Abstract
Background
The prognostic factors for patients with pure ovarian immature teratoma (POIT) and the role of adjuvant chemotherapy in stage IA G2-G3 and IB-IC POIT remains controversial.
Methods
We conducted a retrospective study of 155 POIT patients treated in our hospital between 2000 and 2022. The recurrence-free survival (RFS), disease-specific survival (DSS), and potential prognostic factors of POIT patients were evaluated. Subgroup analysis was conducted in stage I other than stage IA G1 POIT.
Results
The median age at diagnosis was 23.0 years (range: 4.0 − 39.0), and 126 (81.3%), 2 (1.3%), 26 (16.8%), and 1 (0.6%) patients had FIGO stage I, stage II, stage III, and stage IV disease, respectively. Twenty-three patients relapsed and five died of the diseases after a median follow-up of 7.6 years, with a 5-year RFS and DSS rate of 86.0% and 97.0%, respectively. Multivariate analysis showed that positive postoperative tumour markers (TM) were the risk factor for recurrence in the overall cohort (hazard ratio [HR] 4.058, 95% CI 1.175 − 14.019, p = 0.027) and subgroup (HR 10.237, 95% CI 2.175 − 48.179, p = 0.003), and FIGO stage II–IV was the only factor for DSS in overall cohort (HR 7.751, 95% CI 1.281 − 46.895, p = 0.026). In 110 patients subjected to subgroup analysis, 29 patients received surveillance without chemotherapy and 81 patients were administered adjuvant chemotherapy. Multivariate analysis revealed active surveillance significantly increased the recurrence rate (5-year RFS of 75.7% vs. 93.6%, HR 7.562, 95% CI 2.441 − 23.424, p < 0.001) but not the death related to POIT (p = 0.338).
Conclusion
Positive postoperative TM and FIGO stage II–IV were the prognostic factors for POIT. Active surveillance in stage I POIT of any grade may be practical for those with negative postoperative TM.
KEY MESSAGE
Positive postoperative tumour markers and FIGO stage II–IV were the prognostic factors for pure ovarian immature teratoma. Active surveillance in stage I pure ovarian immature teratoma of any grade may be practical for those with negative postoperative tumor markers.
Introduction
Malignant ovarian germ cell tumors (MOGCT) are rare non-epithelial ovarian cancers that only occupy about 5% of ovarian malignancies [Citation1]. Pure ovarian immature teratoma (POIT) accounts for approximately one-third of the MOGCT, and it predominantly affects young patients and presents early-stage fertility-sparing surgery (FSS) with optimal surgical staging is the most commonly applied surgical treatment [Citation2–4]. Patients with MOGCT usually had satisfactory survival outcomes after standard treatment, with a 5-year disease-specific survival (DSS) rate of exceeding 95% for those diagnosed as International Federation of Gynaecology and Obstetrics (FIGO) stage I, and the 5-year DSS rate was nearly 70% even in FIGO stage IV diseases [Citation5]. Nevertheless, older age, advanced stage, tumour grade, yolk-sac components, and elevated both β-human chorionic gonadotropin (β-HCG) and α-fetoprotein (AFP) were proposed risk factors for survival [Citation6–8]. However, most of these studies included varied pathological types and they did not focus on the POIT population. Furthermore, the cut-off value and impact of some factors, such as older age and advanced stage or higher tumour grade on prognosis were inconsistent [Citation8,Citation9]. More explorations remain should be conducted.
Meanwhile, whether active surveillance or adjuvant chemotherapy in POIT patients of stage I except IA G1 remains controversial [Citation2,Citation10]. Recently, researchers suggested that active surveillance may be acceptable in stage IA-IC POIT of any grade [Citation11–15]. However, most of these studies included patients of different pathological subtypes of MOGCT, and the sample size of POIT patients was relatively small [Citation13,Citation14]. Moreover, these studies usually included POIT patients of IA G1 diseases in the surveillance group, which would bias the true impact of adjuvant chemotherapy. Our previous meta-analysis found that surveillance significantly increased the risk of mortality and tended to be related to a higher risk of recurrence in POIT [Citation16]. Therefore, whether surveillance or adjuvant chemotherapy in each corresponding tumour stage and grade has not been well defined [Citation11,Citation12]. The role of adjuvant chemotherapy in patients with stage I POIT except for IA G1 still needs to be addressed.
