Abstract
Objectives
Tracheobronchial Talaromyces marneffei (T. marneffei) infections among non-HIV-infected patients are rare. To improve understanding, we analysed the clinical features, immune mechanisms, treatment, and prognosis.
Methods
Data on hospitalized patients with tracheobronchial T. marneffei infections from September 2013 to May 2022 were collected. The clinical and imaging features were analysed.
Results
Nineteen patients were enrolled, with a median age of 52 years (45–62 years). The most common symptoms were cough, expectoration, fever, weight loss, and anaemia. The total white blood cell and neutrophil counts, erythrocyte sedimentation rate, C-reactive protein, procalcitonin and globulin were increased, and the serum albumin levels were decreased. Chest CT manifestations included patchy shadows, masses, obstructive atelectasis, cavities, pleural effusion, and hilar and mediastinal lymphadenopathy. The fibreoptic bronchoscopy findings included masses, polyps or nodules with mucosal oedema, hypertrophic bulges, lumen stenosis or obstruction, and purulent secretions. T. marneffei infection was confirmed in 10 patients by positive culture, in five by both culture and metagenomic next-generation sequencing (mNGS), in two by mNGS, in one by culture and pathology and in 1 by histopathology. BALF (15/19, 78.9%) had the highest culture positive rate, followed by sputum (3/19), bronchial mucosa (1/1), lung biopsy (1/2); 36.8% of the patients were coinfected with other pathogens. For induction therapy, 7, 6, 2, and 4 patients received voriconazole, amphotericin B, voriconazole combined with amphotericin B, and fluconazole therapy, respectively, and 26.3% received treatment combined with nebulization and/or administration of amphotericin B under fibreoptic bronchoscopy. Four patients were treated for underlying diseases or coinfection, 31.6% were cured, 42.1% improved, and 26.3% died.
Conclusions
T. marneffei infection is common in the tracheobronchial airway tissue or secretions, and bronchoscopy has important diagnostic and treatment value. Antifungal therapy, including systemic therapy, involves triazoles and amphotericin administration, and aerosol inhalation and administration of amphotericin B under bronchoscopy are important.
KEY MESSAGES
T. marneffei infection involving the tracheobronchial region in airway tissue or secretions is high, and bronchoscopy has important value in diagnosing and treating these patients
The use of triazoles and amphotericin and the aerosol inhalation and instillation of amphotericin B under bronchoscopy are essential to antifungal therapy.
Introduction
Talaromycosis marneffei is an invasive fungal disease caused by Talaromyces marneffei (T. marneffei) infection. In Southeast Asia, T. marneffei is the third most common presenting illness in AIDS patients. Talaromycosis marneffei is categorized as either localized or disseminated. T. marneffei disseminates via the blood or the lymphatic system throughout the body, affecting the lung, liver, spleen, lymph nodes, skin, and other tissues and organs. The respiratory system is the earliest and most commonly involved system, and respiratory involvement accounts for 66.7% of cases [Citation1]. It is suggested that the respiratory route may be one of the transmission routes of T. marneffei infection in humans. However, clinical reports of tracheobronchial T. marneffei infections are very rare. Moreover, T. Marneffei infection with respiratory system lesions is often misdiagnosed as pulmonary tuberculosis and lung cancer. Long-term anti-tuberculosis treatment leads to refractory pneumonia and systemic spread. The clinical manifestations we observed in a patient whose case we reported for the first time were very similar to those observed in patients with bronchial tuberculosis, bronchial tumours, and other fungal infections. Due to delayed diagnosis and treatment, extensive lysis and destruction of the airway result in fatal asphyxia [Citation2]. Therefore, it is very important for clinicians to be familiar with tracheobronchial Talaromycosis marneffei to improve the rate of early recognition, facilitate the initiation of treatment, and improve patients’ overall quality of life.
Materials and methods
Study population
We retrospectively analysed patients with tracheobronchial T. marneffei infections between September 2013 and May 2022 at The First Affiliated Hospital of Guangxi Medical University. The medical records included demographic information (sex and age), clinical characteristics, laboratory findings, and clinical outcomes, and data on these variables were collected.
