Abstract
Objective
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare acquired disease characterized by chronic complement-mediated hemolysis. The concentrated outbreak of coronavirus disease 2019 (COVID-19) in China after 6 December 2022, provided an opportunity to observe the disease course of PNH during an active Omicron infection epidemic.
Patients and method
Patients diagnosed with PNH at Peking Union Medical College Hospital (PUMCH) before 6 December 2022, were followed up until 10 April 2023. Clinical data related to coronavirus infection and hemolysis were recorded. Factors influencing the infection and severity rate of Omicron, as well as hemolysis provocation, were analyzed.
Results
In total, 131 patients with PNH were included in this retrospective analysis; 87.8% were infected with Omicron. Among them, 15.7% met the criteria for severity, and 1 patient died (0.87%). No protective factors were identified against Omicron infections. However, patients with severe Omicron infection (n = 18) had a lower vaccination rate than those with non-severe infection (n = 97; p = 0.015). Among those infected (n = 115) with Omicron, there was a significant increase in lactate dehydrogenase (LDH) levels compared with those in the uninfected group (n = 16, p = 0.000). Patients with severe infections (n = 18) had even higher LDH increase rates than those without severe infections (n = 97; p = 0.002). 10 (37.0%) patients treated with complement inhibitors developed breakthrough hemolysis (BTH). Patients treated with complement inhibitors (n = 27) exhibited less severe hemolysis than treatment-naïve patients (n = 104; p = 0.003).
Conclusions
Omicron infection exacerbates hemolytic attacks in patients with PNH. Vaccination helps mitigate the severity of Omicron infection, and using complement inhibitors reduces hemolysis exacerbation.
Introduction
Paroxysmal nocturnal hemoglobinuria (PNH) is a clonal hematopoietic stem cell disease in which patients develop somatic phosphatidylinositol glycan (PIG-A) gene mutations in hematopoietic stem cells, resulting in impaired biosynthesis of glycosylphosphatidylinositol (GPI) anchors. This results in the deletion of proteins attached to the cell surface by GPI anchors. The absence of the two important complement regulatory proteins, CD55 and CD59, can lead to the destruction of red blood cells following complement activation, resulting in hemolysis [Citation1–4]. Thus, PNH hemolysis is driven by complement activation. The clinical manifestations of PNH include intravascular hemolysis, bone marrow failure, and thrombosis [Citation5]. In 2007, the United States Food and Drug Administration (US FDA) approved the first-generation complement inhibitor eculizumab, which greatly improved the related symptoms caused by intravascular hemolysis, prolonged the survival of patients, and enhanced their quality of life [Citation5]. In recent years, newer generation complement inhibitors, such as crovalimab and iptacopan, have been used in premarket clinical studies in China, demonstrating promising efficacy [Citation6–8].
Since December 2019, the outbreak of the novel coronavirus pneumonia (COVID-19) has been recognized by the World Health Organization as a major emergency public health event owing to its strong pathogenicity and transmissibility, causing a global impact on human health and mortality [Citation9]. China initiated a large-scale COVID-19 vaccination campaign in 2021, followed by sporadic vaccinations. Patients with PNH are more susceptible to hemolytic attacks under stress, infections, and other complement-activating factors. In China, there was a concentration of omicron infections from December 2022 to April 2023.
Our center routinely followed up many patients with PNH in the outpatient clinic. Some patients received COVID-19 vaccinations before December 6. In addition, some patients participated in several complement inhibitor clinical trials that began in October 2021 and continued until the time of this paper’s submission. To investigate the infection rate, severity of Omicron disease, and hemolysis-related symptoms following infection, we conducted a retrospective analysis of patients with PNH during this specific period. Patients willing to provide medical records were included in the analysis.
Patients and methods
Data collection
Data were collected from successive patients diagnosed with PNH before 6 December 2022 who were regularly followed up at Peking Union Medical College Hospital (PUMCH) until 10 April 2023. During that period, 150 patients were followed up and 131 patients were included in this study. Patients infected with pathogens other than Omicron were excluded. Demographic information, medical history, physical examination, hemoglobin, LDH, hepatic and renal function, ferritin, PNH clone size (calculated by neutrophil FLAER-negative cell ratio), disease complications, COVID-19 vaccination status, and previous treatment were documented.
The above information and the Omicron-related manifestations during the pandemic were also collected. Hemolysis tests for infected patients were conducted during active Omicron infections, usually within 2 weeks after the diagnosis of Omicron infection. Hemolysis tests for uninfected patients were performed during the same Omicron pandemic period as part of regular follow-up. Data within 1 month before 6 December 2022, were recognized as the baseline data.
Most of the above information was obtained from the medical records of our hospital, some patients’ laboratory examinations came from local hospitals, and follow-up information was obtained from medical records or telephone follow-ups. All patients signed an informed consent form before providing their medical history. This study was approved by the Ethics Committee of the Peking Union Medical College Hospital.