To investigate the survival outcomes and prognostic factors in POIT patients, we conducted a single-center retrospective study. Subgroup analysis was further performed restricted to POIT patients of stage IA G2-3 and stage IB-IC in any grade, and the role of adjuvant chemotherapy in this population was also evaluated.
Materials and methods
This study was approved by the Peking Union Medical College Hospital Ethics Committee. Patients with pathology-confirmed POIT who were treated at our hospital between January 2000 and December 2022 were included. Comprehensive medical information, including patients’ demographic characteristics, and clinical and pathological features, were collected. All the pathology slides of POIT patients in our hospital were reviewed by at least two experienced pathologists to confirm the diagnosis, and additional interinstitutional pathologic consultation would also be performed if necessary. We screened all patients with the ICD-10 code for immature teratoma (ICD-10 code: C56 M9080/3) in medical records, and those with incomplete treatment and follow-up data were excluded (see Supplementary Figure 1).
Exploratory factors related to RFS in the POIT cohort included age at diagnosis (< 18 or ≥ 18 years), tumour stage (FIGO stage I or II–IV), postoperative negative tumor markers (TM, yes or no), tumour size (< 15.0 or ≥ 15.0 cm), coexistence of gliomatosis peritonei (GP) at initial treatment (yes or no), tumour grade (G1, G2, G3), complete surgical staging (yes or no), lymphadenectomy (yes or no), and FSS (yes or no). Complete surgical staging was defined as peritoneal cytology examination, peritoneal biopsy, omentectomy or omental biopsy with or without lymph node dissection. FSS was defined as the preservation of at least one ovary and the uterus. TM (AFP, CA125, and/or CA 19-9) decreasing to or maintaining within normal values in two weeks postoperatively would be considered as postoperative negative TM. Subgroup analysis was conducted on patients with stage I diseases except IA G1 to determine the role of adjuvant chemotherapy in this population. Whether patients were administered adjuvant chemotherapy depends on the discussion between treating physicians and patients and their families in this subgroup. On the contrary, chemotherapy was routinely administered in all patients who had FIGO stage II or higher diseases.
Chemotherapy regimens were represented by bleomycin, etoposide, and cisplatin (BEP), or bleomycin, vincristine, and cisplatin (BVP), and the cycles were according to the treatment response and clinical guidelines. Follow-up plans were also conducted as the recommendation of guidelines [Citation2]. RFS was defined as the date from initial treatment intervention to confirmed immature teratoma recurrence. DSS was defined as the time from the date of the initial diagnosis to death related to POIT or the final follow-up.
Statistical analysis
We performed descriptive analyses to present the characteristics of this POIT population, and variables were described according to their distributions, such as means ± standard deviations, medians and interquartile ranges (IQRs), or counts (percentages). Student’s t-tests/Mann–Whitney U-test or chi-squared test/Fisher’s exact test was used to perform intergroup comparisons, depending on the distributions. The Kaplan-Meier (log-rank test) analysis was used to perform survival analyses. Univariate analyses for RFS and DSS were performed to screen exploratory factors and those with P-value < 0.2 or highly suspected to be related to prognosis were further included in the multivariate analysis. We used the Cox regression model to identify potential independent prognostic predictors. A two-tailed P value < 0.05 was considered statistically significant. Statistical analyses were conducted using SPSS (Version 21.0; SPSS Inc., Chicago, IL, USA) or GraphPad Prism (Version 8.0; GraphPad Software Inc., La Jolla, CA).
