Abstract
Objective
The optimal cycle of neoadjuvant chemotherapy (NAC) for muscle-invasive bladder cancer (MIBC) remains controversial. This study aimed to compare the efficacy of three and four cycles of NAC in the treatment of MIBC through a systematic review and meta-analysis of the literature.
Materials and Methods
Relevant studies were systematically collected and reviewed in PubMed, Medline, Embase, Web of Science Databases, and the Cochrane Library. Relative ratios (RRs), Hazard ratios (HRs) and their 95% confidence intervals (CIs) were used to estimate outcome measures. Studies comparing the pathological response and prognosis of three versus four cycles of NAC for MIBC were included.
Results
Five studies were included in this meta-analysis, including 2190 patients, of whom 1016 underwent three cycles of NAC and 1174 underwent four cycles of NAC. All studies were retrospective cohort studies. We found that 4 cycles of NAC had significantly better cancer-specific survival than 3 cycles (HR = 1.31, 95%CI,1.03–1.67, p = 0.029). There was no significant difference in overall survival between patients who received 3 and 4 cycles of chemotherapy (HR = 1.18, 95%CI = 0.83–1.69, p = 0.345). Similarly, no significant difference was observed in pathological objective response (RR = 0.95, 95%CI= 0.81–1.11, p = 0.515) and complete response rates (RR = 0.87, 95%CI = 0.69–1.11, p = 0.256) in MIBC after 3 or 4 cycles of NAC.
Conclusions
Three and four cycles of NAC had similar pathological responses and prognosis for MIBC, although the cancer-specific survival rate of four cycles was better than that of three cycles.
KEY MESSAGES:
The pathological response rate and overall survival of three and four cycles of neoadjuvant chemotherapy for muscle-invasive bladder cancer were similar.
Four cycles of neoadjuvant chemotherapy may improve the cancer-specific survival of patients with muscle-invasive bladder cancer
It is reasonable and feasible for clinicians to use three or four cycles of neoadjuvant chemotherapy.
1. Introduction
Bladder cancer is widely recognized as the 11th most prevalent cancer globally, with approximately 550,000 new cases diagnosed annually [Citation1,Citation2]. In the United States, bladder cancer is the fourth most common cancer in men and accounts for more than 80000 cancer cases each year [Citation3]. A Initially, around 30% of patients receive a diagnosis of muscle-invasive bladder cancer (MIBC), characterized by a clinical stage of T2 or higher, indicative of its high malignancy and propensity for distant metastasis [Citation4]. Standard treatments for MIBC encompass radical cystectomy and pelvic lymph node dissection [Citation5]. Nonetheless, approximately 50% of patients experience recurrent disease and metastasis within two years following surgery [Citation6]. Platinum-based neoadjuvant chemotherapy (NAC) has shown efficacy in reducing micrometastatic disease occurrence and improving patient survival rates [Citation7].
A meta-analysis demonstrated that cisplatin-based NAC was associated with a 16% lower overall risk of death compared to locoregional therapy alone [Citation8]. Currently, the commonly utilized NAC regimens include methotrexate, vincristine, doxorubicin, and cisplatin (MVAC), as well as gemcitabine-cisplatin (GC), with no significant difference in efficacy between them [Citation9]. Furthermore, GC is characterized by fewer side effects and better tolerability [Citation10]. However, the optimal selection of NAC cycles, particularly the three and four cycles most commonly employed, remains a subject of controversy [Citation11]. The earliest study investigating prognostic factors in clinically node-negative patients after radical cystectomy suggested that four cycles of NAC resulted in better pathological responses [Citation12]. A recent study revealed that patients receiving 1–2 cycles of NAC exhibited lower response rates, while those treated with three or four cycles demonstrated similar pathological responses and prognoses. Further administration of more than four cycles of chemotherapy did not yield additional benefits [Citation13].