Criteria for the diagnosis and classification of PNH
PNH diagnosis was based on clinical manifestations combined with the absence of GPI-anchored proteins (GPI-Aps) on ≥2 lineages [Citation1,Citation5,Citation10,Citation11]. PNH was further classified as PNH with or without concomitant bone marrow failure (BMF). PNH clone size was determined using the proportion of FLAER-negative neutrophils.
The criteria of diagnosis and efficacy
Diagnostic criteria for COVID-19
Patients were required to meet the following criteria for the diagnosis of COVID-19: (1) Present COVID-19 infection-related clinical manifestations; (2) Have one or more of the following etiological and serological test results: (a) positive nucleic acid test for novel coronavirus, (b) test positive for COVID-19 antigen, (c) positive isolation or culture of the novel coronavirus, and (d) the level of novel coronavirus-specific IgG antibody increased four times or more in the convalescent phase than in the acute phase [Citation12,Citation13].
Criteria for severe COVID-19 (severe and/or critical)
Severe cases met any of the following criteria:
Oxygen saturation <90% on room air
Severe pneumonia
Signs of severe respiratory distress (adult use of auxiliary muscles, inability to speak complete sentences, and respiratory rate >30 times/min [Citation12].
Critical case
Critical cases involve acute respiratory distress syndrome (ARDS), sepsis, septic shock, or other conditions usually requiring life-sustaining treatment, such as invasive or noninvasive mechanical ventilation or pressors.
Definition of hemolysis-related manifestations
Hemolysis aggravation: worsening hemolysis and/or new thrombotic events. Worsening hemolysis: an aggravation of hemolysis-related manifestations (hemoglobinuria, jaundice), an increase of >200 U/L in LDH, a decrease of 20 g/L in hemoglobin (HGB) from baseline, or a change from non-transfusion-dependent to transfusion-dependent without other causes.
Thrombotic events are diagnosed by imaging [Citation14].
Definition of breakthrough hemolysis
Since no consensus was reached for the definition of breakthrough hemolysis in patients with PNH, we adopted the criteria from previous reports [Citation15–17]: at least one new or worsening symptom or sign of intravascular hemolysis (fatigue, hemoglobinuria, abdominal pain, shortness of breath [dyspnea], anemia [hemoglobin < 10 g/dL], MAVE [including thrombosis], dysphagia, or erectile dysfunction) in the presence of elevated LDH ≥2 × ULN after prior LDH reduction to <1.5× ULN on treatment.
Statistical analysis
Data analysis was conducted using the SPSS software. The chi-square test was used for categorical variables, and the rank-sum test was used for rank data. An independent samples t-test was used for continuous variables. The chi-square test was used to screen for influencing factors, followed by a binary logistic regression analysis. Statistical significance was set at p < 0.05.
Results
Baseline (data before 6 December 2022) characteristics of patients with PNH
The final analysis included 131 patients, including 66 males (50.4%). The median patient age was 42 years (range 17–81 years). The median time from the diagnosis of PNH to 6 December 2022, was 72 months (range 12–504 months). The final follow-up date was 10 April 2023. The median follow-up duration was 3 months (range 2–4 months).
Before 6 December 2022, the median frequency of hemoglobinuria was two episodes per year (range 0–10). Further, 7 patients (5.3%) had persistent hemoglobinuria and 39 (29.8%) had a history of thrombosis; 20 cases (51.2%) of thrombus occurred in the abdominal vein. Other cases involved deep vein thrombosis (DVT), intracranial venous sinus thrombosis, myocardial infarction, and cerebral infarction. A total of 24 patients (61.5%) received anticoagulant therapy, such as warfarin, low-molecular-weight heparin, and rivaroxaban, and 15 (38.5%) did not receive anticoagulants due to contraindications.
Of all the patients in this study, 27 patients were treated with complement inhibitors, including 11 patients (40.7%) treated with crovalimab (a C5 complement inhibitor) for 9 months (range 6–14 months), 10 patients (37%) treated with iptacopan (Factor B complement inhibitor) for 9 months (range 6–14 months) and 6 patients (22.2%) treated with CAN106 (a C5 complement inhibitor) for 7 months (range 6–9 months). A total of 41 patients (31.3%) received COVID-19 vaccinations, and the median time from the last vaccination to 6 December 2022, was 9 months (range 1–18 months). The vaccines included the Beijing Biological Vaccine (45%) and the Kexing Vaccine (55%); both are inactivated vaccines. All patients received two shots. The baseline characteristics of the patients are shown in .
Table 1. Clinical features of patients with PNH before 6 December 2022.
A total of 115 patients (87.8%) were newly diagnosed with COVID-19 (Omicron). Of these, 86 (74.8%) infections were confirmed via antigen and nucleic acid tests, while 29 (25.2%) were based on close contact history, clinical manifestations during the peak of Omicron infection, and other positive COVID-19 tests. Diagnoses were made between 6 December 2022 and 5 February 2023. No significant differences were found in clinical characteristics, including COVID-19 vaccination status and use of complement inhibitors, between those infected or with Omicron ().