Results
Clinical characteristics of patients with POIT
A total of 155 patients who met the inclusion criteria were enrolled. The clinical characteristics of this cohort are summarized in . The median age at diagnosis was 23.0 years, and 81.3% of the patients were classified as FIGO stage I. Except for 6 patients who had unknown tumour grade, 40, 62, and 47 patients were graded as G1, G2, and G3, respectively. Serum TM was tested preoperatively in 145 patients, of whom 82.8% showed elevated, and the AFP (61.4%) was the most common elevated TM. The median preoperative AFP level in this cohort was 104.6 ng/ml (IQR 8.6 − 495.4), and only three patients had AFP levels over 2000 ng/ml.
Table 1. Clinical characteristics of POIT patients in the overall cohort (N = 155).
Surgery was performed in all patients at first treatment, and fertility was preserved in 94.2% of the patients. Approximately one-half (52.3%) of patients received complete surgical staging, and lymphadenectomy was conducted in 32 patients but only two showed tumours involved. At the initial presentation, GP was observed in 15 (9.7%) patients. Postoperative TM examination revealed that 63 (43.4%) patients had negative TM. Moreover, the median postoperative AFP level was only 11.8 ng/ml (IQR 2.2 − 69.3). Chemotherapy was administered in 117 (75.5%) patients, of whom most (98.3%) were BEP (98 patients, 83.8%) or BVP (17 patients, 14.5%) regimens ().
Table 2. Management and survival outcomes in POIT patients of overall cohort (N = 155).
Survival outcomes and prognostic factors in POIT patients
During the follow-up, one patient did not achieve complete remission (CR), she died of POIT progression 1.4 years after diagnosis. Among the other 154 patients who obtained CR after initial treatment, twenty-three patients relapsed as immature teratoma, with a 5-year and 10-year RFS of 86.0% and 83.3%, respectively (). Importantly, the median time to relapse in these 23 patients was 0.4 years, and 19 (82.6%) of the patients relapsed within one year after initial treatment. Clinical characteristics, pathological features, and surgical options were evaluated to screen potential risk factors for recurrence (Supplementary Table 1a). Positive postoperative TM (p = 0.017, ) and tumour grade (p = 0.226) were further included in multivariate Cox regression, while positive postoperative TM remained statistically significant (hazard ratio [HR] 4.058, 95% CI 1.175 − 14.019, p = 0.027). However, age at diagnosis, FIGO stage, mass size, tumour grade, coexisted with GP, FSS, complete surgical staging, and lymphadenectomy showed no statistical significance on RFS (all p > 0.05) (Supplementary Table S1). Of patients with stage II – IV POIT, only 3 of them achieved negative postoperative TM, which was unavailable to evaluate the role of postoperative TM level in this subgroup.
Figure 1. (A) The Kaplan-Meier (log-rank test) curves showed the RFS and DSS rates in this overall POIT cohort. (B) POIT patients with positive postoperative TM had a significantly higher risk of recurrence compared with those who had negative postoperative TM. (C) FIGO stage II-IV significantly increased the mortality risk in POIT patients compared with stage I. (D) The 5-year RFS and DSS rates in stage I POIT except IA G1 subgroup were 88.8% and 99.0%, respectively. (E) A significant difference in RFS rate was noted based on the postoperative TM level (negative vs. positive) in stage I POIT patients except IA G1. (F) Patients who received adjuvant chemotherapy showed significantly better RFS compared with those with active surveillance in POIT of stage IA G2-G3 and IB-IC of any grade.
