Abstract
Background
Heart failure is considered an epidemic disease in the modern world. Since it presents as a multifactorial, systemic disease, a comprehensive understanding of the underlying mechanism is essential. Epicardial adipose tissue (EAT) is increasingly recognized to be metabolically active and is able to secrete myriad bioactive molecules, including exosomes carrying tRNA-derived small RNAs (tsRNAs). Mounting evidence has suggested that these specific tsRNAs dynamically impact fundamental cellular processes, but no studies have focused on the influence of tsRNA in EAT on cardiac dysfunction.
Methods
To investigate the regulatory mechanism of tsRNAs of EAT associated with HF, we collected EAT from HF (n = 5) patients and controls (n = 5) and used a combination of RNA sequencing, quantitative reverse transcription-polymerase chain reaction (qRT–PCR) and bioinformatics to screen the expression profiles of tsRNAs in HF.
Results
We ultimately identified an expression profile of 343 tsRNAs in EAT. Of those, a total of 24 tsRNAs were significantly differentially expressed between HF and controls: 17 were upregulated and 7 were downregulated (fold change >1.5, p < 0.05). Four tsRNAs (tiRNA-Pro-TGG-001, tRF-Met-CAT-002, tRF-Tyr-GTA-010 and tRF-Tyr-GTA-011) were randomly selected and validated by qRT–PCR. Bioinformatics analyses revealed a dense interaction of target genes between tRF-Tyr-GTA-010 and tRF-Tyr-GTA-011. Based on functional analysis, these two tRFs might play a protective role by regulating sphingolipid and adrenergic signaling pathways by targeting genes mainly contributing to calcium ion transport.
Conclusions
Our study profiled tsRNA expression in EAT with HF and identified a comprehensive dimension of potential target genes and tsRNA-mRNA interactions.
KEY MESSAGES
The development of heart failure involves a complex mechanistic network, which until now, has not been clarified.
Excess epicardial adipose tissue may promote heart failure via unknown mechanisms.
Our current study profiled transfer RNAs expression in epicardial adipose tissue with heart failure and identified a comprehensive dimension of potential target genes and transfer RNAs -mRNA interactions for the pathogenesis of deterioration in cardiac function.
Introduction
Heart failure (HF) constitutes a group of clinical syndromes with various aetiologies leading to cardiac structural abnormalities and functional dysfunction. With the ageing of the population, HF has become a highly frequent, ‘epidemic’ disease in contemporary society, placing constant pressure on the public health system [Citation1]. Therefore, it is of utmost significance to explore the pathophysiological mechanisms of the occurrence and development of HF and block the progression of the disease on this basis for timely, effective and individualized treatment. However, during the development of HF, a complex mechanistic network on macro- and microstructural, cellular and molecular levels associated with the deterioration of cardiac function is involved and, until now, has not been clarified. Recently, epicardial adipose tissue (EAT) has been increasingly utilized to contribute to this investigatory process [Citation2].
EAT is visceral adipose tissue with metabolic activity located in the atrioventricular and interventricular grooves [Citation3]. The accumulation of EAT has been regarded as a significant feature of chronic metabolic status related to HF. Additionally, in past clinical studies, we have reported that EAT volume is independently associated with subclinical left atrial dysfunction and impaired global longitudinal strain of the left ventricle, but the molecular indicators have not been elucidated [Citation3]. As an active metabolic factor, EAT is able to produce a diversity of bioactive molecules, including microparticles carrying small noncoding RNAs (sncRNAs), acting on the atrial myocardium in a paracrine manner.
SncRNAs are RNAs that cannot be translated into proteins but regulate the expression of target genes through posttranslational modification. Recently, a new kind of small RNA derived from transfer RNAs (tsRNAs), which is different from typical sncRNAs, was discovered [Citation4]. These tsRNAs are not non-functional small molecular fragments randomly cut from transfer RNAs but instead exert various biological activities and dynamically affect various cellular functions of organisms. Based on different cleavage positions, tsRNAs can be divided into two categories [Citation5]: tRNA-derived fragments (tRFs) with length of approximately 14–30 nt, which can be divided into four subcategories: tRF-1, tRF-3, tRF-5 and i-tRF; and tRNA-derived stress-induced RNAs (tiRNAs) with a length of 28–36 nt, which are produced by angiopoietin-specific cleavage under exposure to stress conditions such as hypoxia, ultraviolet radiation and oxidative stress. tRFs and tiRNAs accumulate in different biological processes and participate in various biological functions, such as the growth and metastasis of cancer cells [Citation5], the expression of genes and proteins [Citation6], the invasion process of viruses [Citation7], and neurodegenerative diseases [Citation8]. Nevertheless, the role of tsRNAs in extracardiac fat associated with HF has not been illustrated in the literature.