COVID-19 related symptoms
All observed Omicron infections occurred within a month after 6 December 2022, and most hemolysis cases occurred within 2 weeks after Omicron infections. A total of 101 (87.8%) patients exhibited fever with a median maximum body temperature of 38.5 °C (range 37.1–40.5) and an average duration of 2 days (range 1–12). Other symptoms included sore throat (n = 46, 40%), cough (n = 75, 65.2%), phlegm (n = 59, 51.3%), muscle and joint pain (n = 45, 39.1%), fatigue (n = 84, 73%), headache (n = 11, 9.6%), nausea and vomiting (n = 7, 6.1%), nasal congestion (n = 4, 3.5%) and dizziness (n = 4, 3.5%), loss of smell and taste (n = 4, 3.5%), diarrhea (n = 3, 2.6%), shortness of breath in chest (n = 3, 2.6%), loss of appetite (n = 2, 1.7%), abdominal pain (n = 1, 0.9%), sweating (n = 1, 0.9%), bleeding (n = 1, 0.9%), toothache (n = 1, 0.9%), chest pain (n = 1, 0.9%), Lumbago (n = 1, 0.9%), insomnia (n = 1, 0.9%), orbital pain (n = 1, 0.9%), and dry eyes (n = 1, 0.9%). The median duration of COVID-19 infection was 8 days (range 3–16).
Severe infections and possible risk factors
Of all the infected patients, 18 (15.7%) were considered severe. Baseline clinical features were compared between patients with and without severe infection. Only patients who had received vaccination exhibited a significant reduction in severe COVID-19 symptoms (p = 0.015, and ). We further categorized patients who received complement inhibitors as those with proximal or terminal complement inhibitors. Of the 17 patients treated with terminal complement inhibitors (crovalimab and CAN106), 14 (82.4%) were infected with Omicron, and 2 (14.3%) were severe cases. Of the 10 patients treated with a proximal complement inhibitor (iptacopan), 9 (90%, p = 1.000 compared with those with terminal complements) were infected with Omicron, but no serious cases were observed (p = 0.502, ).
Figure 1. Comparison of proximal and terminal complement inhibitors in protecting BTH. Patients treated with terminal (crovalimab and CAN106) or proximal (iptacopan) complement inhibitors were also compared. No differences were found in infection or severe infection rates. However, higher BTH levels were observed in patients treated with terminal complement inhibitors (p = 0.003). Infection rate: Omicron infection rate; severe infection rate: severe Omicron infection rate; BTH: breakthrough hemolysis. p*: <0.05 indicated a statistically significant difference.
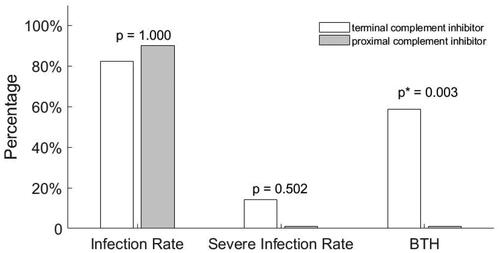
Table 2. Comparison of patients with or without vaccination.
PNH-related manifestations during the COVID-19 pandemic
Hemolysis aggravation
From 6 December 2022, to the end of follow-up, 81 (61.8%) patients experienced hemolysis aggravation, among them, 2 patients had new thrombotic events. Of the 81 patients, 79 patients (97.6%) were infected, and only 2 (2.4%) were uninfected with Omicron (p = 0.000). In the infected group, all severe cases (100%) had hemolysis aggravation, while 62.9% of non-severe cases had hemolysis aggravation (p = 0.002).
Changes in laboratory parameters
During the COVID-19 pandemic, which started on 6 December 2022 hemolytic-related parameters were significantly worse in infected patients, especially those with severe infections. The HGB level was 65 (43–138) g/L, which was significantly lower in infected patients than in non-infected patients [80 (68–136) g/L, p = 0.010]. Severe cases had lower HGB levels than non-severe cases [56 (43–86) g/L vs. 77 (45–138) g/L, p = 0.016]. Similar results were observed for platelet (PLT) counts. PLT counts were lower in infected patients than in non-infected patients [112.5 (26–251) ×109/L vs 151 (75–356) ×109/L, p = 0.025], and even lower in severe cases than in non-severe cases [97 (26–157) ×109/L vs 158 (63–251) ×109/L, p = 0.013). Absolute neutrophil count (ANC) was significantly lower in infected patients than non-infected patients [1.81 (0.18–6.52) ×109/L vs 3.62 (1.01–6.45) ×109/L, p = 0.040], and even lower in severe cases than non-severe cases [1.35 (0.18–3.59) ×109/L vs 2.68 (0.8–6.52) ×109/L, p = 0.013]. Among those infected (n = 115) with Omicron, there was a significant increase in LDH levels compared to the uninfected group (n = 16, 68.7% vs 12.5%, p = 0.000), and individuals with severe infections (n = 18) had an even higher LDH increase rate than those without (n = 97; 100% vs 62.9%, p = 0.002).
