Abstract
Background
Previous reports have not reached consistent results regarding the prognostic significance of the controlling nutritional status (CONUT) score in biliary tract cancer (BTC). Therefore, the present meta-analysis was conducted to investigate the precise role of the CONUT score in predicting the prognosis of BTC.
Methods
Electronic platforms including Web of Science, PubMed, Cochrane Library, and Embase were comprehensively searched up to May 2, 2023. We also determined combined hazard ratios (HRs) and 95% confidence intervals (CIs) to estimate the role of the CONUT score in predicting the prognosis of patients with BTC.
Results
Ten studies involving 1,441 patients were included in the present study. Nine studies treated patients with surgical resection, and one study used percutaneous transhepatic biliary stenting (PTBS) plus 125I seed intracavitary irradiation. Based on the combined data, a higher CONUT score significantly predicted dismal overall survival (OS) (HR = 1.94, 95%CI = 1.41–2.66, p < 0.001), inferior recurrence-free survival (RFS) (HR = 1.79, 95%CI = 1.48–2.17, p < 0.001) in BTC, and low differentiation (OR = 1.57, 95%CI = 1.15–2.14, p = 0.004). Nonetheless, the CONUT score was not related to sex, lymph node metastasis, microvascular invasion, perineural invasion, distant metastasis, TNM stage, or tumor number in patients with BTC.
Conclusion
Higher CONUT scores significantly predicted worse OS and RFS in patients with BTC. Moreover, BTC patients with high CONUT scores tended to have poor tumor differentiation. The CONUT score could help clinicians stratify high-risk patients with BTC and devise individualized treatment plans.
KEY MESSAGES
As far as we know, this study is the first to analyze whether pretreatment CONUT is significant for predicting the prognosis of BTC.
A high CONUT significantly predicted worse OS and RFS in BTC patients.
CONUT could help clinicians stratify high-risk BTC patients and devise individualized treatment plans.
Introduction
Biliary tract cancer (BTC) refers to invasive carcinomas such as gallbladder cancer (GBC), extrahepatic cholangiocarcinoma (ECC), and intrahepatic cholangiocarcinoma (ICC) [Citation1]. BTC accounts for approximately 3% of all gastrointestinal cancers and ranks second among hepatobiliary cancers in terms of morbidity, followed by hepatocellular carcinoma [Citation2,Citation3]. According to the disease stage, BTC has a dismal prognostic outcome, with 5-year survival rates of 2%-15%, 2%-30% and 2%-70% for ICC, ECC, and GBC, respectively [Citation4]. Surgery is a potential radical therapy for BTC. Nonetheless, as many patients with BTC do not have early symptoms, they still have a poor prognosis. Over 65% of patients with BTC are not candidates for surgery at the time of diagnosis, and their 5-year survival and 1-year relapse rates are 5–15% and 67%, respectively [Citation5,Citation6]. Therefore, the identification of new and reliable biomarkers for predicting BTC prognosis is urgently needed.
Increasing evidence has shown that nutritional status is an important factor in cancer patients because it can predict treatment tolerance and tumor development [Citation7]. Many blood test-derived biomarkers, including the prognostic nutritional index (PNI) [Citation8], albumin-to-globulin ratio (AGR) [Citation9], geriatric nutritional risk index (GNRI) [Citation10], and C-reactive protein-to-albumin ratio (CAR) [Citation11] have been identified as effective prognostic markers in various cancers. Notably, the controlling nutritional status (CONUT) score represents a verified nutritional evaluation system, which contributes to the comprehensive evaluation of host immunocompetence and anabolism [Citation12]. The CONUT score is based on serum albumin levels, total cholesterol (TC) content, and lymphocyte count [Citation12] (). The CONUT has attracted wide interest as a biomarker for predicting cancer prognosis in breast cancer (BC) [Citation13], peripheral T-cell lymphoma [Citation14], esophageal cancer [Citation15], and thyroid cancer [Citation16]. Previous studies have analyzed whether the CONUT score could be adopted to predict the prognosis of patients with BTC, but their findings have been inconsistent [Citation17–26]. For instance, some studies have shown that the CONUT score is important for predicting BTC prognosis [Citation19,Citation20,Citation23,Citation26], whereas others have suggested that CONUT score is not related to BTC prognosis [Citation21,Citation24]. Therefore, the present meta-analysis reviewed the most recent data to identify the significance of the CONUT score in predicting the prognosis of patients with BTC.
Table 1. The scoring criteria for the CONUT.
Materials and methods
Study guideline
The present study was conducted in accordance with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement (Supplementary File 1) [Citation27].
Ethnics statement
As our analysis did not directly involve participants, and we extracted the necessary data from previous publications, institutional review board and ethics committee approval was not required for this meta-analysis.
