?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Septic shock is the development of sepsis to refractory circulatory collapse and metabolic derangements, characterized by persistent hypotension and increased lactate levels. Anisodamine hydrobromide (Ani HBr) is a Chinese medicine used to improve blood flow in circulatory disorders. The purpose of this study was to determine the therapeutic efficacy of Ani HBr in the treatment of patients with septic shock.
Methods
This was a prospective, multicenter, randomized controlled trial focusing on patients with septic shock in 16 hospitals in China. Patients were randomly assigned in a 1:1 ratio to either the treatment group or the control group. The primary endpoint was 28-day mortality. The secondary outcomes included 7-day mortality, hospital mortality, hospital length of stay, vasopressor-free days within 7 days, etc. These indicators were measured and collected at 0, 6h, 24h, 48h, 72h and 7d after the diagnosis.
Results
Between September 2017 and March 2021, 404 subjects were enrolled. 203 subjects received Ani HBr and 201 subjects were assigned to the control group. The treated group showed lower 28-day mortality than the control group. Stratified analysis further showed significant differences in 28-day mortality between the two groups for patients with a high level of illness severity. We also observed significant differences in 7-day mortality, hospital mortality and some other clinical indicators between the two groups.
Conclusion
Ani HBr might be an important adjuvant to conventional treatment to reduce 28-day mortality in patients with septic shock. A large-scale prospective randomized multicenter trial is warranted to confirm our results.
1. Background
Sepsis is a life-threatening organ dysfunction caused by a dysregulated host response to infection, which is an increasingly common cause of morbidity and mortality [Citation1–4]. Septic shock is the most severe form of sepsis, which results in refractory circulatory collapse and deranged cellular metabolism. The mortality of septic shock remains high at nearly 35% to 40% [Citation5]. In recent years, a series of treatments for sepsis has been proposed to improve its clinical outcomes, including early fluid resuscitation, empirical anti-infection, use of vasoconstrictors and organ function support. However, due to the complex pathophysiological mechanism and the heterogeneity of the body’s response to infection, the mortality rate of sepsis, especially septic shock, still remains high [Citation4, Citation6]. Exploring innovative therapies is a top research priority for sepsis/septic shock [Citation6,Citation7].
Historically, the treatment for septic shock has focused on restoring the macrodynamics of circulation such as vital signs, mean arterial pressure, and oxygen delivery and extraction ratio. This type of treatment may neglect the upstream events that actually trigger organ dysfunction [Citation8]. Indeed, the cause of alterations in the macrohaemodynamics lies in the microcirculation. Moreover, it has been proved that resuscitating the macrohaemodynamics is not always associated with improved microhaemodynamics [Citation9,Citation10]. In particular, it is concluded that microcirculatory dysfunction appears in the early course of sepsis and can cause decreased oxygen delivery, tissue hypoxia and organ dysfunction [Citation11]. Anisodamine hydrobromide (, Ani HBr) is a type of belladonna alkaloid extracted from the root of a Chinese specialty plant Scopolia tangutica Maxim in 1965. Anisodamine has been synthesized in 1975 and used clinically in China for improving microcirculation by stabilizing endothelium via antagonizing acetylcholine and by the inhibition of platelet and thromboxane synthesis [Citation12]. However, our knowledge on the evidence supporting the use of Ani HBr in septic shock is far from complete and its efficacy is probably yet to be proved. The goal of this study is to determine the therapeutic efficacy of Ani HBr in the treatment of septic shock in a prospective, randomized, controlled trial.
2. Methods
2.1. Study design and setting
This was a prospective, multicenter randomized controlled trial (RCT) conducted in 16 hospitals in China from September 2017 to March 2021. The protocol was reviewed and approved by the Institutional Review Board and Medical Ethics Committee of Beijing Chao-Yang Hospital (Ethical approval number: 2016-ke-181-1) and each participating center. All patient screening and enrollment procedures were performed by the coinvestigators in each participating hospitals. Written informed consents to participate in the study were obtained from participants or their legal guardians prior to study enrollment. The study conformed to the CONSORT statement [Citation13] and was registered on the www.chictr.org.cn website (Registration Number: ChiCTR-IPR-17012035). The study was conducted under the ethical principles of the Declaration of Helsinki.