PNH outcome
At the end of follow-up, 53 patients (65.4%) with aggravated hemolysis returned to the baseline level, 27 patients (33.3%) worsened, and 1 patient (1.2%) died of COVID-19. Infected patients had a lower recovery rate than uninfected patients (64.5 vs 100%, p = 0.542), and severe cases had worse recovery rates than non-severe cases (27.8 vs 78.7%, p = 0.000). One severe patient died from an omicron infection, with a death rate of 0.87%.
Predictors for aggravation of hemolysis and thrombosis
For the entire cohort, a comparison between those with and without hemolysis aggravation showed that the use of complement inhibitors [odds ratio (OR) (95% confidence interval (CI)): 0.311 (0.117–0.822), β < 0; p = 0.019] was independent factors for less hemolysis aggravation and omicron infection [OR (95%CI): 16.016 (3.334–76.946), β > 0; p = 0.001] was independent factors for hemolysis aggravation ().
Table 3. Clinical features before 6 December 2022, for all patients with or without hemolysis aggravation.
Univariate analysis showed that, for the infected patients, being vaccinated, receiving complement inhibitors, and having non-severe infections significantly reduced hemolysis aggravation (p = 0.018, p = 0.004, and p = 0.002, respectively). Only receiving complement inhibitors was the independent factor for protecting hemolysis aggravation on multivariate regression analysis [OR (95%CI): 0.318 (0.118–0.857), β < 0; p = 0.024, ]. In total, 10 (58.8%) patients treated with terminal complement inhibitors developed BTH, whereas none of the patients who received proximal complement inhibitors developed BTH (p = 0.003, ). However, patients with proximal complement inhibitors had a statistical higher vaccination rate than those with C5 inhibitors (p = 0.014), meanwhile, there were 2 severe infections in the C5 complement inhibitor.
Discussion
The COVID-19 pandemic has significantly impacted public health both in China and globally. Patients with PNH are generally more susceptible to various infections, and the use of complement inhibitors increase susceptibility to encapsulated organisms, namely meningococcal and gonococcal infections, potentially leading to different clinical outcomes during a pandemic. Although a few reports have investigated PNH in other countries, their patients have different backgrounds from ours. First, most patients with hemolysis were treated with complement inhibitors or, on rare occasions, had no prior exposure to such complement inhibitors. Owing to different management policies, most countries have experienced a gradual occurrence of COVID-19.
Most importantly, the current publications often involve few participants [Citation18–20]. In Asia, a report from Japan described 5 patients with PNH undergoing complement inhibitor treatment, all of whom had contracted COVID-19. Among them, 4 had mild COVID-19, and 1 had moderate COVID-19. They all encountered new hemolytic events during infection [Citation21].
In this study, we included a relatively large number of patients with various disease types, some of whom were vaccinated or treated with various complement inhibitors (clinical trials), had concentrated outbreaks of infections during the winter of 2022–2023, and were newly infected with COVID-19.
Based on data from 131 cases, the infection rate of COVID-19 was 87.8%, which was similar to our assessment of the general population at the same time in China, although accurate reports have not yet been published. Findings from a South Korean study involving 40 patients and a Chinese study involving 3,715 patients with Omicron disease showed that fever remained the most prevalent symptom, followed by mild dry cough and sore throat [Citation22,Citation23]. In the present study, the most frequently reported symptoms were fever, sore throat, cough, phlegm, muscle and joint pain, fatigue, and headache. Unlike the symptoms from the initial COVID-19 strain [Citation24–26], those associated with the Omicron variant were much milder [Citation27–29]. In addition, the emerging literature concerning the outcome of Omicron, which appeared long after the initial COVID-19 outbreak, showed a substantial reduction in hospitalizations and deaths in the general population [Citation30–33]. In Shanghai, China, the number of severe Omicron infections in the general population was 4.43% between April 2022 and May 2022 [Citation34]. However, there are no data for China on the general population from 6 December 2022 to 10 April 2023. In the context of PNH, we observed a 15.7% incidence of severe infections, higher than that reported in the general population between April 2022 and May 2022.
We next analyzed the factors that could influence the infection rate, severity, and PNH hemolysis-related attacks during this pandemic period. Unlike most reports from other countries that have demonstrated that vaccination can reduce the risk of COVID-19 infection [Citation35,Citation36], we did not identify any factors that may protect patients from Omicron infection. One major difference is the type of vaccine used. Most vaccines from other countries are mRNA vaccines administered concurrently with viral exposure, and China mainly employs inactivated vaccines. Most individuals, including patients with PNH, were vaccinated long before 6 December 2022, and the country adhered to stringent COVID-19 control measures before 6 December 2022.
In addition, most of our patients were younger, whereas many reports from other countries focused on older populations. Variances in vaccine types, timing, age groups, management approaches, and disease status across different countries may explain the different protective effects of vaccinations against different viral strains at different times. However, reports on the protective role of complement inhibitors are scarce. Barcellini et al. found that using complement inhibitors does not decrease susceptibility to COVID-19 [Citation22], which is consistent with our findings.