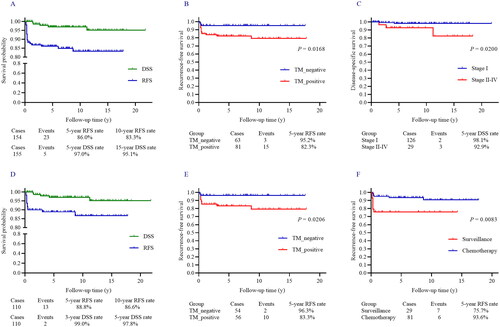
All except one patient received surgery after recurrence, of whom FSS was applied in 17 patients, and 19 (82.6%) achieved R0 resection. Twenty-one patients (91.3%) were administered adjuvant chemotherapy. Of the 19 patients who obtained CR after the first recurrence, 7 had a second relapse (). After a median follow-up of 7.6 years, 148 (95.5%) patients were free of diseases and 2 patients were alive with the diseases, and 5 cases died of the diseases, with a 5-year DSS rate of 97.0% (). FIGO stage (p = 0.020, ), negative TM after surgery, and coexisted GP were included in multivariate Cox regression, while FIGO stage II–IV (HR 7.751, 95% CI 1.281 − 46.895, p = 0.026) was the only risk factor for DSS in POIT cohort (Supplementary Table 1).
Table 3. Clinical characteristics of patients with stage I POIT except IA G1 subgroup (N = 110).
Patients with stage I POIT (except IA G1)
After selection, we included 110 stage IA G2-G3 or IB-IC POIT (the screening details were summarized in Figure S1), and the median follow-up time was 7.7 years. The median age was 23.0 years in this subgroup, and no significant difference was noted in follow-up time or age at diagnosis between those who received adjuvant chemotherapy and surveillance. Of the 110 patients, 29 of them received surgery alone, and the other 81 patients received adjuvant chemotherapy after surgery, the details of the patient’s characteristics were listed in .
Elevated TM was noted in 88 patients, and 54 patients had negative TM after surgery. The number of patients graded G1, G2, and G3, was 23, 57, and 30, respectively. One hundred and five (95.5%) patients received FSS and 46 (41.8%) patients underwent surgical staging. Meanwhile, ovarian cystectomy was applied in 48 patients, and 57 patients received unilateral salpingo-oophorectomy (USO). During the follow-up, 13 patients had relapsed as immature teratoma while 7 and 6 cases occurred in the surveillance group and adjuvant chemotherapy, respectively. The 5-year RFS rate was 88.8% (). Factors that may predict RFS are listed in Supplementary Table 2a. Positive postoperative TM (p = 0.021, ) and chemotherapy (p = 0.008, ) revealed a statistically significant relationship with RFS in univariate analysis. However, tumour grade (), ovarian cystectomy (), and incomplete surgical staging () were not significantly relate to RFS. Mass size, negative TM after surgery, tumour grade, and chemotherapy were further subjected to multivariate analysis, while positive postoperative TM (HR 10.237, 95% CI 2.175 − 48.179, p = 0.003) and surveillance (HR 7.562, 95% CI 2.441 − 23.424, p < 0.001) were two independent prognostic factors predicting poor RFS. Of the 35 paediatric POIT patients in this subgroup, 7 received active surveillance, of which 3 experienced relapses, and all were successfully cured by surgery and adjuvant chemotherapy.
Figure 2. (A–C) Tumor grades (a), surgical options (B), and incomplete surgical staging (C) did not significantly affect the RFS in stage I POIT excluded IA G1. (D) Adjuvant chemotherapy did not significantly improve the DSS rate compared with active surveillance in stage I POIT except IA G1.
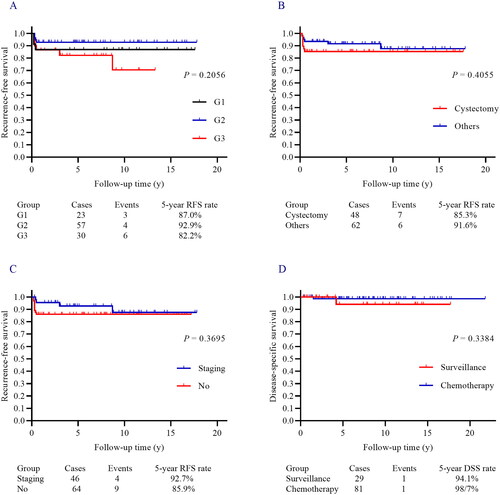
Therapeutic options after recurrence were summarized in , of whom 11 patients received surgery with chemotherapy, and each one received surgery alone and chemotherapy combined with radiotherapy. At the final follow-up, each one who died of the disease was noted in two groups, and 108 patients achieved no evidence of disease. The 5-year DSS rate was 99.0% (). No significant difference in DSS was observed in patients who received surveillance or adjuvant chemotherapy after surgery (p = 0.338, ) (Supplementary Table 2). Similarly, factors that may affect DSS were presented in Table S2b, but no statistically significant risk factor was identified in univariate and multivariate regression.