Several recent studies [Citation14–18] have sought to compare pathological response and -survival outcomes between three and four cycles of NAC but have produced inconsistent findings. The retrospective design of these studies and the limited number of patients receiving four cycles of NAC have also compromised their credibility. Consequently, the objective of this research is to conduct a systematic review and meta-analysis of available studies in order to comprehensively compare the efficacy and survival outcomes between three and four cycles of NAC.
2. Materials and methods
We followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses recommendations) statement (http://www. prisma-statement.org/) to report results [Citation19].
2.1. Search strategy
A systematic literature search was conducted on the PubMed, Medline, Embase, Web of Science databases and the Cochrane Library (up to 8 August 2022), to identify studies investigating the relationship between different cycles of NAC and the treatment outcome of MIBC. The keywords used in our search strategy were as followed: ((‘Urinary Bladder Neoplasms’[Mesh]) OR ((Neoplasm, Urinary Bladder) OR (Urinary Bladder Neoplasm)) OR (Bladder Tumours)) OR (Bladder Tumour)) OR (Tumour, Bladder)) OR (Tumours, Bladder)) OR (Neoplasms, Bladder)) OR (Bladder Neoplasms)) OR (Bladder Neoplasm)) OR (Neoplasm, Bladder)) OR (Urinary Bladder Cancer)) OR (Cancer, Urinary Bladder)) OR (Malignant Tumour of Urinary Bladder)) OR (Cancer of the Bladder)) OR (Bladder Cancer)) OR (Bladder Cancers)) OR (Cancer, Bladder)) OR (Cancer of Bladder))) AND ((‘Neoadjuvant Therapy’[Mesh]) OR (((Neoadjuvant Therapies) OR (Neoadjuvant Treatment)) OR (Neoadjuvant Radiotherapy)) OR (Neoadjuvant Radiation Treatment)) OR (Neoadjuvant Radiation Therapy)) OR (Neoadjuvant Radiation)) OR (Neoadjuvant Systemic Treatment)) OR (Neoadjuvant Chemotherapy)) OR (Neoadjuvant Chemotherapy Treatment)) OR (Neoadjuvant Chemoradiotherapies)) OR (Neoadjuvant Chemoradiation Therapies)) OR (Neoadjuvant Chemoradiation Treatment) OR (Neoadjuvant Chemoradiation). The initial screening process of the literature was conducted independently by two researchers based on titles and abstracts. Full-text reviews were performed for potentially eligible reports, and relevant references were searched for additional studies. Any disagreements were resolved through consensus among the co-investigators.
2.2. Inclusion and exclusion criteria
The studies included in this review must meet the following criteria: (1) Subjects must have been diagnosed with bladder cancer and received NAC prior to radical cystectomy. (2) The intervention group should have received four cycles of NAC, while the control group received three cycles. (3) The study must have compared the pathological downstaging and long-term survival rates between the control and experimental groups. (4) The research design should be a prospective or retrospective cohort study or a randomized clinical trial. The main exclusion criteria encompassed: (1) Patients with non-bladder cancer or those with concurrent cancers. (2) Patients who received less than three cycles or more than four cycles of NAC prior to surgery or who had additional drug treatments alongside NAC. (3) Studies lacking a comparison of NAC effects between the control and experimental groups. (4) Reviews, letters, editorial comments, author responses, and case reports were also excluded.
2.3. Data extraction
Two investigators independently extracted the data, including the first author’s name, publication year, country, study design, patient count, NAC regimen, number of patients receiving three and four cycles of NAC, age, pathological stage and grade, bladder cancer histology, pathological objective response (pOR), pathological complete response (pCR), overall survival (OS), cancer-specific survival (CSS) and recurrence-free survival (RFS). Any disagreements were resolved through consensus with the co-investigators.
2.4. Risk of bias assessment
The quality and risk of bias of the included prospective or retrospective cohort studies were assessed using the Newcastle-Ottawa Scale (NOS) [Citation20]. The NOS is a 9-point scale that evaluates bias in three domains: selection of subjects, comparability of groups, and ascertainment of exposure or outcome. Studies with a total NOS score of more than six are considered to be of high quality.