Therefore, this study evaluated the expression levels of tsRNAs in the EAT of patients with HF through RNA high-throughput sequencing, predicted target genes and evaluated the potential signalling pathways in combination with bioinformatics to provide a sufficient theoretical basis for discovering new therapeutic targets for HF.
Materials and methods
Patients and tissue sampling
This study prospectively selected consecutive patients who were admitted to the Heart Centre of Beijing Chaoyang Hospital from April 2018 to August 2018. Criteria for the diagnosis of heart failure referred to the 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure [Citation9]. The exclusion criteria were as follows: (1) pregnancy; (2) thyroid disease; (3) severe renal dysfunction with an estimated glomerular filtration rate less than 30 ml/min/1.73m2 at baseline; (4) cancers; and (5) severe infection. According to the diagnostic criteria of HF, patients were divided into an acute HF group and a control group. Four patients underwent coronary artery bypass grafting, and one patient underwent heart valve replacement in the two groups. This study protocol was approved by the Ethics Committee of Beijing Chaoyang Hospital Affiliated to Capital Medical University (2021-ke-246) and has been registered in the Chinese Clinical Trial Registry (ChiCTR2100045905, www. chictr.org.cn). All of the participants provided informed consent. EAT samples were taken from the two groups of patients. The detailed procedure was described in our previous study [Citation10].
RNA extraction, library preparation, and tsRNA sequencing
Total RNA was extracted from frozen EAT using a miRNAeasy Mini Kit (Qiagen, Hilden, Germany). Agarose gel electrophoresis and a NanoDrop 1000 were used for quality inspection to ensure the integrity of the RNA samples.
Pre-treatment of tsRNAs, library preparation and small RNA sequencing were conducted as previously described [Citation11]. In brief, after removing some modifications that may interfere with library construction, RNA samples were ligated to 3′ and 5′ small RNA adapters and sequentially amplified with Illumina’s primers to generate cDNA. Amplified cDNA was purified from the PAGE gel and quantified using an Agilent 2100 Bioanalyzer. The prepared libraries were denatured and diluted to a loading volume of 1.3 mL and concentration of 1.8 pM and then loaded onto reagent cartridges and forwarded for sequencing on an Illumina NextSeq 500 system.
Identification of tsRNAs
Generated raw sequencing data that passed the Illumina chastity filter were used for the following analysis. After alignment statistical analysis with mapping ratio, read length and fragment sequence bias, the expression profiling of tsRNAs was screened with the R package edgeR. For statistical computing and graphics, principal component analysis (PCA), correlation analysis, pie plots, Venn plots, hierarchical clustering, scatter and volcano plots were constructed in the R 3.5.1 or Perl environment.
Bioinformatic and functional analysis of tsRNAs
The computational predictions of the relationships between selected tsRNAs and target mRNAs were conducted using TargetScan and miRanda. To determine the potential biological function of the selected tsRNAs, enrichment analyses based on Gene Ontology (GO) (http://www.geneontology.org) and Kyoto Encyclopaedia of Genes and Genomes (KEGG) pathway analysis (http://www.genome.jp/kegg) were performed. Significant GO terms, including biological process, cellular component, and molecular function, were denoted by p ≤ 0.05. KEGG enrichment analysis indicated functional prediction of mapped genes, where a lower P value indicated a more significant pathway.
Quantitative real-time polymerase chain reaction (qRT–PCR)
Total RNA was isolated from EAT with a miRNAeasy Mini Kit (Qiagen, Hilden, Germany), and then cDNA was synthesised with a rtStarTM First-Strand cDNA Synthesis Kit. qRT–PCR was performed on a ViiA 7 Real-time PCR system. The relative expression levels of tsRNAs were calculated by the 2−ΔΔCt method, and U6 was used as an internal control.