Literature search
We comprehensively searched electronic platforms, including Web of Science, PubMed, Cochrane Library, and Embase, using Medical Subject Heading (MeSH) terms combined with text words: (controlling nutritional status or CONUT) and (biliary tract tumor, biliary tract cancer, biliary tract neoplasm, cholangiocarcinoma, bile duct cancer, gallbladder tumor, gallbladder neoplasm, gallbladder cancer, and gallbladder carcinoma). The literature search was conducted from the inception of each database until May 2, 2023. We manually reviewed the reference lists of the included studies to avoid omitting relevant articles.
Study eligibility criteria
Eligible studies were included based on the following criteria: (1) BTC diagnosed through histology, (2) COUNT before treatment was measured, (3) studies that investigated the association between CONUT score and survival outcomes in BTC, (4) hazard ratios (HRs) and associated 95% confidence intervals (CIs) of survival analysis were obtained or calculated based on available data, (5) the threshold CONUT score was determined, and (6) English-language articles. Studies that met the following criteria were excluded: (1) reviews, case reports, meeting abstracts, letters, and comments; (2) studies that did not provide survival data; and (3) animal studies.
Data collection and quality evaluation
Two reviewers (HJ and ZW) reviewed eligible studies and independently collected the required data. Any disagreements were resolved through negotiations until a consensus was reached. The following data were collected from the included articles: first author, country, publication year, sample size, sex, age, study duration, histology, treatment, study design, threshold CONUT score, study center, follow-up, neoadjuvant or adjuvant treatments, survival endpoints, survival analysis type, HRs with corresponding 95%CIs, and clinicopathological characteristics. The Newcastle–Ottawa Scale (NOS) was used to assess the quality of the included studies [Citation28]. The NOS consists of three domains–selection, comparability, and outcome–with a score of 0–9 stars. An article with an NOS score ≥6 was considered high-quality.
Statistical analysis
The pooled HRs and corresponding 95%CIs were calculated to estimate the significance of the CONUT score in predicting the prognosis of patients with BTC. Heterogeneity among studies was assessed using I2 statistics [Citation29] and the chi-square Q test [Citation30]. We selected the random-effects model [Citation31] for the calculation in the case of distinct heterogeneity (I2>50% or p < 0.10); otherwise, we selected the fixed-effects model [Citation32]. An appropriate calculation model was selected based on heterogeneity levels. We also performed subgroup analysis to detect potential sources of heterogeneity. Correlations between the CONUT scores and clinicopathological features were assessed by combining odds ratios (ORs) and 95%CIs. Moreover, a sensitivity analysis was carried out to assess whether individual studies affected the overall outcomes. Funnel plots and Begg’s test were used to assess publication bias. Stata software version 12.0 (Stata Corp., College Station, TX, USA) was used for the statistical analysis. Statistical significance was set at p < 0.05 (two-sided).
Results
Literature search process
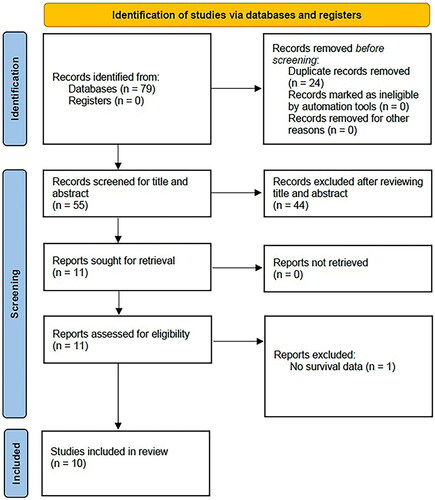
As shown in , 79 articles were initially obtained, and 55 remained after the removal of duplicates. Through title and abstract screening, 44 irrelevant studies or fundamental research in cell biology were removed. Subsequently, we read the full text of 11 articles and discarded one because it did not provide survival information. Finally, the present meta-analysis included ten studies with 1,441 patients [Citation17–26] (; ).
Table 2. Baseline characteristics of included studies.
Features of included articles
displays fundamental features of the included articles [Citation17–26]. Six studies were performed in Japan [Citation18,Citation19,Citation23–26] and four in China [Citation17,Citation20–22]. The sample size of these studies ranged from 45 to 371, with the median value of 121.5. All studies used a retrospective design. Four articles included ECC cases [Citation17,Citation20,Citation23,Citation25]; three, ICC cases [Citation18,Citation21,Citation24]; two, BTC cases [Citation22,Citation26]; and one, GBC cases [Citation19]. Nine studies treated patients with surgical resection [Citation18–26], and one study adopted percutaneous transhepatic biliary stenting (PTBS) plus 125I seed intracavitary irradiation [Citation17]. Five studies used ≥3 as the cut-off value for CONUT [Citation19–21,Citation23,Citation25], four studies selected ≥2 [Citation17,Citation18,Citation22,Citation24], and one study adopted ≥4 [Citation26]. All 10 studies mentioned the role of CONUT in predicting overall survival (OS) in BTC [Citation17–26]. Six studies provided data on the association between CONUT and recurrence-free survival (RFS) in patients with BTC [Citation18,Citation20,Citation21,Citation23,Citation24,Citation26]. Eight studies calculated HRs together with 95%CIs by multivariable regression [Citation17–23,Citation26], and two studies used univariate analysis [Citation24,Citation25]. For the included articles, NOS scores were 6–9 (median, 7), suggesting the articles were high quality (). The details of NOS scores are shown in Supplementary File 2.