2.5. Statistical analyses
We utilized relative risks (RRs), hazard ratios (HRs), and 95% confidence intervals (CIs) to assess the association between tumour pCR, objective response, long-term survival, and CSS following three or four cycles of NAC. Both fixed-effect model (FEM) and random-effect model (REM) were used to calculate pooled RRs and HRs. Heterogeneity was evaluated using the Chi-square-based Q-test and I2 test. FEM [Citation21] was applied if the P of the Q-test was >0.05 or I2 <50%, otherwise, REM [Citation22] was used. Subgroup analyses were conducted based on the NAC regimen and tumour histology to explore their potential impact on the results. Sensitivity analysis involved sequentially removing each study to assess stability and credibility. Publication bias was assessed using Begg’s funnel plot and Egger’s tests [Citation23]. This meta-analysis was performed using Stata software (version 12.0) and Review Manager (version 5.3, Copenhagen: Nordic Cochrane Centre, Cochrane Collaboration Network, 2014).
3. Results
3.1. Study selection and characteristics
Our initial search identified 6687 records; after removing duplicates, 4202 records remained (.). After screening for titles and abstracts, 3672 articles were excluded, and 530 were reviewed in full text. According to our meta-analysis inclusion and exclusion criteria, we finally included five studies [Citation14–18] including 2190 patients, of which 1016 received three cycles of NAC, and 1174 received four cycles of NAC. All studies were retrospective cohort studies. Four were multicentre studies, and the remaining one was a single-centre study [Citation14]. Three studies only used GC as a NAC regimen, and the remaining two [Citation15,Citation18] used a variety of NAC regimens, including GC and dose-dense MVAC (DD-MVAC). Patients with stage T2 accounted for the majority of the included studies, ranging from to 49.1%-83.8%. We summarized the features of the included studies in .
Table 1. Main characters of retrospective cohort studies included in this meta-analysis.
3.2. Bias assessment and evidence quality assessment
The risk assessment of the five included articles is shown in . The cohorts of all included studies were well represented and exposed. However, most studies were slightly less comparable because they were all retrospective studies. In addition, two studies [Citation17,Citation18] had a short follow-up period after surgery. In summary, all five included studies were of high quality.
Table 2. The risk assessment of the included studies.
3.3. Clinical outcomes from meta-analysis
3.3.1. Cancer-specific survival
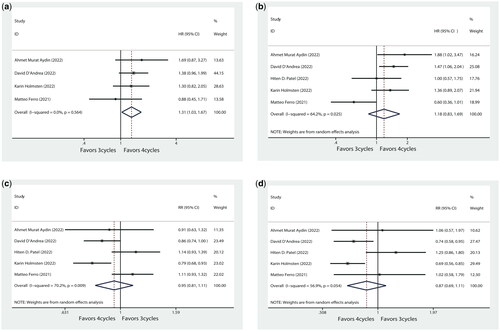
Four studies, including 1919 patients, compared CSS with three and four cycles of NAC. Three cycles of NAC were significantly associated with worse CSS compared with four cycles (HR: 1.31, 95%CI = 1.03–1.67, p = 0.029, ).
3.3.2. Overall survival
All five studies reported data on OS with different cycles of NAC regimens. There was no significant difference in OS between receiving three or four cycles of NAC (HR = 1.18, 95%CI = 0.83–1.69, p = 0.345, ).
3.3.3. Pathological objective response
Five studies including 2,190 patients provided pOR rates for MIBC after three and four cycles of NAC. The pOR rates for three cycles of NAC in the five studies were 40.2% [Citation14], 41.9% [Citation15], 62.3% [Citation18], 45.0% [Citation17], and 80.6% [Citation16], respectively. The pOR rates for four cycles were 44.1% [Citation14], 48.9% [Citation15], 54.8% [Citation18], 56.9% [Citation17], 72.9.0% [Citation16], respectively. The comprehensive summary of the pOR rates in the enrolled studies is displayed in Supplementary Figure S1. The forest plot () and revealed no statistically significant difference in the pOR rates of bladder cancer across cycles of NAC (RR = 0.95, 95% CI = 0.81–1.11, p = 0.515).