According to the strict regulations on a retrospective study of the Ethics Committee of the first affiliated Hospital of Guangxi Medical University, written informed consent was obtained from all patients (signed by the patient or their immediate family) prior to the study, and the ethic approval number was 2022-E343-01 ().
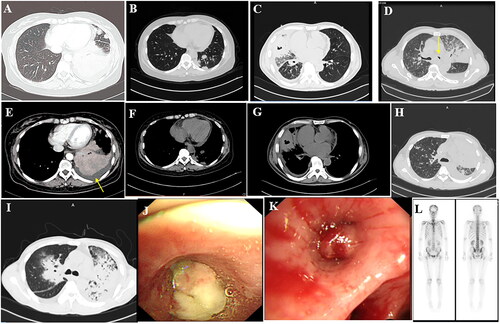
Figure 1. (A,E) Shadow of the left lower lobe, left pleural effusion; (B,F) Nar shadow of the left lower lobe (C,G). The middle lobe of the right lung is incomplete, with internal bronchial inflation and cavity, and right pleural effusion. (D) Irregular mass shadow of the left superior lobe and narrow left main bronchus. (H) Diffuse density increase shadow in the left lung and inflatable bronchial shadow. (I) large solid lesions, even on the left, with bronchial inflation, (J) The left main bronchial terminal mass completely blocked the official lumen, indicating attachment of necromass. (K) The opening of the right middle lobe is completely blocked. (L) The skull is concentrated in multiple parts, including the 12th thoracic vertebra, the first lumbar spine, multiple heel ribs, and a small tablet imaging agent with concentrated poly shadow.
Inclusion and exclusion criteria
The inclusion criteria were as follows: (i) HIV-negative status and (ii) Talaromycosis marneffei patients with airway lesions under bronchoscopy and respiratory specimens from which T. marneffei was isolated.
The exclusion criteria were as follows: (i) Talaromycosis marneffei without respiratory involvement, (ii) Talaromycosis marneffei with clinical manifestations of respiratory but no evidence of positivity for T. marneffei infection in respiratory specimens, or (iii) patients with lung tissue involvement but no tracheobronchial involvement by bronchoscopy.
Methods used to diagnose T. marneffei infection
To diagnose T. marneffei infection, microbiological or pathological findings identified from clinical specimens consistent with any of the following manifestations were utilized: (a) isolation of pathogens from culture; (b) visible detection of fungi (intracellular yeast-like or sausage-shaped cells measuring 2–3 mm in diameter with transverse septa.) by microscopy after Periodic Acid-Schiff or Wright staining. Finally, as a molecular biological method for confirming the diagnosis, metagenomic next-generation sequencing (mNGS) was applied for the early diagnosis of T. marneffei infection. When T. marneffei infection was further confirmed by the traditional methods mentioned above, the diagnosis was confirmed. When only the mNGS analysis of tissue samples confirmed the presence of T. marneffei, other pathogens were excluded and antifungal treatment was effective, these patients were also considered to have a confirmed diagnosis of Talaromycosis marneffei infection in the analysis.
Anti-IFN-γ autoantibody (AIGA) assay
Serum samples were obtained under sterile conditions before the patient received antimicrobial therapy and during the active stage of the infection. Serum was separated by centrifugation at 3000 rpm for 10 min, and the samples were diluted 16-fold. The detection of AIGA in the serum was performed using an enzyme-linked immunosorbent assay kit (Cloud-Clone Corp., Wuhan, China) according to the manufacturer’s protocols. The concentration of anti–IFN-γ in the sample was then determined by comparing the optical density of the sample to the standard curve [Citation3].