Although these factors did not affect the infection rate, some affected the severity of infection. We found that vaccination reduced the incidence of severe COVID-19. Multiple studies have demonstrated that vaccination can significantly reduce the incidence of severe or critical diseases caused by COVID-19 [Citation35,Citation37,Citation38]. For instance, a few studies have shown that higher serum-positive detection rates of vaccines in patients with multiple myeloma and hematopoietic stem cell transplantation may be associated with better prevention [Citation39–42]. However, most of these reports were related to other hematological diseases. A previous study has shown that vaccination can achieve a good SARS-CoV-2 antibody response in patients with PNH [Citation43]. Recent findings have highlighted the importance of neutralizing antibody levels as reliable predictors of protection against symptomatic COVID-19 [Citation44–48].
Contrary to expectations, our study indicated that complement inhibitors do not substantially protect against infection severity. Numerous studies have confirmed the efficacy of complement inhibitors in reducing the severity of pneumonia and lowering the fatality rate in the general population [Citation49–52]. Other studies have shown that complement inhibitors are beneficial both in vivo and in external models of novel coronavirus infection and may help reduce the clinical severity of a novel coronavirus infection [Citation53,Citation54]. This disparity may be attributed to differences in dosage, combined medications, and types of complement inhibitors. Our study used crovalimab, iptacopan, and CAN106, whereas other studies used eculizumab or AMY-101. However, these effects of case reports/series mentioned above were not seen in clinical trials. Although patients treated with different complement inhibitors had similar infection rates, those treated with iptacopan seemed to have less severe cases. However, the difference was not statistically significant, probably because of the limited number of cases.
In our study, COVID-19 infection exacerbated hemolytic-related events, as evidenced by more severe hemolytic attacks in infected patients, particularly those with severe infections. Correspondingly, hemolytic-related laboratory parameters, such as HGB and LDH, were worse in infected patients, especially those with severe infections. COVID-19 is related to aggravated PNH symptoms; this has been shown by other investigators. Pike reported 4 cases of PNH infected with COVID-19, 3 of which had breakthrough hemolysis, and noted that infections increased the risk of breakthrough hemolysis in PNH due to increased complement system activation, overwhelming the complement blockade [Citation55]. Schüller and Cavallaro et al. also reported 2 cases of hemolysis and exacerbation of chronic anemia in patients with PNH due to COVID-19 infection [Citation56,Citation57]. Changes in ANC and platelet counts may stem from the infections themselves, as observed in other infectious diseases [Citation58,Citation59], or potentially from aggravated hemolysis.
Our data revealed that complement inhibitors could reduce hemolytic attacks. This finding is consistent with those reported in the literature. Kulasekararaj et al. reported that 4 COVID-19-infected patients with PNH with complement inhibitors experienced fewer hemolysis-related symptoms than their non-inhibited counterparts [Citation60]. Other small-sample studies have similarly indicated the beneficial role of complement inhibitors during COVID-19 infections [Citation6,Citation61]. Some studies indicated that complement inhibitors can reduce the inflammatory response caused by COVID-19 in both PNH and non-PNH cases [Citation51,Citation60].
Further analysis demonstrated that patients treated with a proximal complement inhibitor (iptacopan) had a significantly lower risk of developing BTH than those treated with a terminal complement inhibitor (p = 0.003). Research on iptacopan showed that this proximal complement inhibitor can effectively control intra- and extravascular hemolysis, leading to transfusion-free and normal HGB levels [Citation7,Citation62]. In addition, iptacopan had a very low BTH level during the experimental period, which is consistent with our results. However, patients with iptacopan had a statistical higher vaccination rate than those with C5 inhibitors (p = 0.014), meanwhile, there were 2 severe infections in the C5 complement inhibitor. Considering the factors above, as well as the limited number of cases, the conclusion ‘proximal complement inhibitors may reduce BTH events in the setting of COVID-19’ needs further studies to be evaluated.
In our study, only 1 patient died from the infection, resulting in a mortality rate of 0.87%, significantly lower than that observed in the early days of the COVID-19 pandemic. The patient had severe COVID-19 and did not receive vaccination or complement inhibitor treatment.
Conclusions
Although the retrospective nature of this study introduced some bias, the prospective data collection remained challenging. With a relatively large sample size of consecutive patients and a short timeframe for exposure to the Omicron-variant strain, we described the characteristics of patients with PNH under specific conditions. Furthermore, our study showed that vaccination can reduce the severity of COVID-19, whereas COVID-19 infection exacerbates hemolytic episodes. Complement inhibitors did not significantly influence infection severity and seemed to mitigate the aggravation of hemolytic-related events in patients with PNH.
Author contributions
Bing Han and Chen Yang were responsible for the initial planning and study design. Leyu Wang, Qinglin Hu, Yuan Yang, Miao Chen, and Chen Yang were responsible for data collection, extraction, and statistical analyses. Leyu Wang was responsible for the data interpretation and drafting of the manuscript. Bing Han and Chen Yang are guarantors and have full access to all data, including statistical reports and tables, and take full responsibility for the integrity of the data and the accuracy of the data analysis.