3.3.4. Pathological complete response and pT0
All five studies reported the rates of pCR in bladder cancer following NAC across various treatment cycles. The pCR rates for three cycles of NAC in the five studies were 21.5% [Citation14], 21.1% [Citation15], 33.3% [Citation18], 30.7% [Citation17], 22.5% [Citation16], respectively. The pCR rates for four cycles were 20.3% [Citation14], 28.4% [Citation15], 26.8% [Citation18], 44.4% [Citation17], and 22.0% [Citation16], respectively. And we summarized the detailed description of pCR rates from the included studies in Supplementary Figure S2. The forest plot () indicates no statistically significant difference in tumour pCR rates between patients who received 3 or 4 cycles of NAC (RR = 0.87, 95%CI = 0.69-1.11, p = 0.256). The pT0 rates for three cycles of NAC in the five studies were 22.1% [Citation15], 33.1% [Citation17], 22.5% [Citation16], respectively. The pT0 rates for four cycles were 29.5% [Citation15], 46.4% [Citation17], 22.0% [Citation16], respectively (Supplementary Figure S3). Notably, patients who underwent four cycles of NAC exhibited a significantly higher likelihood of achieving pT0 status compared to those who received three cycles (RR = 0.81, 95%CI = 0.69–0.94, p = 0.007, Supplementary Figure S4).
3.4. Subgroup analysis on tumour histology and NAC regimens
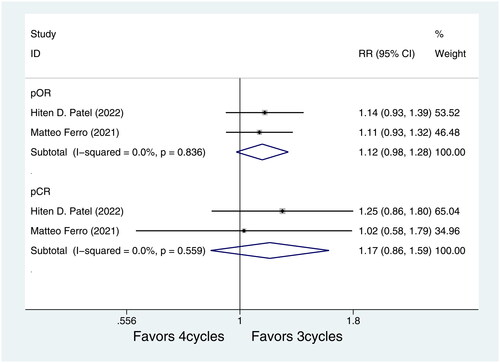
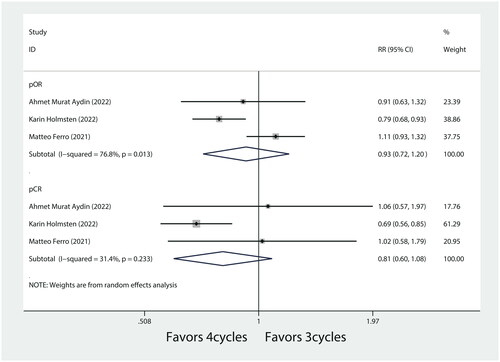
Subgroup analysis based on pure urothelial carcinoma (UC) showed that there was no association between different cycles of NAC in pOR rates of pure UC (RR = 1.12, 95%CI = 0.98–1.28, p = 0.093, .). Similarly, different cycles of NAC were not statistically significantly associated with pCR rates (RR = 1.17, 95%CI = 0.86–1.59, p = 0.306, ) in pure UC. Furthermore, subgroup analysis based on GC regimen showed pOR and pCR were not associated with different cycles of GC regimen (RR = 0.93, 95%CI = 0.72–1.20, p = 0.567, and RR = 0.82, 95%CI = 0.60–1.08, p = 0.154, respectively, ).
3.5. Assessment of sensitivity analyses and publication bias
Sensitivity analyses were used to assess the reliability of the meta-analyses, and the effect of each article on the pooled results was evaluated by excluding each included study. The overall results of our research were reliable and independent of the influence of individual data on the pooled RR. In this meta-analysis, Begg and Egger’s test was also used to detect publication bias. The results showed no publication bias, and the combined results were reliable (p > 0.05).
4. Discussion
Three and four cycles of NAC resulted in comparable treatment response and OS in MIBC patients.