Results
Demographic data and clinical characteristics
During the 9-year study period, 210 patients were diagnosed with T. marneffei infections, and 126 patients had respiratory system infections. In addition, bronchoscopy was performed in 59 patients; finally, 19 patients with tracheobronchial T. marneffei infections were selected. All 19 patients were born in Guangxi Province and stayed in T. marneffei endemic areas in southern China. The study population included 11 men and 8 women with a median age of 52 years (range 45–62 years). Their occupations included being a farmer (n = 14), retired (n = 3), self-employed (n = 1), and unemployed (n = 1). Comorbidities included AIGA-associated immunodeficiency (n = 11), type 2 diabetes mellitus (n = 2), pulmonary tuberculosis (n = 2), breast cancer, Sweet’s syndrome, erythema nodosum and ankylosing spondylitis (n = 2). Seven patients were misdiagnosed with breast cancer, 4 with pulmonary tuberculosis, and 2 with bacterial pneumonia. The time from the onset of clinical symptoms to the diagnosis of T. marneffei infection was 55–224 d (median: 133 d). Common clinical features of T. marneffei infection included cough, expectoration, fever, weight loss, and anaemia, followed by chest pain, fatigue, shortness of breath, rash, anorexia, and bone pain/arthralgia haemoptysis ().
Table 1. Characteristics of the 19 patients with tracheobronchial T. marneffei infection.
Laboratory examination results
Assessments of complete blood count results revealed increased levels of white blood cells and neutrophils in all patients but decreased haemoglobin concentrations and platelet counts in 15 patients and 10 patients, respectively. Serum biochemical analysis revealed that serum albumin concentrations were below the normal range in all patients. The C-reactive protein concentration, erythrocyte sedimentation rate, and procalcitonin (PCT) level were increased in all patients. The serum GM test was performed in 19 patients, but positivity was observed in only 2 patients. The G test was performed in 18 patients, and only one patient had elevated levels (positive: > 100 pg/mL). Moreover, 7 patients exhibited positivity in the bronchoalveolar lavage fluid (BALF) GM test, and 4 patients exhibited positivity in the G-test. Ten patients showed increased globulin levels, and serum IgG levels were increased in 12 patients. Serum IgM levels were increased in 7 patients and decreased in 3 patients. The CD4 and CD8 lymphocyte counts were determined in 16 patients by flow cytometry and were decreased in two patients ().
Table 2. Laboratory work-up results for tracheobronchial T. marneffei infection.
Imaging examination results
Chest CT indicated that all patients had different pulmonary lesions. The most common features of CT images were patchy exudates (n = 16, 84.2%), fibrous cords (n = 12, 63.2%), pleural thickening (n = 7, 36.8%), consolidation and nodular shadows (n = 6, 31.6%), intrapulmonary mass shadow (n = 5, 26.3%), obstructive pneumonia (n = 5, 26.3%), and a cavity (n = 3, 15.8%). Moreover, there were 5 cases of tracheobronchial stenosis, 3 cases of bronchial occlusion, and 1 case of bronchial nodules. There were 14 cases of pleural effusion, 14 cases of hilar and mediastinal lymphadenopathy, and 3 cases of pericardial effusions ().
Emission CT (ECT) was performed in 11 patients and revealed significantly increased uptake in multiple bones. Multiple instances of abnormal imaging agent concentrations or bone destruction were observed in the skull, clavicle, sternum, femur, humerus, thoracic spine, lumbar spine, pelvis, ribs, ilium, shoulder joint, sacroiliac joint, knee joint, and ankle joint ().
Endoscopy results
All patients underwent fibreoptic bronchoscopy. There were 13 neoplasms (5 masses, 7 polyps/nodules, and 1 bulge), 6 cases of mucosal oedema/hypertrophy, 4 cases of mucosal unevenness, 8 cases of purulent secretions, and 8 cases of bronchial stenosis/occlusion. The lesions involved were as follows: 1 in the trachea; 19 in the left bronchus, including 8 in the left main bronchus, 5 in the left upper lobe bronchus, and 6 in the left lower lobe bronchus; and 18 in the right bronchus, including 2 in the right main bronchus, 2 in the right upper lobe bronchus, 10 in the right middle lobe bronchus, and 4 in the right lower lobe bronchus. In addition, 12 patients exhibited involvement of 2 or more regions ().