Discussion
Our study presents one of the largest cohorts of POIT patients concerning the prognosis and risk factors reported in China. We found that the survival outcomes in POIT patients were satisfactory, with a 5-year RFS rate of exceeding 85% and a 5-year DSS rate of over 95%. Positive postoperative TM was the risk factor for RFS in the POIT and stage I subgroup, and active surveillance significantly increased the risk of recurrence but not the DSS in the stage IA G2-G3 and IB-IC POIT. FIGO stage II-IV was the only risk factor for DSS in the overall POIT cohort.
Patients with MOGCT usually present at an early stage and about 70–80% are diagnosed at FIGO stages I–II [Citation4]. Published research demonstrated that MOGCT had a 5-year DSS of over 95% and approximately 70-80% in stage I and stage IV patients, respectively [Citation4,Citation7, Citation8]. The 5-year RFS rate in MOGCT patients was about 80% and varied in different pathological types; and the dysgerminomas had a better RFS and DSS [Citation4,Citation8]. However, most of these studies mixed different types of MOGCT and did not focus on the POIT population, and data from China remained insufficient. Jorge et al. reported 1045 POIT patients from the national cancer database, revealing the 5-year survival rates were 98.3%, 93.2%, 82.7%, and 72.0% for stage I, II, III, and IV disease, respectively [Citation17]. Our study showed a similar result that the 5-year DSS was 97.0% in the overall cohort and 98.1% in stage I patients, confirming the excellent prognosis in POIT patients and presenting data from the Chinese population. Moreover, more than 80% of the relapse occurred within one year after initial treatment, reminding us to apply rigorous follow-up, especially in the first year postoperatively.
Although the risk stratification system has been well-defined in male germ cell tumours (GCT), a practical risk stratification system for MOGCT has not been well established [Citation18,Citation19]. In 2015, Meisel et al. classified MOGCT as good, intermediate, and poor-risk groups to guide the treatment [Citation19]. It could effectively distinguish patients in good or poor risk groups but failed to differentiate well between patients classified as good and intermediate risk. Advanced stage, older age, higher tumour grade, and non-dysgerminoma pathology were common proposed risk factors for prognosis [Citation3,Citation8,Citation14]. Nevertheless, inconsistent results were also noted. Some researchers proposed age over 45 or 50 years as a prognostic factor [Citation4,Citation8,Citation17], but it only occupied a small proportion of cases, and Murugaesu et al. found age at diagnosis did not predict prognosis [Citation7].
Our current study also showed no significant difference in paediatric or adult patients, and no patients were older than 40 years, which may be explained by the different sample sizes and we restricted to POIT patients rather than mixed different types of MOGCT. We also found that the FIGO stage over I had poor DSS in POIT patients, which was in accordance with the previous study [Citation17]. Meanwhile, the postoperative TM level may be an additional factor that clinicians can consider in whether to apply active surveillance or adjuvant chemotherapy in stage I POIT patients. Further study is needed regarding the impact of chemotherapy in this group with elevated postoperative TM. This result extended the previous study of Murugaesu et al. [Citation7] that used preoperatively elevated both AFP and HCG as risk factors. Another finding in our study was that the tumour grade did not relate with RFS and DSS, which was in accordance with Newton et al. [Citation14] but differed from Jorge et al. [Citation17]. It may be attributed to Jorge et al. included older patients and could not distinguish patients who died of POIT or comorbidity due to data from public databases, which impacted the role of tumour grade on survival.