Importantly, the CSS was significantly superior with four cycles of NAC compared to three cycles. Patients who underwent four cycles of chemotherapy demonstrated a higher probability of achieving pT0 status, which could potentially elucidate the favourable CSS associated with four cycles of NAC. Importantly, the CSS was significantly superior with four cycles of NAC compared to three cycles. Hence, administering four cycles of chemotherapy may contribute to mitigating tumour metastasis and recurrence to some degree. Subgroup analysis indicated that the GC regimen and tumour histology of pure urothelial carcinoma were not correlated with the pathological response of cancer. The included articles exhibited no publication bias, thus affirming the robustness of the findings.
MIBC is a highly aggressive disease prone to lymph node and distant metastasis, leading to recurrence in around 50% of post-surgery patients due to the presence of micrometastases at the time of cystectomy [Citation11]. Platinum-based chemotherapy regimens promote apoptosis and enhance immunogenicity in malignant cells [Citation24]. NAC can effectively target micro-metastatic disease and result in primary tumour downstaging, indicating a favourable prognosis [Citation25]. A recent meta-analysis, incorporating data from 15 randomized controlled trials, demonstrated an 8% absolute improvement in 5-year survival with NAC [Citation8]. Moreover, bladder cancer patients who attained pCR following NAC exhibited superior OS and RFS compared to those without pCR [Citation26]. Likewise, patients achieving pCR to NAC demonstrated improved OS following robot-assisted radical cystectomy [Citation27]. RNA N6-methyladenosine (m6A) modification plays a pivotal role in tumour development. METTL14 serves as the primary m6A-related enzyme in bladder cancer, facilitating IncDBET expression through m6A modification. Upregulation of IncDBET stimulates the PPAR signalling pathway, promoting lipid metabolism in cancer cells through direct interaction with FABP5. Consequently, this drives the progression of bladder cancer [Citation28].
In a recently published large prospective randomized clinical trial, the DD-MVAC regimen was compared with the GC regimen, revealing superior rates of local control (pCR, tumour downstaging, or organ preservation) in the DD-MVAC group [Citation29]. Furthermore, DD-MVAC demonstrated higher RFS [Citation30]. However, a study comparing four cycles of DD-MVAC and GC showed no significant disparities in OS or RFS [Citation31,Citation32]. Thus, the disparity in outcomes could potentially be attributed to the varying number of chemotherapy cycles. Conversely, a retrospective study indicated no correlation between the number of platinum-based chemotherapy cycles and survival in advanced UC [Citation33]. Similarly, another multicentre retrospective study reached comparable findings, with multivariate analysis failing to identify any relationship between OS, chemotherapy cycle count, or chemotherapy response [Citation34]. This may suggest that NAC primarily benefits distant metastasis rather than local control [Citation8,Citation35]. Moreover, augmenting the number of cycles did not enhance the survival outcomes of patients with advanced bladder cancer. Importantly, there exists a notable gender disparity in the prognosis of bladder cancer, with females experiencing poorer outcomes. The Gonadotropin-releasing hormone (GNRH) family genes play a fundamental role in sex-related biological processes [Citation36].
The immune microenvironment of MIBC can influence patients’ response to NAC. Infiltration and proliferation of CD8+ T cells and CD204+ cells serve as potential indicators of NAC efficacy, with CD204+ cells being associated with a poor prognosis for these patients [Citation37]. NAC can enhance the expression of genes associated with anti-tumour immune response activation and decrease the expression of genes involved in tumour proliferation pathways in bladder cancer patients, thus improving the inhibitory tumour immune microenvironment. Furthermore, NAC enhances tumour response to and increases the sensitivity of bladder cancer to immune checkpoint inhibitors [Citation38]. Hu et al. intriguingly discovered that combining neoadjuvant immunotherapy with chemotherapy resulted in the highest rates of pCR and downstaging in patients with MIBC [Citation39]. Hence, the integration of chemotherapy and immunotherapy may represent a future treatment approach for MIBC.