Statistical analysis
SPSS 24.0 (IBM Corp, Armonk, NY) statistical software was used for statistical analysis of clinical data. The measurement data conforming to a normal distribution are expressed as the mean ± standard deviation, and those not conforming to a normal distribution are expressed as the median and interquartile range. Independent sample t-tests or nonparametric Mann–Whitney U tests were used for comparisons of group measurement data according to whether they conformed to a normal distribution. The chi-square test or exact probability method was used for the analysis of group counting data. The sequencing data, including PCA, correlation analysis, Venn diagram, hierarchical clustering and GO/KEGG analysis, were analyzed using the R package. All statistical analyses were performed by a two-sided test, and p < 0.05 was considered statistically significant.
Results
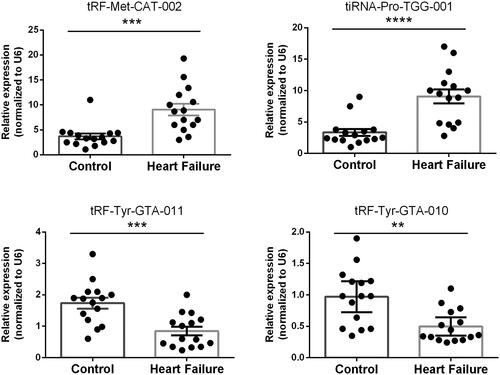
In our study, we identified an expression profile of 343 tsRNAs in EAT. Of those, a total of 24 tsRNAs were significantly differentially expressed between HF and controls: 17 were upregulated and 7 were downregulated (fold change > 1.5, p < 0.05). Four tsRNAs (tiRNA-Pro-TGG-001, tRF-Met-CAT-002, tRF-Tyr-GTA-010 and tRF-Tyr-GTA-011) were randomly selected and validated by qRT–PCR. Bioinformatics analyses revealed a dense interaction of target genes between tRF-Tyr-GTA-010 and tRF-Tyr-GTA-011. Based on functional analysis, these two tRFs might play regulatory roles through sphingolipid and adrenergic signalling pathways by targeting genes mainly contributed to calcium ion transport.
Overview of tRF and tiRNA profiles
The baseline characteristics and clinical parameters are presented in . Based on the heatmap of the correlation coefficients from the included samples, the two compared groups were similar (). PCA is an unsupervised statistical analysis to reduce the dimensionality of large datasets and explore sample classes based on counts per million total aligned reads (CPM) values of tsRNAs. As shown in , these samples presented a distinguishable tRF and tiRNA spectrum. After sequencing, we ultimately identified an expression profile of all 343 tsRNAs between the HF and control groups. The Venn diagrams show a total of 177 commonly expressed tsRNAs, while 29 tsRNAs were specifically expressed in HF samples and 22 tsRNAs were specifically expressed in the control. In addition, 284 detected tsRNAs were described as new tRNA derivatives that had never been previously annotated in the tRF database (). The distribution of tsRNA subtypes in both the HF and control groups is summarized in pie charts (), indicating that most tsRNAs were generated from mature tRNAs. It was also found that tRF-5c, tRF-3a, and tRF-1 were the most abundant isoforms in both groups. Compared with the control, the expression levels of tRF-5c and tRF-3a were decreased, whereas those of other subtypes remained the same or were increased in the context of cardiac dysfunction. Furthermore, based on stacked bar charts (), the tRF and tiRNA subtype distributions came from the same anticodon tRNA, but the length distributions of the subtypes were completely different in these two groups.
Figure 1. The expression level analysis. (A) Heat map of the correlation coefficient from all samples. (B) Principle component analysis (PCA). the PCA results show a distinguishable tRF & tiRNAs spectrum in the epicardial fat depot between heart failure and controls. (C) The volcano plot of the number of commonly expressed and specifically expressed tRF & tiRNAs. (D) a total of 284 detected tsRNAs were described as new tRNA derivatives that had never been annotated in the tRF database yet.