Fungal culture and histopathology results
Ten cases of tracheobronchial T. marneffei infection were identified via positive specimen culture. One case was identified by histopathological examination and culture, 5 cases were confirmed by culture combined with mNGS, and 2 cases were confirmed by mNGS. In addition, seven patients were diagnosed with T. marneffei infection by histopathology of the bronchial mucosa. Before and after T. marneffei infections, 7 patients (36.8%) were also infected with other opportunistic pathogens, including NTM (n = 3; 15.8%), Salmonella spp. (n = 2; 10.5%), Mycobacterium tuberculosis (n = 1; 5.3%), and Staphylococcus haemolytic (n = 1; 5.3%).
Treatments and outcomes
For the initial induction therapy, 7 patients were initially treated with voriconazole, 6 patients were initially treated with amphotericin B, and 2 patients were initially treated with voriconazole combined with amphotericin B. All patients were sequentially treated with voriconazole 0.2 g q12 or itraconazole 0.2 g BID orally. One patient was initially treated with fluconazole, followed by itraconazole, and was finally cured. Three patients were initially treated with fluconazole, but the curative effect was not good. They were switched to voriconazole 0.2 g q12 and amphotericin B 0.6 mg/kg/d, and their symptoms improved. The treatment in 5 patients also involved nebulization (25 mg BID) and/or instillation of amphotericin B under fibreoptic bronchoscopy. The total treatment time was 12 months (8, 15 months), and 2 patients continued to take antifungal drugs for more than 3 years. Two patients received active antifungal treatment immediately after diagnosis but eventually died of multiple organ dysfunction syndrome (MODS), while 3 patients had good initial treatment effects, and the total antifungal course of treatment was more than 10 months. Three patients were treated with anti-NTM medication. According to the drug susceptibility results, 3–4 drugs, including moxifloxacin, clarithromycin, ethambutol, imipenem, and linezolid, were combined with anti-infective treatment, and the total course of treatment was not less than 12 months. One patient received paclitaxel combined with carboplatin chemotherapy for underlying lung cancer. To date, 6 patients were cured, 8 patients improved, and 5 patients (26.3%) died.
Discussion
Talaromycosis marneffei is a severe invasive disseminated fungal disease caused by T. marneffei, which is mainly prevalent in Southeast Asian countries and Southern China [Citation4]. Inhaling T. marneffei spores from the environment and their passage through the respiratory tract may be the key mode of transmission. It has also been clinically observed that HIV-negative Talaromycosis marneffei patients with immunodeficiency are most likely to exhibit respiratory system involvement, which occurs earliest in these patients, and the highest rate of T. marneffei isolation is from respiratory and lung tissue specimens [Citation1]. However, unlike pulmonary involvement, which is easy to diagnose, tracheobronchial involvement is difficult to detect clinically, clinical reports are extremely rare, and bronchoscopy can be performed only when dyspnoea or fatal asphyxia occurs. Therefore, the systematic summary and analysis of its clinical characteristics reported here have clinical importance.
The 19 patients with tracheobronchial Talaromycosis marneffei included in this study were all HIV-negative patients, and 14 were farmers who had been engaged in agricultural activities for a long time. Common clinical features included fever, lymphadenopathy, cough, and dyspnoea. Tracheobronchial Talaromycosis marneffei is often misdiagnosed as pulmonary tuberculosis or lung cancer. The misdiagnosis rate is as high as 68.4% because misdiagnosis leads to inappropriate treatment and to a prolonged course of disease. In this study, the time from onset to diagnosis was as long as 133 d. According to the literature, the detection rate of T. marneffei infection of HIV-negative patients, the time of diagnosis and timely treatment are the main features underlying the clinical prognosis. The detection rate of pathogens associated with Talaromycosis marneffei in AIDS patients is high in clinical specimens, and these infections are easy to diagnose. After being diagnosed with AIDS, these patients need to be transferred to a special AIDS hospital for timely treatment according to China’s epidemic prevention requirements. Therefore, this study did not include HIV-positive hosts. Data show that AIGA-related immunodeficiency, autoimmune diseases, application of glucocorticoids and/or immunosuppressive agents, and malignant tumours are the most common immune disorders in HIV-negative T. marneffei-infected patients [Citation5]. The most common is an anti-cytokine disease, such as AIGA-related immunodeficiency, accounting for 25.1% [Citation5]. Studies have shown that the AIGA titre is an independent risk factor for T. marneffei infection and recurrence as well as multiple infections [Citation5]. Immunodeficiency caused by AIGA is an emerging adult-onset immunodeficiency syndrome first described in 2004 [Citation6]. INF-γ plays a very important role in the body’s defence against intracellular pathogen infection. Increased antibody titres can inhibit the signal transduction of INF-γ and the production of TNF-α and IL-12, resulting in severely impaired Th1 responses. There is also increased susceptibility of the body to pathogens, especially intracellular infectious pathogens, such as T. marneffei, NTM, nontyphoid Salmonella, Burkholderia cepacia, varicella-zoster virus, cytomegalovirus [Citation7]. Studies have demonstrated that autoantibodies against IFN-γ are the main aetiology of T. marneffei infections in HIV-negative individuals, in which the risk HLA–class II alleles HLA-DRB1*16:02 and -DQB1*05:02 are highly prevalent [Citation8] There were 11 patients (57.9%) with increased AIGA levels in this group. Therefore, autoantibodies against IFN-γ may predispose these patients to T. marneffei infection [Citation9].