Immunotherapy is also becoming more prevalent in treatment of bladder cancer. Since only a minority of patients respond to immune checkpoint inhibitors, there is a need to explore new targets. S100A5 hinders the secretion of proinflammatory chemokines and impairs the recruitment, proliferation, and cytotoxicity of CD8+ T cells, thereby creating a non-inflammatory tumour microenvironment in bladder cancer. Targeting S100A5 has the potential to convert cold tumours into hot tumours, presenting it as a promising new immunosuppressive target for bladder cancer [Citation40]. Similarly, BCAT2 contributes to the formation of a non-inflammatory tumour microenvironment in bladder cancer. It achieves this by suppressing the recruitment of cytotoxic lymphocytes through inhibition of proinflammatory cytokine/chemokine-related pathways and t cell chemotactic pathway. CD8+ T cell-associated chemokine secretion shows an inverse correlation with BCAT2 expression. In addition, BCAT2 deficiency and combination therapy with anti-PD-1 antibody exhibit synergistic effects in vivo, highlighting the potential of BCAT2 as a target for combination therapy [Citation41].
Despite being the primary treatment for invasive bladder cancer, there is a lack of identified biomarkers for predicting NAC response. In a previous retrospective study, the gene expression model (GEM) score, generated by the co-expression extrapolation algorithm, was validated as a biomarker in patients who underwent radical cystectomy [Citation32]. Likewise, an NLR >3 correlated with diminished response to NAC, as well as shorter disease-specific survival and OS [Citation42]. A retrospective cohort analysis indicated that mutations in the fibroblast growth factor receptor 3 (FGFR3) gene could be linked to reduced responses and shorter recurrence times [Citation43]. Additionally, Ecke et al. discovered an association between high pCR rates and intraluminal tumours expressing high levels of KRT20 mRNA, while the double-negative subgroup with elevated FGFR1 expression showed no response to pCR [Citation44]. In a retrospective study, a positive association was observed between deleterious mutations in ERCC2 and pathological response to NAC, supporting the notion that bladder cancer patients with ERCC2 mutations frequently exhibit a favourable response to chemotherapy [Citation45]. The luminal-like (GU and Uro) subtypes of urothelial carcinoma (UC) exhibit heightened sensitivity to cisplatin-based NAC. Vollmer et al. identified the intratumoral CXCR3 chemokine system as a crucial factor in eradicating tumours with NAC in MIBC. They also demonstrated the stimulatory effect of the CXCR3alt-CXCL11 chemokine system on CD8+ T cells, which can serve as a predictor of chemotherapy responsiveness in MIBC [Citation46]. Positive expression of Ki-67 and PD-L1 in radical cystectomy specimens following NAC correlated with decreased OS and absence of tumour downstaging. Immunohistochemistry analysis of Ki-67 and PD-L1 can aid in identifying patients with post-NAC MIBC who may benefit from adjuvant therapy [Citation47]. Luminal-like (GU and Uro) subtypes of urothelial carcinoma (UC) exhibit enhanced sensitivity to cisplatin-based NAC. Second-generation subtype-specific biomarkers, like SPP1, hold potential for the development of more targeted NAC treatments for MIBC [Citation48]. Siglec15 is linked to a non-inflammatory tumour microenvironment in bladder cancer. Elevated levels of Siglec15 indicate a luminal subtype of bladder cancer, which is characterized by reduced immune infiltration and a diminished response to immunotherapy and NAC [Citation49].
This study constitutes the inaugural meta-analysis of three- and four-cycle NAC regimens. A comprehensive and systematic literature search was conducted, incorporating the most recent findings published within the last two years and encompassing a substantial patient cohort. Nevertheless, this paper acknowledges several inherent limitations. Primarily, the included articles solely comprised retrospective studies, lacking data from prospective trials, thereby introducing potential selection bias that may have influenced the final outcomes. For example, Patel et al. [Citation18] reported a significant proportion (over 60%) of patients initially intended to undergo four cycles of NAC but ultimately received only three cycles due to various side effects. Furthermore, the scarcity of available information concerning side effects on NAC in the reviewed literature precluded a comparative analysis of toxicities associated with different chemotherapy cycles, an aspect often of paramount concern when determining chemotherapy regimens. Although surgical-related indicators are important in evaluating NAC for bladder cancer, further analysis was hindered due to the absence of reported data in the included studies. Moreover, the limited number of articles incorporated and the evident heterogeneity observed in the data analysis may impact the final results.