The need for adjuvant chemotherapy in stage IA G2-G3 and IB-IC of any grade POIT patients remains controversial. The ESMO guidelines recommend 3–4 cycles of BEP chemotherapy after proper surgery in stage IB-IC POIT, while active surveillance could be an option in properly staged IA G2-G3 POIT patients with negative postoperative TM [Citation2]. However, surveillance is recommended only in stage IA G1 POIT patients in NCCN guidelines [Citation10]. Due to the excellent survival outcomes of POIT and chemotherapy-induced toxicities, current research has aimed to reduce unnecessary chemotherapy in stage I MOGCT. Pashankar et al. [Citation20] reported adjuvant chemotherapy did not decrease the recurrence rate in 98 paediatric POIT patients. This finding was supported by Pavone et al. [Citation15] who found that only one relapsed in 35 paediatric of any grade or stage, and no death occurred. In our study, 35 paediatric POIT patients of stage IA G2-G3 or IB-IC were identified, 5 relapsed but none died, again indicating excellent survival in paediatric stage I POIT patients.
Recently, researchers also suggested that active surveillance may be acceptable in stage IA-IC POIT of any grade, even in adult patients [Citation6,Citation11–14,Citation21–23]. In these studies, no significant difference was noted in recurrence and death between patients who received surveillance and adjuvant chemotherapy. However, we found that surveillance significantly increased the risk of relapse (RR 7.562, p < 0.001), although it did not increase the mortality rate (p = 0.338). These inconsistencies could be explained by the different inclusion criteria, we only included POIT patients of stage IA G2-G3 and IB-IC of any grade. Most previous studies [Citation13,Citation14,Citation22] also included POIT of IA G1 diseases in the surveillance group, which would bias the true impact of adjuvant chemotherapy. And in the previous study of the largest sample published by Bergamini et al. patients who had persistent elevated TM were all given adjuvant chemotherapy [Citation12]. The only study excluding IA G1 patients only examined POIT of IA-IB G2-G3 diseases [Citation23]. Furthermore, most of the studies enrolled different pathological subtypes of MOGCT, and the sample size of POIT patients was relatively small [Citation13,Citation14]. Our previous meta-analysis indicated that adjuvant chemotherapy significantly decreased the mortality rate (RR 0.31, 95% CI 0.11–0.88, p = 0.03), and it appeared to significantly lower both the recurrence (RR 0.17, 95% CI 0.03–0.83, p = 0.03) and death (RR 0.04, 95% CI 0.00–1.00, p = 0.05) in adult POIT [Citation16]. We also firstly evaluated the predictive value of postoperative TM level in RFS and DSS in this cohort, which demonstrated that positive postoperative TM was strongly correlated with increased risk of recurrence (HR = 10.237, p = 0.003), and only 3.7% of patients with negative postoperative TM experienced a relapse. Combined with the results that surveillance did not increase the mortality rate in our cohort, we proposed that surveillance may be better to apply in stage I POIT patients with postoperative negative TM.
The role of complete surgical staging in apparent stage I POIT patients has also been long argued. Bergamini et al. [Citation12] and Mangili et al. [Citation6] reported incomplete surgical staging was related to a higher risk of recurrence, but Wang et al. [Citation11] found that surgical staging may be omitted in apparent stage I adult POIT patients and restaging was negative in three patients. Our study found no different RFS and DSS in patients who received complete surgical staging or not, and a similar result was also noted in lymphadenectomy. The inconsistency among different studies was mainly due to the sample sizes and varied inclusion criteria. Liu et al. also found that comprehensive surgical staging may not be superior to USO in RFS and DSS, and they suggested comprehensive intraoperative exploration with biopsy of suspicious sites may be safer in MOGCT [Citation24]. Graham et al. in 2022 found that the absence of surgical staging did not alter the RFS or OS in stage I MOGCT of all histologies and ages [Citation22]. Indeed, although nearly half of the patients did not undergo surgical staging in our cohort, they all received thorough exploration during surgery, which could greatly reduce the chance of mistakenly downstaging even without surgical staging. Hence, we proposed that USO with a comprehensive exploration of the abdominopelvic cavity may be an alternative option for apparent stage I POIT patients.