Acknowledgement
The funding agencies played no role in the study design, collection, analysis, and interpretation of data, writing of the report, or the decision to submit the article for publication. The authors have no financial or non-financial conflicts of interest to disclose.
This study was supported by the National Natural Science Foundation [82370121], Beijing Natural Science Foundation [7232109], National High-Level Hospital Clinical Research Funding [2022-PUMCH-C-026, 2022-PUMCH-D-002, and 2022-PUMCH-B-046], CAMS, and the Innovation Fund for Medical Sciences [CIFMS 2021-I2M-1-003].
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All the data are available in the main text. All the detailed metadata are available upon reasonable request from the corresponding author.
Additional information
Funding
References
- Brodsky RA. How I treat paroxysmal nocturnal hemoglobinuria. Blood. 2021;137(10):1–10. doi: 10.1182/blood.2019003812.
- Hill A, DeZern AE, Kinoshita T, et al. Paroxysmal nocturnal haemoglobinuria. Nat Rev Dis Primers. 2017;3(1):17028. doi: 10.1038/nrdp.2017.28.
- Luzzatto L. PNH phenotypes and their genesis. Br J Haematol. 2020;189(5):802–805. doi: 10.1111/bjh.16473.
- Kinoshita T, Fujita M. Biosynthesis of GPI-anchored proteins: special emphasis on GPI lipid remodeling. J Lipid Res. 2016;57(1):6–24. doi: 10.1194/jlr.R063313.
- Brodsky RA. Paroxysmal nocturnal hemoglobinuria. Blood. 2014;124(18):2804–2811. doi: 10.1182/blood-2014-02-522128.
- Röth A, Nishimura JI, Nagy Z, et al. The complement C5 inhibitor crovalimab in paroxysmal nocturnal hemoglobinuria. Blood. 2020;135(12):912–920. doi: 10.1182/blood.2019003399.
- Risitano AM, Röth A, Soret J, et al. Addition of iptacopan, an oral factor B inhibitor, to eculizumab in patients with paroxysmal nocturnal haemoglobinuria and active haemolysis: an open-label, single-arm, phase 2, proof-of-concept trial. Lancet Haematol. 2021;8(5):e344–e354. doi: 10.1016/S2352-3026(21)00028-4.
- Risitano AM, Marotta S, Ricci P, et al. Anti-complement treatment for paroxysmal nocturnal hemoglobinuria: time for proximal complement inhibition? A position paper from the SAAWP of the EBMT. Front Immunol. 2019;10:1157. doi: 10.3389/fimmu.2019.01157.
- WHO. WHO coronavirus disease (COVID-19) dashboard. COVID 19 Special Issue 2020; 10; 2021 [cited 2021 March 1]. Available from: https://covid19.who.int/.
- Parker C, Omine M, Richards S, International PNH Interest Group., et al. Diagnosis and management of paroxysmal nocturnal hemoglobinuria. Blood. 2005;106(12):3699–3709. doi: 10.1182/blood-2005-04-1717.
- Sutherland DR, Keeney M, Illingworth A. Practical guidelines for the high-sensitivity detection and monitoring of paroxysmal nocturnal hemoglobinuria clones by flow cytometry. Cytometry B Clin Cytom. 2012;82(4):195–208. doi: 10.1002/cyto.b.21023.
- Clinical management of COVID-19: living guideline. Geneva: World Health Organization; 2023.
- Yüce M, Filiztekin E, Özkaya KG. COVID-19 diagnosis -a review of current methods. Biosens Bioelectron. 2021;172:112752. doi: 10.1016/j.bios.2020.112752.
- Baskin JL, Pui CH, Reiss U, et al. Management of occlusion and thrombosis associated with long-term indwelling central venous catheters. Lancet. 2009;374(9684):159–169. doi: 10.1016/S0140-6736(09)60220-8.
- Brodsky RA, Peffault de Latour R, Rottinghaus ST, et al. Characterization of breakthrough hemolysis events observed in the phase 3 randomized studies of ravulizumab versus eculizumab in adults with paroxysmal nocturnal hemoglobinuria. Haematologica. 2021;106(1):230–237. doi: 10.3324/haematol.2019.236877.
- Yenerel MN, Sicre de Fontbrune F, Piatek C, et al. Phase 3 study of subcutaneous versus intravenous ravulizumab in eculizumab-experienced adult patients with PNH: primary analysis and 1-year follow-up. Adv Ther. 2023;40(1):211–232. doi: 10.1007/s12325-022-02339-3.
- Schrezenmeier H, Kulasekararaj A, Mitchell L, et al. One-year efficacy and safety of ravulizumab in adults with paroxysmal nocturnal hemoglobinuria naïve to complement inhibitor therapy: open-label extension of a randomized study. Ther Adv Hematol. 2020; 11:2040620720966137. doi: 10.1177/2040620720966137.
- Otieno SB, Altahan A, Kaweeta F, et al. Severe hemolysis in a COVID-19 patient with paroxysmal nocturnal hemoglobinuria. Case Rep Hematol. 2021;2021:6619177. doi: 10.1155/2021/6619177.