Conclusion
Both three and four cycles of NAC yielded comparable pathological responses and prognoses in patients with MIBC. While four cycles exhibited superior CSS compared to three cycles, no significant enhancements were observed in OS or pathological response. Larger cohort studies and prospective randomized clinical trials are needed to validate these results in the future.
Authors’ contributions
Conception and design: YLW and YXS; Analysis and interpretation of the data: YLW, YXS, and CPQ; Drafting and revision of the paper: YLW and YXS; Revision of the paper: CLZ, YQD and TX; All authors read and approved the final manuscript.
Supplemental Material
Download Zip (138.4 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All the data generated or analyzed during the current study were obtained from the included studies.
Additional information
Funding
References
- Teoh JYC, Huang J, Ko WYK, et al. Global trends of bladder cancer incidence and mortality, and their associations with tobacco use and gross domestic product per capita. Eur Urol. 2020;78(6):1–11. doi: 10.1016/j.eururo.2020.09.006.
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492.
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi: 10.3322/caac.21654.
- Jacobs BL, Lee CT, Montie JE. Bladder cancer in 2010: how far have we come? CA Cancer J Clin. 2010;60(4):244–272. doi: 10.3322/caac.20077.
- Witjes JA, Bruins HM, Cathomas R, et al. European association of urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2020 guidelines. Eur Urol. 2021;79(1):82–104. doi: 10.1016/j.eururo.2020.03.055.
- Witjes JA, Compérat E, Cowan NC, et al. EAU guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2013 guidelines. Eur Urol. 2014;65(4):778–792. doi: 10.1016/j.eururo.2013.11.046.
- Abol-Enein H, Bono AV, Boyer M, et al. Neoadjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis. Lancet. 2003;361:1927–1934.
- Yin M, Joshi M, Meijer RP, et al. Neoadjuvant chemotherapy for muscle-invasive bladder cancer: a systematic review and two-step meta-analysis. Oncologist. 2016;21(6):708–715. doi: 10.1634/theoncologist.2015-0440.
- Zargar H, Espiritu PN, Fairey AS, et al. Multicenter assessment of neoadjuvant chemotherapy for muscle-invasive bladder cancer. Eur Urol. 2015;67(2):241–249. doi: 10.1016/j.eururo.2014.09.007.
- Von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18(17):3068–3077. doi: 10.1200/JCO.2000.18.17.3068.
- Ruiz de Porras V, Pardo JC, Etxaniz O, et al. Neoadjuvant therapy for muscle-invasive bladder cancer: current clinical scenario, future perspectives, and unsolved questions. Crit Rev Oncol Hematol. 2022;178:103795. doi: 10.1016/j.critrevonc.2022.103795.
- Zargar-Shoshtari K, Zargar H, Dinney CP, et al. Clinical and therapeutic factors associated with adverse pathological outcomes in clinically node-negative patients treated with neoadjuvant cisplatin-based chemotherapy and radical cystectomy. World J Urol. 2016;34(5):695–701. doi: 10.1007/s00345-015-1667-4.
- Blanco-Martinez E, Patel HD, Patel SH, et al. Deviations in cycles of neoadjuvant chemotherapy for muscle-invasive bladder cancer and implications for pathologic response and survival. J Urol. 2021;206(Supplement 3):e764–e765. doi: 10.1097/JU.0000000000002062.11.
- Aydin AM, Cheriyan SK, Reich R, et al. Comparative analysis of three vs. four cycles of neoadjuvant gemcitabine and cisplatin for muscle invasive bladder cancer. Urol Oncol. 2022;40(10):453.e19–453.e26. doi: 10.1016/j.urolonc.2022.05.023.