The 19 Talaromycosis marneffei patients in this study showed prominent clinical symptoms of respiratory system involvement, such as cough, expectoration, chest tightness, shortness of breath, and lung shadows, accompanied by systemic inflammatory responses that were manifested as fever, anaemia, weight loss, hypoalbuminemia, significantly increased levels of WBCs and neutrophils, elevated platelet counts, and varying degrees of increased anaemia and levels of the inflammatory indicators ESR, CRP, and PCT. The rates of positivity for G and GM antigens in bronchoalveolar lavage fluid were higher than those in peripheral blood. The pathological findings in sites of airway involvement included purulent changes dominated by neutrophil infiltration, which was accompanied by the formation of chronic granulomas. In this study, all the affected tissue specimens, including blood, lymph node, and skin tissue samples, obtained from the patients were sent to be examined for aetiology. Respiratory tract specimens, including mucosal tissue and bronchoalveolar lavage fluid, had the highest rate of positivity for T. marneffei isolation, and the diagnosis methods included routine culture and pathological tissue assessments. MNGS examination was performed on 7 specimens. The mNGS detection results in 6 patients were consistent with the results of conventional detection methods, but the diagnosis time was advanced by more than 1 week. In addition, 2 patients with T. marneffei infection had confirmed diagnoses only with mNGS but exhibited negativity in conventional culture, suggesting that mNGS is a sensitive detection method. In addition, 3 patients whose infections were combined NTM infections, especially slow-growing NTM infections, were identified using mNGS, which indicates that the use of mNGS can reduce the chance of early missed diagnosis. The mNGS assessment has obvious advantages for immunosuppressed hosts, those clinically suspected to exhibit multiple infections, and those with intracellular bacterial infections with a low rate of positivity in routine culture including T. marneffei [Citation10]. For HIV-negative Talaromycosis hosts, 94.8% of patients have immunodeficiency caused by high titres of AIGA [Citation8] ,so the possibility of infection with other opportunistic pathogens is high. Bronchoscopy is recommended for all patients with Talaromycosis with respiratory symptoms, with or without abnormal lung manifestations. In addition to obtaining an early understanding of whether the respiratory tract is involved, the obtained respiratory tract specimens can be evaluated as soon as possible, and corresponding treatment plans can be formulated. All patients in this study showed diverse results in assessments of abnormal chest imaging findings, including multiple patchy densities or consolidation shadows; intrapulmonary mass-like lesions or cavities; mediastinal and/or hilar lymph node enlargement; ground glass opacity; diffuse or miliary nodules; and pleural or pericardial effusion. During bronchoscopy, nodules, masses, and new organisms were observed in the involved airways, which could block the bronchial lumen or cause lumen stenosis, accompanied by mucosal congestion, hypertrophy, and purulent secretions in the airways. Rare tumours or mucosal erosions, ulcers, and other features were also observed. T. marneffei infections can involve the trachea, main bronchi, lobar bronchi, and segmental bronchi, with the bronchi being more commonly involved than the trachea. These microscopic manifestations are similar to those of bronchial lung cancer, bronchial aspergillosis, and tracheal tuberculosis, which are difficult to differentiate under the microscope. Thus, T. marneffei infections must be diagnosed by histopathology and pathogenic bacterial examination. If malignant tumour cells are found, T. marneffei infection may be diagnosed as a tumour, but if a chronic granuloma is identified, the disease may be tuberculosis or may be due to an Aspergillus infection, an NTM infection, a T. marneffei infection, or other infections. Special staining, such as acid-fast staining, PAS staining, and D-PAS staining, as well as molecular biotechnology approaches, such as culture and mNGS, are used to detect and identify pathogens. We found T. marneffei in lung cancer tissue in 1 patient in this group, and T. marneffei was found in the bronchoalveolar lavage fluid, blood, and bronchial mucosal tissue of 7 patients. It is also an opportunistic infection often associated with other intracellular pathogens.