- Sokol J, Nehaj F, Mokan M, et al. COVID19 infection in a patient with paroxysmal nocturnal hemoglobinuria: a case report. Medicine. 2021;100(20):e25456. doi: 10.1097/MD.0000000000025456.
- Genthon A, Chiarabini T, Baylac P, et al. Severe COVID-19 infection in a patient with paroxysmal nocturnal hemoglobinuria on eculizumab therapy. Leuk Lymphoma. 2021;62(6):1502–1505. doi: 10.1080/10428194.2020.1869963.
- Shino M, Iizuka H, Fukushima H, et al. COVID-19 development during the treatment of paroxysmal nocturnal hemoglobinuria. Rinsho Ketsueki. 2023;64(3):224–229.
- Kim MK, Lee B, Choi YY, et al. Clinical characteristic of 40 patients infected with the SARS-CoV-2 omicron variant in Korea. J Korean Med Sci. 2022;37(3):e31. doi: 10.3346/jkms.2022.37.e31.
- Sha J, Meng C, Sun J, et al. Clinical and upper airway characteristics of 3715 patients with the omicron variant of SARS-Cov-2 in Changchun, China. J Infect Public Health. 2023;16(3):422–429. doi: 10.1016/j.jiph.2023.01.013.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5.
- Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994.
- Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of covid-19 in New York City. N Engl J Med. 2020;382(24):2372–2374. doi: 10.1056/NEJMc2010419.
- Fan Y, Li X, Zhang L, et al. SARS-CoV-2 omicron variant: recent progress and future perspectives. Signal Transduct Target Ther. 2022;7(1):141.
- Kumar S, Thambiraja TS, Karuppanan K, et al. Omicron and Delta variant of SARS-CoV-2: a comparative computational study of spike protein. J Med Virol. 2022;94(4):1641–1649. doi: 10.1002/jmv.27526.
- Tian D, Sun Y, Xu H, et al. The emergence and epidemic characteristics of the highly mutated SARS-CoV-2 omicron variant. J Med Virol. 2022;94(6):2376–2383. doi: 10.1002/jmv.27643.
- Jassat W, Karim S, Mudara C. Clinical severity of covid-19 patients admitted to hospitals in Gauteng, South Africa during the omicron-dominant fourth wave. Preprint at SSRN. Available from: https://ssrn.com/abstract=3996320. 2021).
- Iuliano AD, Brunkard JM, Boehmer TK, et al. Trends in disease severity and health care utilization during the early omicron variant period compared with previous SARS-CoV-2 high transmission periods – United States, December 2020–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(4):146–152. doi: 10.15585/mmwr.mm7104e4.
- Abdullah F, Myers J, Basu D, et al. Decreased severity of disease during the first global omicron variant covid-19 outbreak in a large hospital in Tshwane, South Africa. Int J Infect Dis. 2022;116:38–42. doi: 10.1016/j.ijid.2021.12.357.
- Ulloa AC, Buchan SA, Daneman N, et al. Estimates of SARS-CoV-2 omicron variant severity in Ontario, Canada. JAMA. 2022;327(13):1286–1288. doi: 10.1001/jama.2022.2274.
- Lu G, Zhang Y, Zhang H, et al. Geriatric risk and protective factors for serious COVID-19 outcomes among older adults in shanghai omicron wave. Emerg Microbes Infect. 2022;11(1):2045–2054. doi: 10.1080/22221751.2022.2109517.
- Tregoning JS, Flight KE, Higham SL, et al. Progress of the COVID-19 vaccine effort: viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat Rev Immunol. 2021;21(10):626–636. doi: 10.1038/s41577-021-00592-1.
- Knoll MD, Wonodi C. Oxford-AstraZeneca COVID-19 vaccine efficacy. Lancet. 2021;397(10269):72–74. doi: 10.1016/S0140-6736(20)32623-4.
- Graña C, Ghosn L, Evrenoglou T, et al. Efficacy and safety of COVID-19 vaccines. Cochrane Database Syst Rev. 2022;12(12):CD015477.
- Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384(15):1412–1423. doi: 10.1056/NEJMoa2101765.
- Cesaro S, Ljungman P, Mikulska M, et al. Recommendations for the management of COVID-19 in patients with haematological malignancies or haematopoietic cell transplantation, from the 2021 European Conference on Infections in Leukaemia (ECIL 9). Leukemia. 2023;37(9):1933–1938. doi: 10.1038/s41375-022-01578-1.
- Riccardi N, Falcone M, Yahav D. Vaccination for SARS-CoV-2 in hematological patients. Acta Haematol. 2022;145(3):257–266. doi: 10.1159/000523753.
- Redjoul R, Le Bouter A, Beckerich F, et al. Antibody response after second BNT162b2 dose in allogeneic HSCT recipients. Lancet. 2021;398(10297):298–299. doi: 10.1016/S0140-6736(21)01594-4.