2.2. Participants
All patients aged 18 years or older who were admitted to the emergency department and met the diagnostic criteria of septic shock according to Sepsis-3 criteria [Citation4, Citation6] were assessed for eligibility. Septic shock can be clinically defined as a persistent hypotension (a required vasoconstrictor to maintain a mean arterial pressure of 65 mmHg despite adequate volume resuscitation, or an arterial systolic blood pressure less than 40% of the baseline level), and/or serum lactate concentration greater than 2 mmol/L in the absence of hypovolemia. Suspected or documented infection of the following sites were considered: respiratory system, urinary system, central nervous system, digestive system, bloodstream, skin and unknown.
Patients were excluded if they: (1) were immunocompromised or with agranulocytosis; (2) have been using immunosuppressants for a long time; (3) were discharged within 24 hours of admission; (4) were in unrecoverable dying state; (5) have contraindications to Ani HBr, including glaucoma, acute phase of intracranial hemorrhage, elevated intracranial pressure, untreated bowel obstruction, and enlargement of prostate without urinary catheterization; (6) were allergic to the components of this medicine; (7) were pregnant or planning to become pregnant and lactating women and (8) participated in other clinical trials in the past three months.
2.3. Randomization
As soon as the diagnosis of septic shock was established and informed consent was obtained from participants, the patients were allocated at random in a 1:1 ratio stratified by participating hospitals to either the control or the treated group. Randomization was performed by a computer-generated randomization table using SAS version 9.2. This process was performed by one principal investigator, who prepared the sequentially numbered, opaque, sealed envelopes containing the information about the assignment of the patients to each participating hospital. The on-duty pharmacists at each hospital, who were responsible for preparing medications, were aware of the study assignments. However, the patients and their legal representatives, the technicians who performed the laboratory tests, and the investigator who examined the outcomes were all blinded to the study assignment.
2.4. Intervention
The treated group received Ani HBr in addition to conventional treatment. A bolus of 0.5 mg/kg Ani HBr, with a minimum of 20 mg and a maximum of 40 mg, was given intravenously within 3 min as the loading dose immediately after enrollment, followed by a dosage of 0.02–0.1 mg/kg/h via pump infusion, with a maximum of 200 mg per day. The maintenance doses were decided by the treating physicians, who took the actual circumstances into consideration, including the patients’ vital signs, lactate levels and side effects. For example, physicians may increase the infusion rate if the serum lactate level continued to elevate and on the contrary reduce the rate if the use of Ani HBr led to tachycardia. Ani HBr was terminated only under the following three conditions: (i) the patient was recovered from shock (normalization of serum lactate and vasopressor discontinuation), (ii) the occurrence of intolerable complications (e.g. mental disorders), or (iii) death. The control group received conventional treatment that did not include the use of Ani HBr. Conventional treatment of septic shock included fluid resuscitation, use of vasopressors, empirical antibiotics and organ support.
2.5. Outcomes
Patients were followed for up to 28 days. The primary outcome was 28-day mortality. The secondary outcomes included 7-day mortality, hospital mortality, hospital length of stay, vasopressor-free days within 7 days, serum lactate level, lactate clearance (at 6 and 24 hours), sequential organ failure (SOFA) score, important clinical indicators, mechanical ventilation (MV) weaning rates and continuous renal replacement therapy (CRRT) weaning rates (the definitions of MV/CRRT weaning rate will be given in Section 3.2). The clinical indicators were measured and collected at 0h, 6h, 24h, 48h, 72h and 7 days after the diagnosis.
According to pharmacological mechanism of anisodamine, we defined the major adverse events as tachycardia,Footnote1 arrhythmia and bowel obstruction. Minor adverse events included but not limited to dry mouth, facial flushing, mild pupil dilation, blurred near vision, distended abdomen, and urine retention. All these adverse events were screened and reported at different time points.
2.6. Sample size and statistical analysis
The sample size was calculated based on the expected 28-day mortality. Following [Citation5, Citation14] and preliminary clinical observation, we expected that the 28-day mortality in the control group to be 40 and that in the treated group to be 25%. We set the significance level (i.e. type I error rate) to be 5% and the type II error to be 0.1 (i.e. the power is 90%). Assume the dropout rate is 15% in this study, it turned out that 191 subjects per group is the sample size (total
). All continuous variables were first used for normality test. Measurement data conforming to the normal distribution were expressed as the mean and standard deviation (
s). Skewed numeric variables were expressed as the median, the first interquartile (Q1), and third interquartile (Q3). Two independent sample t-test was then used for comparison between the two groups. Non-normally distributed measurement data were used in the non-parametric test. The count data were expressed as the number of cases (percentage) using chi-square test. A Kaplan-Meier method survival curve was constructed to compare survival trends at day 28. The results were visualized with survival curves. A two-tailed
was considered statistically significant. All statistical analyses were performed using SPSS version 24.0 and SAS version 9.2.