CONUT and OS in BTC
Ten articles, including 1,441 patients, mentioned the relationship between the CONUT score and OS in BTC [Citation17–26]. According to the pooled data ( and ), a higher CONUT score significantly predicted a dismal OS in BTC (HR = 1.94, 95%CI = 1.41–2.66, p < 0.001). Owing to obvious heterogeneity, we selected the random-effects model (I2=79.8%, p < 0.001). Subgroup analysis revealed that a higher CONUT score was still a prognostic factor for OS regardless of country, sample size, treatment, threshold, neoadjuvant/adjuvant treatments, treatment intent, and type of survival analysis (all p < 0.05; ). Moreover, the CONUT score significantly predicted worse OS in patients with ECC, BTC, and GBC ().
Figure 2. Forest plot verify the association between the CONUT and overall survival (OS) in patients with BTC.
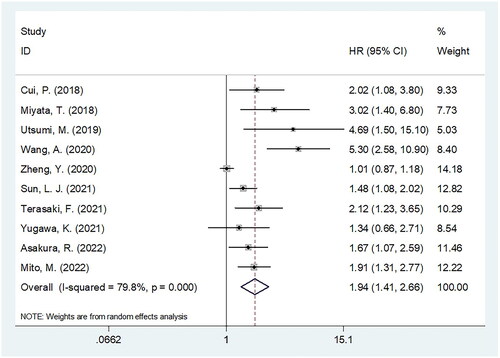
Table 3. Subgroup analysis of association between CONUT and OS in patients with BTC.
CONUT and RFS in BTC
Six studies, including 783 patients [Citation18,Citation20,Citation21,Citation23,Citation24,Citation26] investigated whether the CONUT score was significant in predicting RFS in patients with BTC. According to the combined results ( and ), a higher CONUT score was significantly associated with shortened RFS in patients with BTC (HR = 1.79, 95%CI = 1.48–2.17, p < 0.001). In the subgroup analysis, a higher CONUT score still significantly predicted RFS, regardless of country, sample size, histology, or cutoff value (all p < 0.05; ).
Figure 3. Forest plot verify the association between the CONUT and recurrence-free survival (RFS) in patients with BTC.
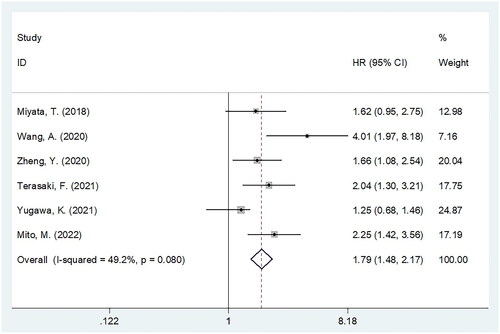
Table 4. Subgroup analysis of association between CONUT and RFS in patients with BTC.
CONUT and clinicopathological factors
Six studies with 1,076 patients [Citation18,Citation20–23,Citation26] provided information on the relationship between the CONUT score and the clinicopathological characteristics of BTC. Based on the pooled results, a higher CONUT score significantly predicted dismal differentiation (OR = 1.57, 95%CI = 1.15–2.14, p = 0.004; ). However, as shown in , there was a non-significant relationship between CONUT score and sex (OR = 0.94, 95%CI = 0.72–1.21, p = 0.617), lymph node metastasis (OR = 1.64, 95%CI = 0.95–2.82, p = 0.074), microvascular invasion (OR = 1.22, 95%CI = 0.92–1.62, p = 0.158), perineural invasion (OR = 1.15, 95%CI = 0.74–1.80, p = 0.530), distant metastasis (OR = 1.15, 95%CI = 0.76–1.76, p = 0.505), TNM stage (OR = 1.23, 95%CI = 0.86–1.76, p = 0.251), and tumor number (OR = 1.02, 95%CI = 0.34–3.03, p = 0.977) of BTC cases.
Table 5. The Correlation between CONUT score and clinicopathological features in patients with BTC.
