgor F. Effect of the COVID-19 pandemic on female sexual behavior. Int J Gynaecol Obstet. 2020;150(1):98–102. doi:10.1002/ijgo.13193.
- Aparisi Á, Ybarra-Falcón C, García-Gómez M, et al. Exercise ventilatory inefficiency in post-COVID-19 syndrome: insights from a prospective evaluation. J Clin Med. 2021;10(12):2591.
- Culha MG, Demir O, Sahin O, et al. Sexual attitudes of healthcare professionals during the COVID-19 outbreak. Int J Impot Res. 2021;33(1):102–109. doi:10.1038/s41443-020-00381-9.
- Güzel A, Döndü A. Changes in sexual functions and habits of healthcare workers during the ongoing COVID-19 outbreak: a cross-sectional survey study. Ir J Med Sci. 2022;191(3):1013–1021. doi:10.1007/s11845-021-02691-3.
- Gebrewahd GT, Gebremeskel GG, Tadesse DB. Intimate partner violence against reproductive age women during COVID-19 pandemic in Northern Ethiopia 2020: a community-based cross-sectional study. Reprod Health. 2020;17(1):152. doi:10.1186/s12978-020-01002-w.
- Warren E, Post N, Hossain M, et al. Systematic review of the evidence on the effectiveness of sexual and reproductive health interventions in humanitarian crises. BMJ Open. 2015;5(12):e008226. doi:10.1136/bmjopen-2015-008226.