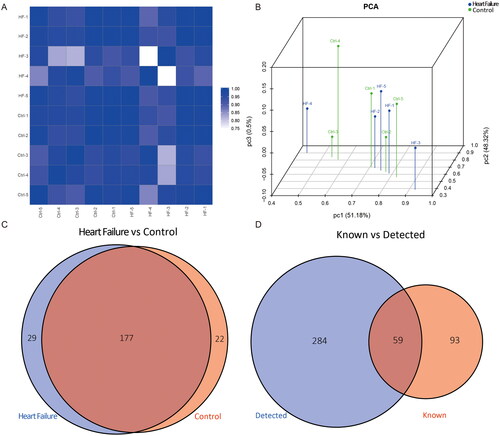
Figure 2. (A) Pie charts of the distribution of subtypes of tRFs & tiRNAs of epicardial adipose tissue in controls and heart failure. Stacked bar charts showed the number of subtypes of tRFs and tiRNAs against tRNA isodecoders (B) and the frequency of subtype against the length of tRFs and tiRNAs (C) in the two groups.
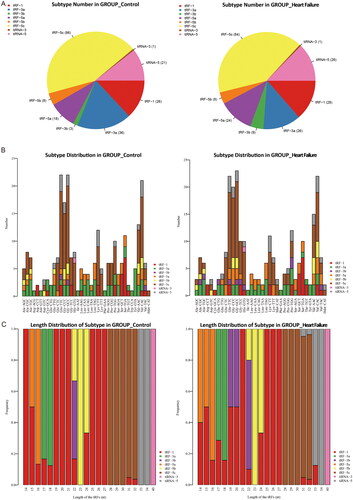
Table 1. Demographic and clinical characteristics of patients.
Expression profiles of tRFs and tiRNAs
As shown in the hierarchical clustering (), the tRF and tiRNA expression profiles were distinguishable among EAT samples. A scatter plot () was created to present the tsRNA expression variation between the HF and control groups, while the volcano plot () showed fold changes and P values in differentially expressed tsRNAs between the two groups. Overall, we identified a total of 24 tsRNAs that were significantly differentially expressed between HF and controls: 17 were upregulated, and 7 were downregulated (fold change > 1.5, p < 0.05, ).
Figure 3. Sequencing analysis of tsRNAs. (A) The hierarchical clustering heatmap for tRFs and tiRNAs in all sapmles. (B) Scatter plots of differentially expressed tsRNAs. (C) The volcano plots of differentially expressed tsRNAs.
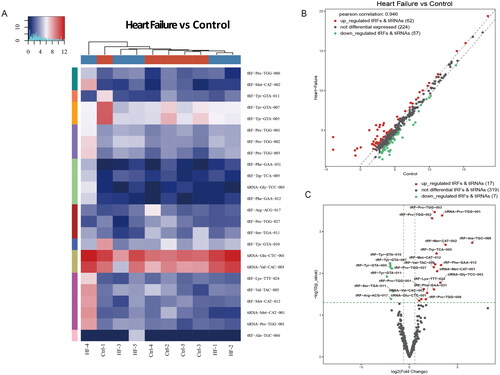
Table 2. Up- and down-regulated tsRNAs in epicardial adipose tissue of heart failure.
Validation by qRT–PCR
To verify the reliability of the high-throughput sequencing data, four tsRNAs, namely, tiRNA-Pro-TGG-001, tRF-Met-CAT-002, tRF-Tyr-GTA-010 and tRF-Tyr-GTA-011, were selected for qRT–PCR in a larger cohort (HF vs. control, n = 15 in each group). Compared with the non-HF group, tiRNA-Pro-TGG-001 and tRF-Met-CAT-002 were significantly upregulated, whereas tRF-Tyr-GTA-010 and tRF-Tyr-GTA-011 were significantly downregulated in HF, indicating a similar expression pattern in both sequencing and PCR data ().
Predicted target genes of tsRNAs and functional analysis
The mechanisms underlying the biological roles of tsRNAs in EAT associated with AF are not yet clear, while there is some evidence indicating that tsRNAs could manifest a miRNA-like mode of action. As a result, the four candidate differentially expressed tsRNAs were subsequently used to predict their target genes with TargetScan and miRanda. Based on the tsRNA/mRNA interaction network, we identified various target genes associated with four validated tsRNAs (). Specifically, there was a dense interaction of target genes between tRF-Tyr-GTA-010 and tRF-Tyr-GTA-011 ().