The large sample size strengthened the reliability and clinical significance of this study. However, there were several limitations. The retrospective nature, single-center experience, and non-randomized division of patients who received surveillance or adjuvant chemotherapy were the major limitations. Moreover, the very limited number of recurrence or death events in this cohort might impact the ability to detect statistically significant differences. Further prospective study research is warranted.
Conclusion
The survival outcomes in POIT patients were satisfactory, and positive postoperative TM was the risk factor for RFS in POIT patients, while FIGO stage II–IV was the prognostic factor for DSS. Surveillance significantly increased the risk of recurrence but not mortality in stage IA G2-G3 and IB-IC POIT. Active surveillance in stage I POIT of any grade may be practical for those with negative postoperative TM.
Ethics approval and consent to participate
This retrospective study was approved by the Ethics Committee of Peking Union Medical College Hospital (reference number: I-23PJ444) and all methods were carried out following the Declaration of Helsinki. Written informed consent to participate in the study was waived by the Peking Union Medical College Hospital ethics committee due to the retrospective nature of the study.
Consent for publication
Not applicable.
Authors’ contributions
Sijian Li wrote the manuscript, participated in the study design, and conducted statistical analysis; Xinyue Zhang, Tianyu Zhang, and Min Yin completed the work of follow-up and participated in statistical analysis; Dongyan Cao and Yang Xiang participated in the manuscript modification; Jiaxin Yang conceived the study design and modified the manuscript. All authors read and approved the manuscript.
Supplemental Material
Download Zip (137 KB)Acknowledgments
Sijian Li want to thank Xin Li for her support in this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data generated or analyzed during this study are included in this published article and the supplementary information files. The datasets used and/or analyzed during the current study can be obtained from the corresponding author upon reasonable request.
Additional information
Funding
References
- Gatta G, van der Zwan JM, Casali PG, et al. Rare cancers are not so rare: the rare cancer burden in Europe. Eur J Cancer. 2011;47(17):1–9. doi:10.1016/j.ejca.2011.08.008.
- Ray-Coquard I, Morice P, Lorusso D, et al. Non-epithelial ovarian cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv1–iv18. doi:10.1093/annonc/mdy001.
- Smith HO, Berwick M, Verschraegen CF, et al. Incidence and survival rates for female malignant germ cell tumors. Obstet Gynecol. 2006;107(5):1075–1085. doi:10.1097/01.AOG.0000216004.22588.ce.
- Park M, Lim J, Lee JA, et al. Incidence and outcomes of malignant ovarian germ cell tumors in korea, 1999-2017. Gynecol Oncol. 2021;163(1):79–84. doi:10.1016/j.ygyno.2021.07.037.
- Torre LA, Trabert B, DeSantis CE, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68(4):284–296. doi:10.3322/caac.21456.
- Mangili G, Sigismondi C, Lorusso D, et al. The role of staging and adjuvant chemotherapy in stage I malignant ovarian germ cell tumors (MOGTs): the MITO-9 study. Ann Oncol. 2017;28(2):333–338. doi:10.1093/annonc/mdw563.
- Murugaesu N, Schmid P, Dancey G, et al. Malignant ovarian germ cell tumors: identification of novel prognostic markers and long-term outcome after multimodality treatment. J Clin Oncol. 2006;24(30):4862–4866. doi:10.1200/JCO.2006.06.2489.
- Mangili G, Sigismondi C, Gadducci A, et al. Outcome and risk factors for recurrence in malignant ovarian germ cell tumors: a MITO-9 retrospective study. Int J Gynecol Cancer. 2011;21(8):1414–1421. doi:10.1097/IGC.0b013e3182236582.
- Patterson DM, Murugaesu N, Holden L, et al. A review of the close surveillance policy for stage I female germ cell tumors of the ovary and other sites. Int J Gynecol Cancer. 2008;18(1):43–50. doi:10.1111/j.1525-1438.2007.00969.x.