Sensitivity analysis
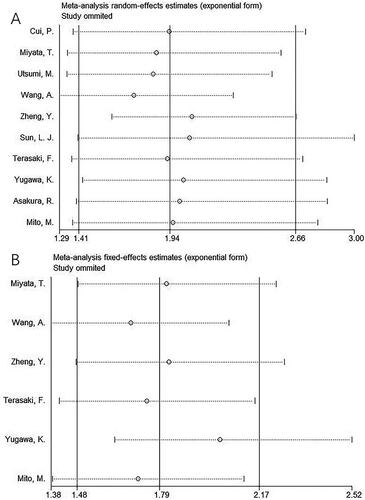
A sensitivity analysis was performed to examine whether each study affected the pooled outcomes. As shown in , after removing the included studies individually, the pooled HRs and 95%CIs for OS and RFS remained almost unchanged, indicating the reliability of our results.
Publication bias
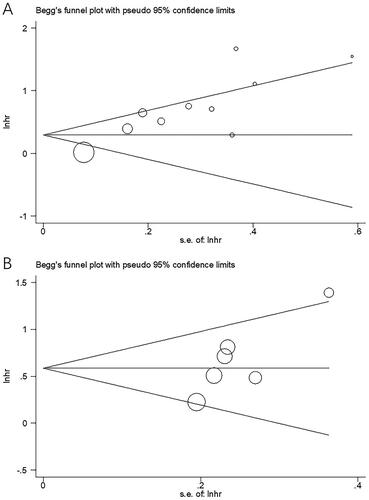
Publication bias in the included studies was assessed using Begg’s test and funnel plots. Symmetric plots were observed in the funnel plots for OS and RFS (). Begg’s test results (p = 0.097 and 0.080 for OS and RFS, respectively) indicated an absence of publication bias in the present study.
Discussion
The prognostic effect of the CONUT score in patients with BTC has not been consistent in prior studies. This study extracted information from ten articles involving 1,441 patients [Citation17–26] and found that a higher CONUT score predicted inferior OS and RFS in BTC patients. Moreover, the CONUT score had a stable prognostic effect among diverse subgroups. We also identified a significant connection between the CONUT score and poor differentiation in BTC, indicating that BTC patients with higher CONUT scores had more aggressive and malignant tumors. Therefore, an elevated CONUT score could be a strong predictor of tumor recurrence and poor long-term prognosis of BTC. To the best of our knowledge, this study is the first to analyze whether the pretreatment CONUT score is significant in predicting the prognosis of patients with BTC.
The CONUT score involves the serum albumin level, TC concentration, and lymphocyte count; therefore, the potential mechanisms of the prognostic effect of the CONUT score for BTC are interpreted from several aspects. First, serum albumin level is a critical predictor of nutritional status as a lower serum albumin level indicates protein loss due to systemic responses [Citation33]. In cancer patients, hypoalbuminemia indicates malnutrition and cachexia. Hypoalbuminemia has been found to impair immune responses because nutrition is a crucial determinant [Citation34]. Lymphocytes play major roles in cell-mediated immunity, cancer immune surveillance, and immune editing. As tumor suppressors, lymphocytes produce cytokines that inhibit the proliferation and metastatic activity of cancer cells and induce cell death [Citation35]. Cholesterol is a major component of mammalian cell membranes [Citation36]. Circulating blood is a source of cholesterol, which is composed of endocytosed low-density lipoprotein receptors. In patients with cancer, low cholesterol levels are associated with poor prognoses [Citation37]. Thus, as a combined index of albumin level, TC content, and lymphocyte count, the CONUT score can be a valuable prognostic marker for BTC.
Significant heterogeneity was observed among the studies in the OS analysis (). Heterogeneity is derived from many variables, such as study type, sample size, study subjects, selection bias, and cutoff value. We selected a random-effects model based on the significant heterogeneity. The studies included in this meta-analysis remained comparable because they were selected based on uniform inclusion and exclusion criteria. The CONUT cutoff values were not uniform in the included studies because they used different methods to determine the cutoff values. This is a limitation of this meta-analysis, as shown below. Moreover, a subgroup analysis was conducted to evaluate the impact of the cutoff values on our results. The results showed that various cutoff values did not affect the prognostic value of CONUT for OS and RFS in patients with BTC ( and ).
It should also be noted that BTC is a heterogeneous disease that primarily comprises CCA and GBC. GBC is the most common BTC, comprising 80–90% of cases according to autopsy studies [Citation38]. Previous studies have indicated that patients with eCCA, iCCA, and GBC have different prognoses [Citation39]. The histological types used in the meta-analysis are listed in . We also conducted a subgroup analysis according to the histological subtype. As shown in and , CONUT score significantly predicted worse OS in patients with ECC, BTC, and GBC, but not in those with ICC. Moreover, a higher CONUT score significantly predicted RFS regardless of histology. This finding indicates that ICC patients with a high CONUT score may experience tumor recurrence, but the OS duration is not significantly different from those with a low CONUT score.