3. Results
3.1. Patient characteristics
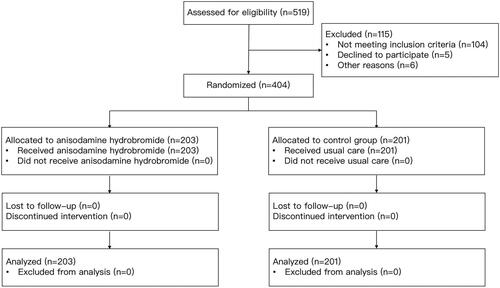
A total of 519 subjects were recruited between September 2017 and March 2021 from the participating hospitals, and 115 were excluded based on the exclusion criteria. Finally, a total of 404 patients provided consent and they were randomized and followed up for 28 days. 203 subjects were allocated in the treated group and the rest 201 subjects were assigned to the control group (see ). The baseline characteristics of the two groups were similar and comparable (see ). Patients recruited to our study were critically ill, with the mean of SOFA scores being 9.64 (there were 279 patients (69.06%) whose SOFA scores were greater than or equal to 8) and that of lactate levels being 6.06. The majority of them were admitted from the emergency room (59.41%).
Table 1. Baseline characteristics in the treated and control groups.
3.2. Clinical outcomes of the patients
3.2.1. Primary outcome
As the primary outcome, the treated group showed lower 28-day mortality than the control group (26.1% vs. 35.8%; ). The log-rank test comparing Kaplan-Meier survival curves showed evidence of a survival difference between the two groups (
, ). We then used stratified analysis to further analyze the difference in mortality outcomes between the two groups (see ). The chi-square test showed that when the SOFA score was less than 8, there was no significant difference in 28-day mortality between the two groups (
), whereas the 28-day mortality of the treated group was significantly lower than that of the control group (
) for patients whose SOFA scores were greater than or equal to 8. We can observe a similar phenomenon from for critically ill patients whose lactate levels were greater than or equal to 4. Finally, we note that Ani HBr was used for
days in the treated group, with total dosage of
mg. The average speed of pumping infusion is
mg/kg/h.
Figure 2. Kaplan-Meier estimates of the probability of survival to day 28. The p value for the log-rank test is 0.026.
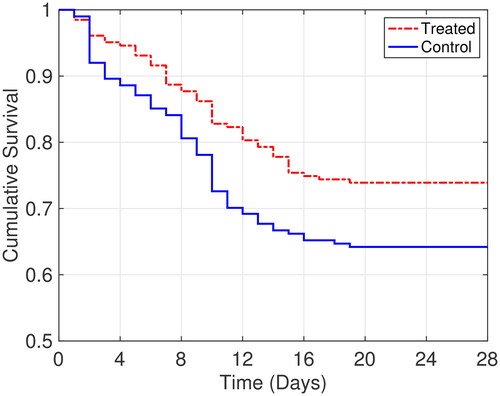
Table 2. Stratified analysis of 28-day mortality.
3.2.2. Secondary outcome and adverse events
The treated group showed lower 7-day mortality and hospital mortality than the control group, and the differences were statistically significant. Moreover, the treated group showed longer vasopressor free days in 7 days. We refer the reader to for more details.
Table 3. Comparison of secondary clinical outcomes between the treated and control groups.
Next, we assessed the lactate levels, SOFA scores as well as some important clinical indicators of the patients at 0, 6, 24, 48, and 72 hours and 7 days after enrollment. The lactate levels of the treated group was lower than that of the control group at 6, 24, 48 and 72 hours, and the SOFA scores of the treated group was lower than that of control group at 7 days after the diagnosis (see . We also computed lactate clearance at 6 hours and 24 hours after the diagnosis. It turned out that the lactate clearance of treated group was higher than that of control group at both 6 and 24 hours
. We further examined several important clinical indicators that represent liver and kidney functions. indicate that the treated group had lower blood urea nitrogen (BUN) levels at 48 hours, 72 hours and 7 days and lower creatinine levels at both 48 and 72 hours compared to the control group. From we observe no significant differences in liver function indicators between the two groups.