Figure 5. The network of candidate tsRNAs and potential target mRNAs. All results have a threshold of ≥1.5-fold change. (A) The gene regulatory module network construction including putative genes of tiRNA-Pro-TGG-001, tRF-Met-CAT-002, tRF-Tyr-GTA-010 and tRF-Tyr-GTA-011. (B) The limited network showed a dense interaction of targets genes between tRF-Tyr-GTA-010 and tRF-Tyr-GTA-011.
We identified the potential roles of the two downregulated tRFs with GO and KEGG analyses. GO enrichment analysis revealed that the target genes of the two tRFs mainly contributed to calcium ion transport (GO: 00006816) and nitric-oxide synthase binding (GO: 0050998), along with the cellular component of the somatodendritic compartment (GO: 0036477) (). In the KEGG pathway analysis, 14 significantly enriched signalling pathways were identified: the top three relevant pathways regulated by candidate tRFs were the sphingolipid signalling pathway (hsa04071), adrenergic signalling in cardiomyocytes (hsa04261), and the mRNA surveillance pathway (hsa03015) (, ).
Figure 6. GO enrichment analysis of target mRNAs of the two candidate tRFs: (A) Bar plot with enrichment score: top ten enriched items in three domains. (B-D) Dot plot with gene ratio values of the top ten enriched items in biological processes (B), cellular components (C) and molecular functions (D). KEGG pathway analysis of target mRNAs of the two candidate tRFs: (E) Pathway bar plot with enrichment score values of the top ten significantly enriched signalling pathways. (F) Dot plot with gene ratio values of the top ten significantly enriched signalling pathways.
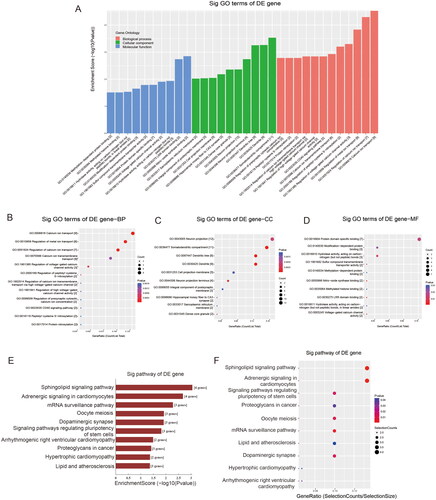
Table 3. Top 10 KEGG pathways of target mRNAs based on candidate tsRNAs in epicardial adipose tissue associated with heart failure.
Discussion
Until now, the complex network on macro- and microstructural, cellular and molecular levels associated with the deterioration of cardiac function has not been clearly clarified. Given the indispensable roles of tsRNAs in tumour development [Citation5], neurodegeneration [Citation12] and metabolic dysfunction [Citation13], the present work aimed to determine whether tsRNAs in the epicardial fat depot are involved in the pathophysiology of impaired cardiac function. Accordingly, integrated small RNA-sequencing analysis of tsRNAs was performed, and our findings offer the first comprehensive assessment of tsRNA expression in human EAT involved in the development of HF. In this study, an expression profile of 343 tsRNAs in EAT was identified. A total of 24 tsRNAs were significantly differentially expressed between HF and control groups, and the number of subtypes of tsRNAs against tRNA isodecoders and the frequency comparison against length between groups were diverse, implying that these tRNA derivatives may participate in the HF process with undefined roles.