Notably, this meta-analysis focused on CONUT [Citation12], a nutritional assessment tool based on serum albumin level, TC content, and lymphocyte count (). The total CONUT score is the sum of the three components. CONUT was established in 2005 [Citation12] and the scoring system has been used until now. is consistent with the original CONUT scoring system in the literature [Citation40]. Compared to other nutritional tools and parameters, such as the Glasgow prognostic score (GPS), modified GPS [Citation41,Citation42], PNI [Citation43,Citation44], AGR [Citation45,Citation46], systemic immune inflammation index [Citation47–50], GNRI [Citation51–53], and CAR [Citation40,Citation54,Citation55], CONUT has the following advantages. First, CONUT is a standardized scoring system with clear scoring criteria (). Therefore, promoting and disseminating CONUT scores is easier. Second, the CONUT score consists of three elements: serum albumin level, TC content, and lymphocyte count. These indicators comprehensively reflect nutritional status. Therefore, CONUT is more reliable and comprehensive. Third, CONUT scores ranged from 0 to 12. The CONUT score is a more refined and easily quantifiable indicator than other nutritional evaluation tools.
CONUT is a promising tool for guiding the management of patients with BTC. When a low pretreatment CONUT score is detected in an individual BTC patient, nutritional support should be provided. Moreover, the risk of tumor recurrence and poor survival and more aggressive therapeutic strategies should be considered. During follow-up, the CONUT score should also be monitored to facilitate the early detection of tumor progression.
Recently, many meta-analyses have reported that the CONUT score is significant for predicting the prognosis of solid tumors [Citation56–61]. According to Peng et al. in a meta-analysis of 5,410 cases, a higher CONUT score indicated worse OS, RFS, disease-free survival (DFS), and cancer-specific survival (CSS) in upper urinary tract urothelial carcinoma and renal cell carcinoma [Citation62]. Zhang et al. demonstrated that a high CONUT score had an unfavorable impact on OS, DFS, CSS, and progression-free survival (PFS) compared to low CONUT scores [Citation57]. According to a recent meta-analysis involving 2,294 patients, a higher CONUT score predicted dismal OS in pancreatic cancer [Citation59]. Takagi et al. conducted a meta-analysis of 2,601 cases and found that a higher CONUT score predicted dismal OS, CSS, and RFS in colorectal cancer patients undergoing surgery [Citation61]. In a meta-analysis by Lu et al. that included 1,811 patients, a high CONUT score predicted worse OS and PFS in patients with hematological cancer [Citation63].
This study had some limitations. First, all included studies were conducted in Asia, which may make them less applicable to other populations when applied on a large scale. Second, all the studies had a retrospective design, which may have reduced the power of our analysis. Third, the threshold for defining the high CONUT score group was not uniform among the studies, which could have introduced a selection bias. Fourth, most included studies (9 out of 10) enrolled patients with BTC who underwent surgical resection. Therefore, our results may be more applicable to BTC patients undergoing surgery. Therefore, prospective studies with larger sample sizes are needed to validate our results.
In summary, a high CONUT score significantly predicted worse OS and RFS in patients with BTC. Additionally, BTC patients with higher CONUT scores tended to have poor tumor differentiation. The CONUT score could help clinicians stratify high-risk BTC patients and devise individualized treatment plans.
Authors’ contributions
HJ and ZW design the project; HZ and ZW searched databases and performed literature screen; HZ and ZW extracted and analyzed the data, analysis; HZ and ZW evaluated the quality of included literature; HZ contributed to writing the manuscript. Final draft was approved by all the authors. All authors contributed to the article and approved the submitted version.
| Abbreviations | ||
| CONUT | = | controlling nutritional status |
| BTC | = | biliary tract cancer |
| HR | = | hazard ratio |
| CI | = | confidence interval |
| OS | = | overall survival |
| RFS | = | recurrence-free survival |
| GBC | = | gallbladder cancer |
| ECC | = | extrahepatic cholangiocarcinoma |
| ICC | = | intrahepatic cholangiocarcinoma |
| HCC | = | hepatocellular carcinoma |
| PNI | = | prognostic nutritional index |
| AGR | = | albumin to globulin ratio |
| GNRI | = | geriatric nutritional risk index |
| CAR | = | C-reactive protein to albumin ratio |
| TC | = | total cholesterol |
| BC | = | breast cancer |
| PRISMA | = | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| MeSH | = | Medical Subject Headings |
| NOS | = | Newcastle-Ottawa Scale |
| OR | = | odds ratio |
| PTBS | = | percutaneous transhepatic biliary stenting |
| OS | = | overall survival |
| RFS | = | recurrence-free survival |
| LNM | = | lymph node metastasis |
| DFS | = | disease-free survival |
| CSS | = | cancer-specific survival |
| PFS | = | progression-free survival. |
Supplemental Material
Download Zip (41.9 KB)Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Disclosure statement
The authors declare that there is no conflict of interest.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Valle JW, Kelley RK, Nervi B, et al. Biliary tract cancer. Lancet. 2021;397(10272):1–13. doi: 10.1016/s0140-6736(21)00153-7.