Figure 3. Comparisons of secondary outcomes between the treated and control groups. The error bar in (a)-(e) indicates the standard deviations. The box plots in (f) and (g) show the median, P25 and P75 (whiskers are at P5 and P95).
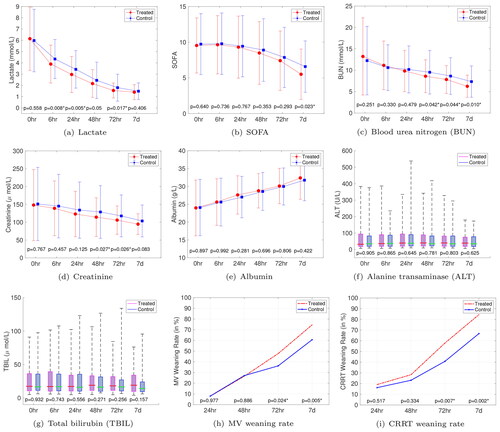
Furthermore, all of the patients required MV and 277 patients (68.56%) required CRRT (141 (69.46%) vs. 136 (67.66%), ) within 6 h after enrollment, and the rest 127 patients (31.44%) did not require CRRT within 7 days. We notice that none of the enrolled patients had been re-intubated (resp., reinitiated of CRRT) within 7 days after discontinuation of MV (resp., CRRT). Therefore we calculated the proportion of the patients who initially received MV or CRRT and then were weaned from the ventilator or renal replacement as a function of the above time points, as depicted in , from which we can have a claim that there were no significant differences of MV and CRRT weaning rates between the two groups in the first two days. However, the treated group showed higher MV weaning rate and CRRT weaning rate at both 72 hours and 7 days than the control group.
Finally, we note that five adverse events occurred during the study period, out of which four were from the treated group (two tachycardia, one distended abdomen, and one urine retention), and one was from the control group (transient tachycardia). However, there was no significant difference in the incidence of adverse events between the two groups (1.97% vs. 0.50%, ). We present the characteristics of the four adverse events in the treated group in , including the total dosage of Ani HBr (the initial loading dose plus the maintenance dose), average speed of pumping infusion, age, BMI, and gender. We find that the two patients with tachycardia received particularly high doses of Ani HBr with high pumping speed for maintenance dose compared to the average, and the one with distended abdomen also received a high total dosage of Ani HBr. This suggests that the adverse events, particularly tachycardia, might be related to the high dose of Ani HBr and high pumping speed. Particular attention should be paid to the side effects on the cardiovascular system, and further study on large-scale clinical experiments is needed to clarify the causality.
Table 4. Characteristics of the four adverse events in the treated group.
4. Discussion
In this multicenter, prospective RCT, we found that critically ill patients with septic shock treated by Ani HBr had a significantly higher 28-day survival rate than those receiving conventional treatment alone. In particular, the 28-day mortality of patients whose SOFA scores were greater than or equal to 8 in the treated group was significantly lower than that of the control group, and similarly, there is significant difference in the survival rate for the patients whose lactate levels were greater than or equal to 4 between the two groups. Therefore, our findings provide evidence that Ani HBr is likely to be beneficial for patients with a high level of illness severity.
Another significant advantage of using Ani HBr is improvement of microcirculation in the early phase of septic shock, as supported by longer vasopressor free days within 7 days, lower lactate levels and higher lactate clearance in the treated group at both 6 and 24 hours after enrollment. However, we only observed significant differences in BUN, creatinine, MV and CRRT weaning rates, and SOFA scores between the two groups consecutively starting from day 2. This can be attributed to the fact that early improvement of microcirculation does not always result in immediate changes in the macrocirculation of various organs. Multiple organ failure, including kidney failure, is the hallmark of sepsis. Acute kidney injury is often reversible with active intervention [Citation15]. In our study, treated group showed lower BUN and creatinine that represent renal function at 48 and 72 hours, which somehow provide another indicator for the therapeutic effect of including the use of Ani HBr into conventional care on septic shock.