- Armstrong DK, Alvarez RD, Bakkum-Gamez JN, et al. Ovarian cancer, version 2.2020. J Natl Compr Canc Netw. 2021;19(2):191–226. doi: 10.6004/jnccn.2021.0007.
- Wang D, Zhu S, Jia C, et al. Role of staging surgery and adjuvant chemotherapy in adult patients with apparent stage I pure immature ovarian teratoma after fertility-sparing surgery. Int J Gynecol Cancer. 2020;30(5):664–669. doi:10.1136/ijgc-2019-001116.
- Bergamini A, Sarwar N, Ferrandina G, et al. Can we replace adjuvant chemotherapy with surveillance for stage IA-C immature ovarian teratomas of any grade? An international multicenter analysis. Eur J Cancer. 2020;137:136–143. doi:10.1016/j.ejca.2020.06.033.
- Mangili G, Giorda G, Ferrandina G, et al. Surveillance alone in stage I malignant ovarian germ cell tumors: a MITO (multicenter Italian trials in ovarian cancer) prospective observational study. Int J Gynecol Cancer. 2021;31(9):1242–1247. doi:10.1136/ijgc-2021-002575.
- Newton C, Murali K, Ahmad A, et al. A multicentre retrospective cohort study of ovarian germ cell tumours: evidence for chemotherapy de-escalation and alignment of paediatric and adult practice. Eur J Cancer. 2019;113:19–27. doi:10.1016/j.ejca.2019.03.001.
- Pavone R, Dijoud F, Galmiche L, et al. Pure pediatric ovarian immature teratomas: the French experience. Pediatr Blood Cancer. 2020;67(4):e28186.
- Li S, Wang Y, Zhang X, et al. Role of adjuvant chemotherapy in stage I pure ovarian immature teratoma: a systematic review and meta-analysis. Cancers. 2023. doi:10.3390/cancers15061741.
- Jorge S, Jones NL, Chen L, et al. Characteristics, treatment and outcomes of women with immature ovarian teratoma, 1998-2012. Gynecol Oncol. 2016;142(2):261–266. doi:10.1016/j.ygyno.2016.05.024.
- International germ cell consensus classification: a prognostic factor-based staging system for metastatic germ cell cancers. International germ cell cancer collaborative group. J Clin Oncol. 1997;15(2):594–603.
- Meisel JL, Woo KM, Sudarsan N, et al. Development of a risk stratification system to guide treatment for female germ cell tumors. Gynecol Oncol. 2015;138(3):566–572. doi:10.1016/j.ygyno.2015.06.029.
- Pashankar F, Hale JP, Dang H, et al. Is adjuvant chemotherapy indicated in ovarian immature teratomas? A combined data analysis from the malignant germ cell tumor international collaborative. Cancer. 2016;122(2):230–237. doi:10.1002/cncr.29732.
- Yuksel D, Ayhan S, Korkmaz V, et al. Retrospective analysis of pure ovarian immature teratoma in patients aged 15–39 years: a Turkish multicenter study. J Adolesc Young Adult Oncol. 2021;10(6):697–702. doi:10.1089/jayao.2020.0155.
- Graham R, MacDonald ND, Lockley M, et al. Surgical management and outcomes for stage 1 malignant ovarian germ cell tumours: a UK multicentre retrospective cohort study. Eur J Obstet Gynecol Reprod Biol. 2022;271:138–144. doi:10.1016/j.ejogrb.2022.02.013.
- Nasioudis D, Frey MK, Chapman-Davis E, et al. Surveillance only for high-risk FIGO stage IA/IB malignant ovarian germ cell tumors: results from a national cancer database. Am J Clin Oncol. 2021;44(5):195–199. doi:10.1097/COC.0000000000000805.
- Liu Q, Ding X, Yang J, et al. The significance of comprehensive staging surgery in malignant ovarian germ cell tumors. Gynecol Oncol. 2013;131(3):551–554. doi:10.1016/j.ygyno.2013.08.016.