- Charbel H, Al-Kawas FH. Cholangiocarcinoma: epidemiology, risk factors, pathogenesis, and diagnosis. Curr Gastroenterol Rep. 2011;13(2):182–187. doi: 10.1007/s11894-011-0178-8.
- Tariq NU, McNamara MG, Valle JW. Biliary tract cancers: current knowledge, clinical candidates and future challenges. Cancer Manag Res. 2019;11:2623–2642. doi: 10.2147/cmar.S157092.
- Shroff RT, Kennedy EB, Bachini M, et al. Adjuvant therapy for resected biliary tract cancer: ASCO clinical practice guideline. J Clin Oncol. 2019;37(12):1015–1027. doi: 10.1200/jco.18.02178.
- Bridgewater J, Lopes A, Wasan H, et al. Prognostic factors for progression-free and overall survival in advanced biliary tract cancer. Ann Oncol. 2016;27(1):134–140. doi: 10.1093/annonc/mdv483.
- Shaib YH, Davila JA, McGlynn K, et al. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol. 2004;40(3):472–477. doi: 10.1016/j.jhep.2003.11.030.
- Mantzorou M, Koutelidakis A, Theocharis S, et al. Clinical value of nutritional status in cancer: what is its impact and how it affects disease progression and prognosis? Nutr Cancer. 2017;69(8):1151–1176. doi: 10.1080/01635581.2017.1367947.
- Kazi M, Gori J, Sasi S, et al. Prognostic nutritional index prior to rectal cancer resection predicts overall survival. Nutr Cancer. 2022;74(9):3228–3235. 2022/05/10. doi: 10.1080/01635581.2022.2072906.
- Liu J, Dai Y, Zhou F, et al. The prognostic role of preoperative serum albumin/globulin ratio in patients with bladder urothelial carcinoma undergoing radical cystectomy. Urol Oncol. 2016;34(11):484.e481–484.e488. doi: 10.1016/j.urolonc.2016.05.024.
- Fang P, Yang Q, Zhou J, et al. The impact of geriatric nutritional risk index on esophageal squamous cell carcinoma patients with neoadjuvant therapy followed by esophagectomy. Front Nutr. 2022;9:983038. doi: 10.3389/fnut.2022.983038.
- Jia-Min Z, Wei D, Ye L, et al. Correlation between C-reactive protein/albumin ratio and prognosis in patients with lung adenocarcinoma. J Int Med Res. 2022;50(6):3000605221105372. doi: 10.1177/03000605221105372.
- Ignacio de Ulíbarri J, González-Madroño A, de Villar NG, et al. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutricion Hospitalaria. 2005;20:38–45.
- Zhu M, Chen L, Kong X, et al. Controlling nutritional status (CONUT) as a novel postoperative prognostic marker in breast cancer patients: a retrospective study. Biomed Res Int. 2022;2022:3254523–3254581. doi: 10.1155/2022/3254581.
- Nakamura N, Kanemura N, Lee S, et al. Prognostic impact of the controlling nutritional status score in patients with peripheral T-cell lymphoma. Leuk Lymphoma. 2022;63(6):1323–1330. doi: 10.1080/10428194.2021.2020777.
- Demir M, Demircan NC. The CONUT score is prognostic in esophageal cancer treated with chemoradiotherapy. Saudi J Gastroenterol. 2023;29(2):119–126. doi: 10.4103/sjg.sjg_384_22.
- Dalmiglio C, Brilli L, Campanile M, et al. CONUT score: a new tool for predicting prognosis in patients with advanced thyroid cancer treated with TKI. Cancers (Basel). 2022;14(3):724. doi: 10.3390/cancers14030724.
- Cui P, Pang Q, Wang Y, et al. Nutritional prognostic scores in patients with hilar cholangiocarcinoma treated by percutaneous transhepatic biliary stenting combined with 125I seed intracavitary irradiation: a retrospective observational study. Medicine (Baltimore). 2018;97(22):e11000. doi: 10.1097/md.0000000000011000.
- Miyata T, Yamashita YI, Higashi T, et al. The prognostic impact of controlling nutritional status (CONUT) in intrahepatic cholangiocarcinoma following curative hepatectomy: a retrospective single institution study. World J Surg. 2018;42(4):1085–1091. doi: 10.1007/s00268-017-4214-1.
- Utsumi M, Aoki H, Nishimura S, et al. Safety of surgical treatment for elderly patients with gallbladder carcinoma. Acta Med Okayama. 2019;73(3):241–246. doi: 10.18926/amo/56867.
- Wang A, He Z, Cong P, et al. Controlling nutritional status (CONUT) score as a new indicator of prognosis in patients with hilar cholangiocarcinoma is superior to NLR and PNI: a Single-Center retrospective study. Front Oncol. 2020;10:593452. doi: 10.3389/fonc.2020.593452.