Anisodamine is a belladonna alkaloid extracted from the Chinese medicinal herb Scopolia tangutica Maxim and was first synthesized in 1975. It has been employed therapeutically as an anti-shock medicine [Citation16,Citation17]. Despite evidence of efficacy in clinical practice, the pharmacological effects and anti-shock action of anisodamine are still under investigation. Many hypotheses have been proposed, and the majority of which are based upon the assumption that anisodamine works as a contributor to the improvement of blood flow in the microcirculation. These mechanisms include: (i) As an M-type acetylcholine receptor blocker, anisodamine can indirectly antagonize -adrenergic receptors and relieve vasospasm [Citation18], (ii) As an antagonist against endotoxin, anisodamine is able to protect ischemic intestine from releasing shock factors and prevent the irreversible development of shock [Citation19,Citation20]. It is also able to counteract endothelial cell activation by inhibiting lipopolysaccharide-induced plasminogen activator inhibitor-1 and tissue factor expression possibly through the NF-
B pathway [Citation16], which plays a key role in inflammatory diseases; and (iii) It was also shown that the effect of anisodamine can be explained in part by inhibition of platelet, granulocyte, and thromboxane synthesis [Citation21]. Other possible mechanisms include direct or indirect stimulation of the heart, reversal of endotoxin-induced vascular leakage, and stabilization of lysosomes and cathepsin inhibition [Citation12]. Several recent studies also investigated the effects of Ani HBr on inflammation by in vitro/vivo experiments. For example, it was implicated that Ani HBr inhibits inflammatory cytokines and protects against lipopolysaccharide-induced acute kidney injury in septic rats by attenuating mitochondrial dysfunction [Citation22]. Ani HBr administration could also protect endothelial cells against lipopolysaccharide-induced increase in cell barrier permeability and nitric oxide production via preserving the glycocalyx [Citation23]. In addition, it was demonstrated that Ani HBr protects against endothelial injury and inhibits excessive inflammatory reactions by improving visceral microcirculation [Citation24]. Our study fills the gap between laboratory results and clinical effectiveness.
The clinical effectiveness of anisodamine on sepsis has been studied in [Citation25,Citation26]. Specifically, Chai et al. reported that the incidence of sepsis can be decreased from 40% to 20% through the administration of anisodamine for patients with extensive burns [Citation25]. This report also demonstrated the benefits of anisodamine in restoring intestinal circulation in both the sepsis development phase and the shock phase. In a prospective, multicenter RCT, Yu et al. found no significant effect of anisodamine in reducing the hospital mortality rate [Citation26]. However, both [Citation26] and our study showed that the mortality rate in the treated group was lower than that in the control group, though the difference between the two groups in [Citation26] was not prominent, which might be because of the limited sample size. Compared with [Citation26], our study has a higher loading dose of Ani HBr (20–40 mg vs. 10 mg), and this might be the reason why the treated group in our study had lower lactate levels compared to the control group in the early days (6, 24, 48 and 72 hours) while the treated group in [Citation26] started to have the same phenomenon in later days (from days 4 to 6). On the other hand, as we already discussed in Section 3.2, the high total dosage of Ani HBr, essentially the high maintenance dose, might lead to various physiological effects on the cardiovascular system. Therefore, a high loading dose followed by a moderate maintenance dose could potentially be a better strategy. Nonetheless, both our studies showed that anisodamine was able to improve microcirculation in patients with septic shock.
Our study included 404 patients with septic shock, including 5 (1.2%) with side effects (4 in the treated group and 1 in the control group). According to the package insert of anisodamine, other anticholinergics (including amantadine, phenothiazines, etc.) and monoamine oxidase preparations (including furazolidone and procarbazine) can have interactions with anisodamine. However, the aforementioned drugs are not typically used for the treatment of septic shock. On the other hand, the anti-muscarinic activity of anisodamine can potentially lead to various physiological effects on the cardiovascular system, especially atrial arrhythmias [Citation27]. In the meanwhile, the use of vasopressor, where in most cases septic shock patients are treated with norepinephrine and/or dopamine, may also induce tachyarrhythmia [Citation28]. Hence, it is difficult to ascribe the side effects to Ani HBr administration, the vasopressor, or the synergistic correlation.