TsRNAs were first discovered in the urine of cancer patients in the late 1970s [Citation14], but they did not capture more attention until recent years. With the advent of high-throughput sequencing and advanced bioinformatics technology, these specific tsRNAs are not regarded as derivatives randomly cleaved from tRNAs, but rather have multiple biological functions during different biological processes. TsRNAs can assume a miRNA-like mode and participate in the regulation of gene expression by affecting mRNA stability via a posttranslational pathway [Citation5]. As reported in gastric tumors [Citation15], tRF-3017A binds to Argonautes and downregulates the expression of tumour suppressor genes, contributing to cancer cell migration and invasion. In addition, it has been reported that tsRNAs can act as protein decoys that competitively bind RNA-binding proteins to influence the stability of target RNAs [Citation16]. Srikar and colleagues revealed differentiation-dependent enrichment of 5′-tsRNA in the context of dynamic cell-state transitions: these specific 5′-tsRNA can preferentially interact with the RNA-binding protein to influence the transcript stability and subsequent translation of the pluripotency-promoting factor, c-Myc, thus modulating the stem cell state in mouse embryonic stem cells [Citation17]. Furthermore, tsRNAs can regulate biological epigenetics by regulating genes associated with germ cell development. Several lines of evidence indicate that sperm tRFs promote intergenerational transmission of specific metabolic phenotypes in mice [Citation13]. As illustrated by Upasna [Citation18], paternal diet can alter the level of tRNA fragments throughout the male reproductive tract and in mature sperm, and the tRNA fragment tRNA-Gly-GCC repressed the expression of transcripts driven by endogenous retroelements, contributing to downstream effects on metabolism. Overall, these small RNAs could carry out multiple biological functions through several molecular mechanisms, including regulation of gene and protein expression and epigenetic modification.
Studies have identified the roles of these tsRNAs in participating in the simplest to most complex biological processes, including coping with environmental stress conditions, rapid proliferation in tumorigenic cells, stem cell differentiation, and the development of neurological disorders. In the context of cardiovascular disorders, studies have shown that tsRNAs are potential therapeutic targets for caloric restriction pre-treatment to improve myocardial ischaemic injury [Citation19]. Nevertheless, regarding the biological roles of tsRNAs in cardiac dysfunction, no previous studies have performed relevant work. Our study described altered expression patterns of tsRNAs in epicardial adipose samples from patients with HF, indicating that the tsRNA spectrum was significantly altered in EAT after the deterioration of cardiac output. Since tsRNAs may have miRNA-like biological activities, we then predicted potential target-binding mRNAs for candidate tsRNAs and constructed a tsRNA-mRNA interactive network for bioinformatic analysis. Additionally, in GO enrichment analysis, we found that the target genes of tRF-Tyr-GTA-010 and tRF-Tyr-GTA-011 mainly focus on the regulation of calcium ion transport. It is well known that HF is a proarrhythmic state and that arrhythmias are a major cause of death in advanced HF. Considering that Ca2+ overlap could result in delayed afterdepolarization and trigger arrhythmias, the regulation of Ca2+ concentration is crucial. Philipp and colleagues demonstrated that increased cytosolic calcium buffering contributes to a cellular arrhythmogenic substrate in induced pluripotent stem cell-derived cardiomyocytes from patients with dilated cardiomyopathy [Citation20]. In addition, David C. discovered a novel sarcolemmal background Ca2+ influx via the TRPC 6 channel that is responsible for sarcoplasmic reticulum Ca2+ overload and Ca2+ waves in HF [Citation21]. It has also been shown that the Bcl-2 19 kilodalton interacting protein 3 contributes to myocardial remodelling and progression in HF with reduced ejection fraction by influencing endoplasmic reticulum-mitochondrial calcium and ion homeostasis [Citation22].
In the KEGG pathway analysis, the sphingolipid signalling pathway and adrenergic signalling in cardiomyocytes were among the top three signalling pathways affected by the candidate tRF-mRNA network. Sphingolipids and associated metabolites are increasingly associated with cellular survival, senescence and metabolism. Altered sphingolipid metabolism has been suggested to be relevant in the pathogenesis of HF [Citation23], and accumulating evidence identifies elevated levels of sphingolipid ceramide in circulation and cardiac tissue in the context of HF [Citation24,Citation25]. Brittany et al. reported that the increase in long-chain ceramides contributed to lipotoxic cardiomyopathy partly via mitochondrial dysfunction, oxidative stress and mitophagy [Citation26]. In addition, Linda and colleagues found that in pressure-overloaded hearts, the loss of inhibition of limiting enzymes of sphingolipid biosynthesis could result in ceramide accrual in cardiomyocytes, suppressing the onset of ‘protective autophagy’ and contributing to severe cardiac dysfunction [Citation27]. In addition, it is well known that during HF, the activation of adrenergic signalling is triggered to maintain cardiac output and plays a crucial role in the regulation of vascular tone [Citation28]. However, prolonged sympathetic stimulation with elevated plasma catecholamine levels promotes subendocardial and interstitial fibrosis, vacuolization of the cytoplasm and mineralization [Citation29,Citation30]. As a result, β-blockers are a mainstay of current HF therapy. In general, biological changes from these tsRNA and their co-association with other factors may be responsive to the deterioration of cardiac function.