- Zheng Y, Wu F, Rong W, et al. Prognostic value of the controlling nutritional status (CONUT) score in intrahepatic cholangiocarcinoma patients especially who had long-time alcohol consumption. J Clin Biochem Nutr. 2020;67(3):323–331. doi: 10.3164/jcbn.20-27.
- Sun LJ, Su S, Xiong JP, et al. Controlling nutritional status score as a prognostic marker to predict overall survival in resected biliary tract cancers. Ann Transl Med. 2021;9(8):644–644. doi: 10.21037/atm-20-6770.
- Terasaki F, Sugiura T, Okamura Y, et al. Use of preoperative controlling nutritional status (CONUT) score as a better prognostic marker for distal cholangiocarcinoma after pancreatoduodenectomy. Surg Today. 2021;51(3):358–365. doi: 10.1007/s00595-020-02098-0.
- Yugawa K, Itoh S, Yoshizumi T, et al. Lymphocyte–C-reactive protein ratio as a prognostic marker associated with the tumor immune microenvironment in intrahepatic cholangiocarcinoma. Int J Clin Oncol. 2021;26(10):1901–1910. doi: 10.1007/s10147-021-01962-4.
- Asakura R, Yanagimoto H, Ajiki T, et al. Prognostic impact of Inflammation-Based scores for extrahepatic cholangiocarcinoma. Dig Surg. 2022;39(2-3):65–74. doi: 10.1159/000521969.
- Mito M, Sakata J, Hirose Y, et al. Preoperative controlling nutritional status score predicts systemic disease recurrence in patients with resectable biliary tract cancer. Eur J Surg Oncol. 2023;49(2):399–409. doi: 10.1016/j.ejso.2022.11.003.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71.
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z.
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186.
- Cochran W. The combination of estimates from different experiments. Biometrics. 1954;10(1):101–129. doi: 10.2307/3001666.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2.
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute. 1959;22:719–748.
- Sun LC, Chu KS, Cheng SC, et al. Preoperative serum carcinoembryonic antigen, albumin and age are supplementary to UICC staging systems in predicting survival for colorectal cancer patients undergoing surgical treatment. BMC Cancer. 2009;9(1):288. doi: 10.1186/1471-2407-9-288.
- Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010;6(1):149–163. doi: 10.2217/fon.09.136.
- Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205.
- Cortes VA, Busso D, Maiz A, et al. Physiological and pathological implications of cholesterol. Front Biosci (Landmark Ed). 2014;19(3):416–428. doi: 10.2741/4216.
- Shor R, Wainstein J, Oz D, et al. Low serum LDL cholesterol levels and the risk of fever, sepsis, and malignancy. Ann Clin Lab Sci. 2007;37(4):343–348.
- Oneda E, Abu Hilal M, Zaniboni A. Biliary tract cancer: current medical treatment strategies. Cancers (Basel). 2020;12(5):12. doi: 10.3390/cancers12051237.
- Personeni N, Lleo A, Pressiani T, et al. Biliary tract cancers: molecular heterogeneity and new treatment options. Cancers (Basel). 2020;12(11):12. doi: 10.3390/cancers12113370.
- Dou L, Shi M, Song J, et al. The prognostic significance of C-Reactive protein to albumin ratio in newly diagnosed acute myeloid leukaemia patients. Cancer Manag Res. 2022;14:303–316. doi: 10.2147/cmar.S343580.
- Demirelli B, Babacan NA, Ercelep O, et al. Modified glasgow prognostic score, prognostic nutritional index and ECOG performance score predicts survival better than sarcopenia, cachexia and some inflammatory indices in metastatic gastric cancer. Nutr Cancer. 2021;73(2):230–238. doi: 10.1080/01635581.2020.1749290.
- Kimura S, D’ Andrea D, Soria F, et al. Prognostic value of modified glasgow prognostic score in non-muscle-invasive bladder cancer. Urol Oncol. 2019;37(3):179.e19–179.e28. doi: 10.1016/j.urolonc.2018.11.005.
- Momokita M, Abe A, Shibata K, et al. Prognostic nutritional index in patients with End-Stage oral cancer. Am J Hosp Palliat Care. 2023;40(4):396–400. doi: 10.1177/10499091221102581.
- Nogueiro J, Santos-Sousa H, Pereira A, et al. The impact of the prognostic nutritional index (PNI) in gastric cancer. Langenbecks Arch Surg. 2022;407(7):2703–2714. doi: 10.1007/s00423-022-02627-0.
- Quhal F, Pradere B, Laukhtina E, et al. Prognostic value of albumin to globulin ratio in non-muscle-invasive bladder cancer. World J Urol. 2021;39(9):3345–3352. doi: 10.1007/s00345-020-03586-1.
- Taguchi S, Kawai T, Nakagawa T, et al. Prognostic significance of the albumin-to-globulin ratio for advanced urothelial carcinoma treated with pembrolizumab: a multicenter retrospective study. Sci Rep. 2021;11(1):15623. doi: 10.1038/s41598-021-95061-z.