- Yamamoto S, Maeda K, Matsuda K, et al. Coronavirus disease 2019 (COVID-19) breakthrough infection and post-vaccination neutralizing antibodies among healthcare workers in a referral hospital in Tokyo: a case-control matching study. Clin Infect Dis. 2022;75(1):e683–e691. doi: 10.1093/cid/ciab1048.
- Pike A, McKinley C, Forrest B, et al. COVID-19 vaccination antibody responses in patients with aplastic anaemia and paroxysmal nocturnal haemoglobinuria. Lancet Haematol. 2022;9(8):e553–e556. doi: 10.1016/S2352-3026(22)00183-1.
- Jin P, Li J, Pan H, et al. Immunological surrogate endpoints of COVID-2019 vaccines: the evidence we have versus the evidence we need. Signal Transduct Target Ther. 2021;6(1):48. doi: 10.1038/s41392-021-00481-y.
- Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–1211. doi: 10.1038/s41591-021-01377-8.
- Earle KA, Ambrosino DM, Fiore-Gartland A, et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine. 2021;39(32):4423–4428. doi: 10.1016/j.vaccine.2021.05.063.
- Garcia-Beltran WF, Lam EC, Astudillo MG, et al. COVID-19-neutralizing antibodies predict disease severity and survival. Cell. 2021;184(2):476–488.e11. doi: 10.1016/j.cell.2020.12.015.
- Lee ARYB, Wong SY, Chai LYA, et al. Efficacy of covid-19 vaccines in immunocompromised patients: systematic review and meta-analysis. BMJ. 2022;376:e068632. doi: 10.1136/bmj-2021-068632.
- Mastellos DC, Pires da Silva BGP, Fonseca BAL, et al. Complement C3 vs C5 inhibition in severe COVID-19: early clinical findings reveal differential biological efficacy. Clin Immunol. 2020;220:108598. doi: 10.1016/j.clim.2020.108598.
- Mastaglio S, Ruggeri A, Risitano AM, et al. The first case of COVID-19 treated with the complement C3 inhibitor AMY-101. Clin Immunol. 2020;215:108450. doi: 10.1016/j.clim.2020.108450.
- Diurno F, Numis FG, Porta G, et al. Eculizumab treatment in patients with COVID-19: preliminary results from real life ASL Napoli 2 Nord experience. Eur Rev Med Pharmacol Sci. 2020;24(7):4040–4047.
- Giudice V, Pagliano P, Vatrella A, et al. Combination of ruxolitinib and eculizumab for treatment of severe SARS-CoV-2-related acute respiratory distress syndrome: a controlled study. Front Pharmacol. 2020;11:857. doi: 10.3389/fphar.2020.00857.
- Peffault de Latour R, Bergeron A, Lengline E, et al. Complement C5 inhibition in patients with COVID-19 – a promising target? Haematologica. 2020;105(12):2847–2850.
- Yu J, Yuan X, Chen H, et al. Direct activation of the alternative complement pathway by SARS-CoV-2 spike proteins is blocked by factor D inhibition. Blood. 2020;136(18):2080–2089. doi: 10.1182/blood.2020008248.
- Pike A, Muus P, Munir T, et al. COVID-19 infection in patients on anti-complement therapy: the leeds national paroxysmal nocturnal haemoglobinuria service experience. Br J Haematol. 2020;191(1):e1–e4. doi: 10.1111/bjh.17097.
- Schüller H, Klein F, Lübbert M, et al. Hemolytic crisis in a patient treated with eculizumab for paroxysmal nocturnal hemoglobinuria possibly triggered by SARS-CoV-2 (COVID-19): a case report. Ann Hematol. 2021;100(3):841–842. doi: 10.1007/s00277-020-04318-6.
- Cavallaro F, Pasquini MC, Giannotta JA, et al. Breakthrough haemolysis in paroxysmal nocturnal haemoglobinuria after COVID-19 infection and COVID vaccination: what is worse? Blood Transfus. 2022;20(5):437–440.
- Borges L, Pithon-Curi TC, Curi R, et al. COVID-19 and neutrophils: the relationship between hyperinflammation and neutrophil extracellular traps. Mediators Inflamm. 2020;2020:8829674–8829677. doi: 10.1155/2020/8829674.
- Liao D, Zhou F, Luo L, et al. Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID-19: a retrospective cohort study. Lancet Haematol. 2020;7(9):e671–e678. doi: 10.1016/S2352-3026(20)30217-9.
- Kulasekararaj AG, Lazana I, Large J, et al. Terminal complement inhibition dampens the inflammation during COVID-19. Br J Haematol. 2020;190(3):e141–e143. doi: 10.1111/bjh.16916.
- Hillmen P, Szer J, Weitz I, et al. Pegcetacoplan versus eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2021;384(11):1028–1037. doi: 10.1056/NEJMoa2029073.
- Jang JH, Wong L, Ko BS, et al. Iptacopan monotherapy in patients with paroxysmal nocturnal hemoglobinuria: a 2-cohort open-label proof-of-concept study. Blood Adv. 2022;6(15):4450–4460. doi: 10.1182/bloodadvances.2022006960.