There are several limitations that should be considered in our study. First, our tsRNA-mRNA cross-talk network was mainly based on an in silico approach, and the expression of tsRNAs in EAT associated with HF should be further identified in vitro and in vivo. Second, the tsRNA spectrum should be validated again in a large-scale study, since our present sequencing data were analyzed for only ten patients and a relatively small cohort limited further valuable information on the role of candidate tsRNA. Finally, the profiling of tsRNAs was roughly depicted, and future studies focused on the biological functions and interactions between tsRNAs and mRNAs are necessary.
Conclusions
In conclusion, the current study, for the first time, profiled tsRNA expression in EAT with HF and identified a comprehensive dimension of potential target genes and tsRNA-mRNA interactions for the pathogenesis of deterioration in cardiac function. Our study inspires more novel perspectives on the role of EAT in neighbouring myocardium and is beneficial for developing prophylactic and therapeutic treatments for cardiac protection.
Authors’ contributions
LZ and PXS designed the study. LZ acquired the data. YSP did the analysis and interpretation of data. LZ wrote the manuscript and revised the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are openly available in Gene Expression Omnibus (GEO) at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE210707, reference number (GSE210707).
Additional information
Funding
References
- Tanai E, Frantz S. Pathophysiology of heart failure. Compr Physiol. 2015;6:1–12.
- Iacobellis G. Epicardial adipose tissue in contemporary cardiology. Nat Rev Cardiol. 2022;19(9):593–606. doi: 10.1038/s41569-022-00679-9.
- Zhao L, Harrop DL, Ng A, et al. Epicardial adipose tissue is associated with left atrial dysfunction in people without obstructive coronary artery disease or atrial fibrillation. Can J Cardiol. 2018;34(8):1019–1025. doi: 10.1016/j.cjca.2018.05.002.
- Orellana EA, Siegal E, Gregory RI. TRNA dysregulation and disease. Nat Rev Genet. 2022;23(11):651–664. doi: 10.1038/s41576-022-00501-9.
- Gu X, Zhang Y, Qin X, et al. Transfer RNA-derived small RNA: an emerging small non-coding RNA with key roles in cancer. Exp Hematol Oncol. 2022;11(1):35. doi: 10.1186/s40164-022-00290-1.
- Krishna S, Raghavan S, DasGupta R, et al. TRNA-derived fragments (tRFs): establishing their turf in post-transcriptional gene regulation. Cell Mol Life Sci. 2021;78(6):2607–2619. doi: 10.1007/s00018-020-03720-7.
- Yu X, Xie Y, Zhang S, et al. TRNA-derived fragments: mechanisms underlying their regulation of gene expression and potential applications as therapeutic targets in cancers and virus infections. Theranostics. 2021;11(1):461–469. doi: 10.7150/thno.51963.
- Wu W, Lee I, Spratt H, et al. TRNA-derived fragments in alzheimer’s disease: implications for new disease biomarkers and neuropathological mechanisms. J Alzheimers Dis. 2021;79(2):793–806. doi: 10.3233/JAD-200917.
- McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–3726. doi: 10.1093/eurheartj/ehab368.
- Zhao L, Ma Z, Guo Z, et al. Analysis of long non-coding RNA and mRNA profiles in epicardial adipose tissue of patients with atrial fibrillation. Biomed Pharmacother. 2020;121:109634. doi: 10.1016/j.biopha.2019.109634.
- Qin C, Feng H, Zhang C, et al. Differential expression profiles and functional prediction of tRNA-derived small RNAs in rats after traumatic spinal cord injury. Front Mol Neurosci. 2019;12:326. doi: 10.3389/fnmol.2019.00326.
- Liu Y, Cheng X, Li H, et al. Non-coding RNAs as novel regulators of neuroinflammation in alzheimer’s disease. Front Immunol. 2022;13:908076. doi: 10.3389/fimmu.2022.908076.