Our study had several limitations. First, the clinical outcomes were evaluated by one independent investigator. This strategy may introduce biased observations toward one of the treatment groups. However, given that the outcome assessor had no knowledge of the treatment assignment and did not work in the participating centers, the possibility of bias should be very low in this trial. Second, our study did not include the direct assessment of microcirculation, such as the filling degree of sublingual microvascular and nail-fold capillary. These factors will be considered in our future research. Third, although this was a multicenter study, the sample size is relatively small, and the patients were severely ill. Note that septic shock comprises a heterogeneous population. As such, our results may not be generalizable to other patient populations. A prospective randomized multicenter trial enrolling more patients is needed to determine the benefits of Ani HBr for sepsis/septic shock patients.
5. Conclusion
In conclusion, our study showed that Ani HBr can reduce 28-day mortality for critically ill patients with septic shock, especially for patients with a high level of illness severity. A large-scale, prospective, comprehensive multicenter clinical trial is required to ascertain whether the potential benefits of Ani HBr administration do indeed translate into survival benefit for critically ill patients with septic shock.
Authors contributions
FZ performed data analysis, and drafted the manuscript; XM helped designing the study and performed data collection; PZ, YPT, JXL, XD, DSY, ZFL, LZ, JHL, AJL, XD, MZC, SYY, JJZ, BS, ZHL collected and prepared the clinical data; SBG designed the study. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The protocol was reviewed and approved by the Institutional Review Board and Medical Ethics Committee of Beijing Chao-Yang Hospital (Ethical approval number: 2016-ke-181-1) and each participating center. Written informed consents to participate in the study were obtained from participants or their legal guardians.
| Abbreviations | ||
| Ani HBr | = | anisodamine hydrobromide |
| APACHE | = | acute physiology and chronic health evaluation |
| BMI | = | body mass index |
| BUN | = | blood urea nitrogen |
| CNS | = | central nervous system |
| COPD | = | chronic obstructive pulmonary disease |
| CRRT | = | continuous renal replacement therapy |
| GCS | = | Glasgow coma scale |
| HCT | = | hematocrit |
| LOS | = | length of stay |
| MAP | = | mean arterial pressure |
| MV | = | mechanical ventilation |
| PCT | = | procalcitonin |
| PLT | = | platelet |
| SOFA | = | sequential organ failure assessment |
| SpO2 | = | peripheral capillary oxygen saturation |
| WBC | = | white blood cell |
Acknowledgements
The authors would like to thank Beijing Gongcuo Pharmaceutical R&D Co., Ltd for project monitoring and data curation, and the School of Public Health in Chengdu University of Traditional Chinese Medicine for statistical analysis.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Disclosure statement
The authors declare that they have no competing interests.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
Notes
1 We acknowledge that tachycardia is pretty common in patients with septic shock. The way we define tachycardia as an “adverse event” is the one that cannot be explained by shock or does not match the shock recovery (such as increased blood pressure, increased urine output, decreased lactate level, and disappearance of skin ecchymosis, etc.), or the one that has a clear positive correlation with the increase in drug dosage.
References
- Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47(11):1–9. doi: 10.1007/s00134-021-06506-y.
- Martín S, Pérez A, Aldecoa C. Sepsis and immunosenescence in the elderly patient: a review. Front Med (Lausanne). 2017;4:20. doi: 10.3389/fmed.2017.00020.
- Oh SY, Cho S, Kim GH, et al. Incidence and outcomes of sepsis in korea: a nationwide cohort study from 2007 to 2016. Crit Care Med. 2019;47(12):e993–e998. doi: 10.1097/CCM.0000000000004041.
- Petros S, John S. The guideline of surviving sepsis campaign sepsis 2016. Med Klin Intensivmed Notfmed. 2017;112(5):454–458. doi: 10.1007/s00063-017-0298-5.
- Bauer M, Gerlach H, Vogelmann T, et al. Mortality in sepsis and septic shock in Europe, North america and Australia between 2009 and 2019- results from a systematic review and meta-analysis. Crit Care. 2020;24(1):239. doi: 10.1186/s13054-020-02950-2.
- Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). J Am Med Assoc. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287.
- Coopersmith CM, De Backer D, Deutschman CS, et al. Surviving sepsis campaign: research priorities for sepsis and septic shock. Intensive Care Med. 2018;44(9):1400–1426. doi: 10.1007/s00134-018-5175-z.
- Tarbell JM, Cancel LM. The glycocalyx and its significance in human medicine. J Intern Med. 2016;280(1):97–113. doi: 10.1111/joim.12465.