For disseminated Talaromycosis marneffei, antifungal therapy should be based on systemic therapy. In vitro experiments show that yeast-phase T. marneffei has high sensitivity to amphotericin B, posaconazole, voriconazole, and itraconazole; it is also sensitive to fluconazole to a certain extent. However, during clinical treatment, drug resistance can be induced [Citation11]. At present, there is no standard treatment for Talaromycosis in HIV-negative hosts. Clinical treatment guidelines often refer to the treatment of HIV-positive Talaromycosis patients and include the intravenous infusion of amphotericin B (0.6 mg/kg d) followed by oral itraconazole (400 mg/d) maintenance therapy [Citation12]. Voriconazole is a second-generation triazole antifungal drug with a definite effect on T. marneffei infections. It can be the first-choice treatment or used in patients who cannot tolerate amphotericin B [Citation13]. The patients in this group were treated with amphotericin B, itraconazole, and voriconazole for antifungal therapy. If the initial treatment with fluconazole was not effective, voriconazole or amphotericin B was administered, which exhibited satisfactory clinical efficacy. The total treatment time in most of the patients was more than 1 year; 2 patients were treated with antifungal therapy for more than 3 years, and they are still being monitored in follow up. We reported a case of T. marneffei infection involving the main tracheal structure that was treated with only intravenous antifungal drugs. Although the effect was obvious in some regions, such as the lungs, airway cartilage and areas of tube wall destruction, pathologies including airway collapse, asphyxia, and other serious complications were observed [Citation2]. It has been suggested that the use of systemic antifungal therapy alone is not sufficient in Talaromycosis marneffei patients with tracheobronchial involvement. Therefore, we recommend the use of nebulized inhalation or intratracheal instillation of amphotericin B in the treatment of invasive pulmonary aspergillosis [Citation14]. In our study, 5 patients were treated with the combined use of intravenous administration of effective antifungal drugs and local nebulized inhalation of amphotericin B (25 mg BID for 1 month); this treatment strategy was associated with the rapid absorption of the drug into the lung, trachea and bronchial lesions and no adverse reactions. The indications for nebulization therapy include Talaromycosis marneffei patients with tracheal, bronchial or lung tissue involvement confirmed by imaging or bronchoscopy. Combined local therapy is particularly important, especially when the disease is complicated with obstructive atelectasis, tracheal stenosis, and other complications. When a combination of infections is identified that includes opportunistic nonfungal infections, it is necessary to choose the appropriate treatment plan for the different pathogens involved. In addition, treatment of coexisting basic diseases, such as lung cancer, should also be included.
Conclusions
In short, T. marneffei involvement of the airway should be considered an important clinical feature of Talaromycosis marneffei. However, the clinical understanding is not sufficient, so missed diagnoses are rarely reported. Early bronchoscopy has important clinical value for the early diagnosis and treatment of T. marneffei. Nebulized inhaled amphotericin B and/or bronchoscopy drops may have better efficacy.