- Zhang Y, Ren L, Sun X, et al. Angiogenin mediates paternal inflammation-induced metabolic disorders in offspring through sperm tsRNAs. Nat Commun. 2021;12(1):6673. doi: 10.1038/s41467-021-26909-1.
- Borek E, Baliga BS, Gehrke CW, et al. High turnover rate of transfer RNA in tumor tissue. Cancer Res. 1977;37(9):3362–3366.
- Tong L, Zhang W, Qu B, et al. The tRNA-derived fragment-3017A promotes metastasis by inhibiting NELL2 in human gastric cancer. Front Oncol. 2020;10:570916. doi: 10.3389/fonc.2020.570916.
- Li J, Zhu L, Cheng J, et al. Transfer RNA-derived small RNA: a rising star in oncology. Semin Cancer Biol. 2021;75:29–37. doi: 10.1016/j.semcancer.2021.05.024.
- Krishna S, Yim DG, Lakshmanan V, et al. Dynamic expression of tRNA-derived small RNAs define cellular states. Embo Rep. 2019;20:e47789.
- Sharma U, Conine CC, Shea JM, et al. Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science. 2016;351(6271):391–396. doi: 10.1126/science.aad6780.
- Liu W, Liu Y, Pan Z, et al. Systematic analysis of tRNA-derived small RNAs discloses new therapeutic targets of caloric restriction in myocardial ischemic rats. Front Cell Dev Biol. 2020;8:568116. doi: 10.3389/fcell.2020.568116.
- Jung P, Seibertz F, Fakuade FE, et al. Increased cytosolic calcium buffering contributes to a cellular arrhythmogenic substrate in iPSC-cardiomyocytes from patients with dilated cardiomyopathy. Basic Res Cardiol. 2022;117(1):5. doi: 10.1007/s00395-022-00912-z.
- Hutchings DC, Madders GWP, Niort BC, et al. Interaction of background Ca2+ influx, sarcoplasmic reticulum threshold and heart failure in determining propensity for Ca2+ waves in sheep heart. J Physiol. 2022;600(11):2637–2650. doi: 10.1113/JP282168.
- Chaanine AH, Higgins L, Lauterboeck L, et al. Multiomics approach reveals an important role of BNIP3 in myocardial remodeling and the pathogenesis of heart failure with reduced ejection fraction. Cells. 2022;11(9):1572. doi: 10.3390/cells11091572.
- Doenst T, Nguyen TD, Abel ED. Cardiac metabolism in heart failure: implications beyond ATP production. Circ Res. 2013;113(6):709–724. doi: 10.1161/CIRCRESAHA.113.300376.
- Lemaitre RN, Jensen PN, Hoofnagle A, et al. Plasma ceramides and sphingomyelins in relation to heart failure risk. Circ Heart Fail. 2019;12:e5708.
- Yu J, Pan W, Shi R, et al. Ceramide is upregulated and associated with mortality in patients with chronic heart failure. Can J Cardiol. 2015;31(3):357–363. doi: 10.1016/j.cjca.2014.12.007.
- Law BA, Liao X, Moore KS, et al. Lipotoxic very‐long‐chain ceramides cause mitochondrial dysfunction, oxidative stress, and cell death in cardiomyocytes. Faseb J. 2018;32(3):1403–1416. doi: 10.1096/fj.201700300R.
- Sasset L, Manzo OL, Zhang Y, et al. Nogo-A reduces ceramidede novo biosynthesis to protect from heart failure. Cardiovasc Res. 2023;119(2):506–519. doi: 10.1093/cvr/cvac108.
- Santulli G, Iaccarino G. Adrenergic signaling in heart failure and cardiovascular aging. Maturitas. 2016;93:65–72. doi: 10.1016/j.maturitas.2016.03.022.
- Lin LY, Wu CK, Juang JM, et al. Myocardial regional interstitial fibrosis is associated with left intra-ventricular dyssynchrony in patients with heart failure: a cardiovascular magnetic resonance study. Sci Rep. 2016;6(1):20711. doi: 10.1038/srep20711.
- Trial J, Entman ML, Cieslik KA. Mesenchymal stem cell-derived inflammatory fibroblasts mediate interstitial fibrosis in the aging heart. J Mol Cell Cardiol. 2016;91:28–34. doi: 10.1016/j.yjmcc.2015.12.017.