- Cesur IB, Özçelik Z. Systemic Immune-Inflammation index may predict mortality in neuroblastoma. Cureus. 2023;15(3):e35705. doi: 10.7759/cureus.35705.
- Jan HC, Wu KY, Tai TY, et al. The systemic Immune-Inflammation index (SII) increases the prognostic significance of lymphovascular invasion in upper tract urothelial carcinoma after radical nephroureterectomy. Cancer Manag Res. 2022;14:3139–3149. doi: 10.2147/cmar.S378768.
- Han W, Weng K, Zhang P, et al. Predictive value of systemic immune-inflammation index for pathological complete response in patients receiving neoadjuvant immunochemotherapy for locally advanced esophageal cancer. Front Surg. 2022;9:1091601. doi: 10.3389/fsurg.2022.1091601.
- Feng L, Xu R, Lin L, et al. Effect of the systemic immune-inflammation index on postoperative complications and the long-term prognosis of patients with colorectal cancer: a retrospective cohort study. J Gastrointest Oncol. 2022;13(5):2333–2339. doi: 10.21037/jgo-22-716.
- Sakamoto T, Makinoya M, Sunaguchi T, et al. Geriatric nutritional risk index as a prognostic factor in patients with recurrent pancreatic cancer. PLoS One. 2022;17(7):e0271073. doi: 10.1371/journal.pone.0271073.
- Si Y, Xu P, Xu A, et al. Geriatric nutritional risk index as a prognostic factor in patients with hepatocellular carcinoma following transarterial chemoembolization: a retrospective study. Medicine (Baltimore). 2022;101(51):e32322. doi: 10.1097/md.0000000000032322.
- Riveros C, Chalfant V, Bazargani S, et al. The geriatric nutritional risk index predicts complications after nephrectomy for renal cancer. Int Braz J Urol. 2023;49(1):97–109. doi: 10.1590/s1677-5538.Ibju.2022.0380.
- Huang YY, Liu X, Liang SH, et al. Prognostic value of preoperative C-reactive protein to albumin ratio in patients with thymic epithelial tumors: a retrospective study. BMC Cancer. 2022;22(1):1183. doi: 10.1186/s12885-022-10234-x.
- Liu Z, Chen L, Sun F, et al. C-Reactive protein/albumin ratio on the first day after surgery predicts Short-Term complications of gastrectomy for gastric cancer. Nutr Cancer. 2022;74(10):3574–3581. doi: 10.1080/01635581.2022.2083190.
- Xue W, Hu X, Zhang Y. The association of controlling nutritional status (CONUT) score with survival in patients with surgically treated renal cell carcinoma and upper tract urothelial carcinoma: a systematic review and Meta-Analysis. Nutr Cancer. 2022;74(6):1907–1916. doi: 10.1080/01635581.2021.1974894.
- Zhang C, Li XK, Cong ZZ, et al. Controlling nutritional status is a prognostic factor for patients with lung cancer: a systematic review and meta-analysis. Ann Palliat Med. 2021;10(4):3896–3905. doi: 10.21037/apm-20-2328.
- Peng J, Hao Y, Rao B, et al. Prognostic impact of the pre-treatment controlling nutritional status score in patients with non-small cell lung cancer: a meta-analysis. Medicine (Baltimore). 2021;100(26):e26488. doi: 10.1097/md.0000000000026488.
- Ma X, Zou W, Sun Y. Prognostic value of pretreatment controlling nutritional status score for patients with pancreatic cancer: a Meta-Analysis. Front Oncol. 2021;11:770894. doi: 10.3389/fonc.2021.770894.
- Takagi K, Buettner S, Ijzermans JNM, et al. Systematic review on the controlling nutritional status (CONUT) score in patients undergoing esophagectomy for esophageal cancer. Anticancer Res. 2020;40(10):5343–5349. doi: 10.21873/anticanres.14541.
- Takagi K, Buettner S, Ijzermans JNM. Prognostic significance of the controlling nutritional status (CONUT) score in patients with colorectal cancer: a systematic review and meta-analysis. Int J Surg. 2020;78:91–96. doi: 10.1016/j.ijsu.2020.04.046.
- Peng L, Meng C, Li J, et al. The prognostic significance of controlling nutritional status (CONUT) score for surgically treated renal cell cancer and upper urinary tract urothelial cancer: a systematic review and meta-analysis. Eur J Clin Nutr. 2022;76(6):801–810. doi: 10.1038/s41430-021-01014-0.
- Lu C, Chen Q, Fei L, et al. Prognostic impact of the controlling nutritional status score in patients with hematologic malignancies: a systematic review and meta-analysis. Front Immunol. 2022;13:952802. doi: 10.3389/fimmu.2022.952802.