Authors contributions
MP, GF and FZ designed the study and analysed the data. FL, WZ, YQ, JD and XC contributed to data collection. JZ contributed to the study concept and design, data curation, data visualization, writing-review, and supervision. All authors contributed to data analysis and the drafting or revising of the article and gave their final approval of the version to be published, and all authors agreed to be accountable for all aspects of the work.
Supplemental Material
Download MS Word (17.4 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Additional information
Funding
References
- Qiu Y, Zhang JQ, Pan ML, et al. Determinants of prognosis in Talaromyces marneffei infections with respiratory system lesions. Chin Med J. 2019;132(16):1–15. doi: 10.1097/CM9.0000000000000345.
- Qiu Y, Zhang J, Liu G, et al. A case of Penicillium marneffei infection involving the main tracheal structure. BMC Infect Dis. 2014;14(1):242. doi: 10.1186/1471-2334-14-242.
- Zeng W, Qiu Y, Tang S, et al. Characterization of anti-Interferon-γ antibodies in HIV-negative patients infected with disseminated Talaromyces marneffei and cryptococcosis. Open Forum Infect Dis. 2019;6(10):ofz208. Oct 11
- Vanittanakom N, Cooper CR, Fisher MC, et al. Penicillium marneffei infection and recent advances in the epidemiology and molecular biology aspects. Clin Microbiol Rev. 2006;19(1):95–110. doi: 10.1128/CMR.19.1.95-110.2006.
- Qiu Y, Feng X, Zhang J, et al. Immunodeficiency disease spectrum in HIV-negative individuals with talaromycosis. J Clin Immunol. 2021;41(1):221–223. doi: 10.1007/s10875-020-00869-5.
- Chan JF, Lau SK, Woo PC, et al. Talaromyces (penicillium) marneffei infection in non-HIV-infected patients. Emerg Microbes Infect. 2016;5(3):e19–9. doi: 10.1038/emi.2016.18.
- Chi CY, Lin CH, Ku CL, et al. Clinical manifestations, course, and outcome of patients with neutralizing anti-interferon-γ autoantibodies and disseminated nontuberculous mycobacterial infections. Medicine. 2016;95(25):e3927. Jundoi: 10.1097/MD.0000000000003927.
- Guo J, Ning XQ, Cao CW, et al. Anti-IFN-γ autoantibodies underlie disseminated Talaromyces marneffei infections. J Exp Med. 2020;217(12):e20190502. Dec 7
- Tang BS, Chan JF, Yuen KY, et al. Disseminated penicilliosis, recurrent bacteremic nontyphoidal salmonellosis, and burkholderiosis associated with acquired immunodeficiency due to autoantibody against gamma interferon. Clin Vaccine Immunol. 2010;17(7):1132–1138. Juldoi: 10.1128/CVI.00053-10.
- Simner PJ, Miller S, Carroll KC. Understanding the promises and hurdles of metagenomic next-generation sequencing as a diagnostic tool for infectious diseases. Clin Infect Dis. 2018;66(5):778–788. doi: 10.1093/cid/cix881.
- Lei HL, Li LH, Chen WS, et al. Susceptibility profile of echinocandins, azoles and amphotericin B against yeast phase of Talaromyces marneffei isolated from HIV-infected patients in Guangdong, China. Eur J Clin Microbiol Infect Dis. 2018;37(6):1099–1102. doi: 10.1007/s10096-018-3222-x.
- Kaplan JE, Benson C, Masur H, et al. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2009;58(RR-4):1–207.
- Huang W, Li T, Zhou C, et al. Voriconazole Versus Amphotericin B as Induction Therapy for Talaromycosis in HIV/AIDS Patients: A Retrospective Study. Mycopathologia. 2021 May;186(2):269-276. doi: 10.1007/s11046-021-00533-5.
- Rijnders BJ, Cornelissen JJ, de Marie S, et al. Aerosolized liposomal amphotericin B for the prevention of invasive pulmonary aspergillosis during prolonged neutropenia: a randomized, placebo-controlled trial. Clin Infect Dis. 2008;46(9):1401–1408. doi: 10.1086/586739.