- Gattinoni L, Brazzi L, Pelosi P, et al. A trial of goal-oriented hemodynamic therapy in critically ill patients. N Engl J Med. 1995;333(16):1025–1032. doi: 10.1056/NEJM199510193331601.
- Sakr Y, Dubois MJ, De Backer D, et al. Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit Care Med. 2004;32(9):1825–1831. doi: 10.1097/01.ccm.0000138558.16257.3f.
- Charlton M, Sims M, Coats T, et al. The microcirculation and its measurement in sepsis. J Intensive Care Soc. 2017;18(3):221–227. doi: 10.1177/1751143716678638.
- Poupko JM, Baskin SI, Moore E. The pharmacological properties of anisodamine. J Appl Toxicol. 2007;27(2):116–121. doi: 10.1002/jat.1154.
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8(1):18. (doi: 10.1186/1741-7015-8-18.
- Das S, Mitra K, Mandal M. Sample size calculation: basic principles. Indian J Anaesth. 2016;60(9):652–656. doi: 10.4103/0019-5049.190621.
- Poston JT, Koyner JL. Sepsis associated acute kidney injury. BMJ. 2019;364:k4891. doi: 10.1136/bmj.k4891.
- Ruan QR, Zhang WJ, Hufnagl P, et al. Anisodamine counteracts lipopolysaccharide-induced tissue factor and plasminogen activator inhibitor-1 expression in human endothelial cells: contribution of the NF-kappa b pathway. J Vasc Res. 2001;38(1):13–19. doi: 10.1159/000051025.
- Sheng CY, Gao WY, Guo ZR, et al. Anisodamine restores bowel circulation in burn shock. Burns. 1997;23(2):142–146. doi: 10.1016/s0305-4179(96)00086-1.
- Zhang Y, Zou J, Wan F, et al. Update on the sources, pharmacokinetics, pharmacological action, and clinical application of anisodamine. Biomed Pharmacother. 2023;161:114522. doi: 10.1016/j.biopha.2023.114522.
- Su JY. Cell protection mechanism of antishock action of anisodamine. Chin Med J (Engl). 1992;105(12):976–979.
- Su JY, Wu L, Tang C. Experimental study in rabbits of the antishock effect of anisodamine (654-2), and its mechanism of action. Resuscitation. 1983;10(3):173–184. doi: 10.1016/0300-9572(83)90005-9.
- Xiu RJ, Hammerschmidt DE, Coppo PA. Anisodamine inhibits thromboxane synthesis, granulocyte aggregation and platelet aggregation. J Am Med Assoc. 1982;247(10):1458–1460.
- Wan F, Du X, Liu H, et al. Protective effect of anisodamine hydrobromide on lipopolysaccharide-induced acute kidney injury. Biosci Rep. 2020;40(7):BSR20201812. doi: 10.1042/BSR20201812.
- Du X, Liu H, Yue Y, et al. Anisodamine hydrobromide protects glycocalyx and against the lipopolysaccharide-induced increases in microvascular endothelial layer permeability and nitric oxide production. Cardiovasc Eng Technol. 2021;12(1):91–100. doi: 10.1007/s13239-020-00486-8.
- Yang J, Dong GJ, Wang HW, et al. Influence of microcirculatory dysfunction on myocardial injury after cardiopulmonary resuscitation. Biomed Environ Sci. 2022;35(4):334–344.
- Chai J, Yang H, Sheng Z, et al. Anisodamine in prevention and treatment of sepsis of severely burned patients. Zhonghua Wai Ke Za Zhi. 2000;38(9):686–689.
- Yu Y, Zhu C, Hong Y, et al. Effectiveness of anisodamine for the treatment of critically ill patients with septic shock: a multicentre randomized controlled trial. Crit Care. 2021;25(1):349. doi: 10.1186/s13054-021-03774-4.
- Zhu CP, Jiang F, Wang RQ, et al. Comparison of efficacy and safety of hyoscine butylbromide versus anisodamine for acute gastric or intestinal spasm-like pain: a randomized, double-blinded, multicenter phase III trial. J Dig Dis. 2017;18(8):453–460. doi: 10.1111/1751-2980.12504.
- Backer DD, Biston P, Devriendt J, et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010;362(9):779–789. doi: 10.1056/NEJMoa0907118.