- D’Andrea D, Black PC, Zargar H, et al. Identifying the optimal number of neoadjuvant chemotherapy cycles in patients with muscle invasive bladder cancer. J Urol. 2022;207(1):70–76. doi: 10.1097/JU.0000000000002190.
- Ferro M, de Cobelli O, Musi G, et al. Three vs. Four cycles of neoadjuvant chemotherapy for localized muscle invasive bladder cancer undergoing radical cystectomy: a retrospective multi-institutional analysis. Front Oncol. 2021;11:651745. doi: 10.3389/fonc.2021.651745.
- Holmsten K, Omland LH, Als AB, et al. Implications for efficacy and safety of total dose and dose-intensity of neoadjuvant gemcitabine-cisplatin in muscle-invasive bladder cancer: three-week versus four-week regimen. BLC. 2022;8(1):71–80. doi: 10.3233/BLC-211556.
- Patel HD, Patel SH, Blanco-Martinez E, et al. Four versus 3 cycles of neoadjuvant chemotherapy for muscle-invasive bladder cancer: implications for pathological response and survival. J Urol. 2022;207(1):77–85. doi: 10.1097/JU.0000000000002189.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLOS Med. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100.
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z.
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J National Cancer Inst. 1959;22:719–748.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2.
- Hayashino Y, Noguchi Y, Fukui T. Systematic evaluation and comparison of statistical tests for publication bias. J Epidemiol. 2005;15(6):235–243. doi: 10.2188/jea.15.235.
- Hato SV, Khong A, de Vries IJ, et al. Molecular pathways: the immunogenic effects of platinum-based chemotherapeutics. Clin Cancer Res. 2014;20(11):2831–2837. doi: 10.1158/1078-0432.CCR-13-3141.
- Rosenblatt R, Sherif A, Rintala E, et al. Pathologic downstaging is a surrogate marker for efficacy and increased survival following neoadjuvant chemotherapy and radical cystectomy for muscle-invasive urothelial bladder cancer. Eur Urol. 2012;61(6):1229–1238. doi: 10.1016/j.eururo.2011.12.010.
- Petrelli F, Coinu A, Cabiddu M, et al. Correlation of pathologic complete response with survival after neoadjuvant chemotherapy in bladder cancer treated with cystectomy: a meta-analysis. Eur Urol. 2014;65(2):350–357. doi: 10.1016/j.eururo.2013.06.049.
- Anceschi U, Brassetti A, Tuderti G, et al. Impact of clinical response to neoadjuvant chemotherapy in the era of robot assisted radical cystectomy: results of a single-center experience. J Clin Med. 2020;9(9):9.
- Liu P, Fan B, Othmane B, et al. m(6)A-induced lncDBET promotes the malignant progression of bladder cancer through FABP5-mediated lipid metabolism. Theranostics. 2022;12(14):6291–6307. doi: 10.7150/thno.71456.
- Pfister C, Gravis G, Fléchon A, et al. Randomized phase III trial of dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin, or gemcitabine and cisplatin as perioperative chemotherapy for patients with muscle-invasive bladder cancer. Analysis of the GETUG/AFU V05 VESPER trial secondary endpoints: chemotherapy toxicity and pathological responses[formula presented. Eur Urol. 2021;79(2):214–221. doi: 10.1016/j.eururo.2020.08.024.
- Pfister C, Gravis G, Fléchon A, et al. Dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin or gemcitabine and cisplatin as perioperative chemotherapy for patients with nonmetastatic muscle-invasive bladder cancer: results of the GETUG-AFU V05 VESPER trial. J Clin Oncol. 2022;40(18):2013–2022. doi: 10.1200/JCO.21.02051.
- Flaig TW, Tangen C, Daneshmand S, et al. SWOG S1314: a randomized phase II study of co-expression extrapolation (COXEN) with neoadjuvant chemotherapy for localized, muscle-invasive bladder cancer with overall survival follow up. J Clin Oncol. 2022;40(6_suppl):536–536. doi: 10.1200/JCO.2022.40.6_suppl.536.
- Flaig TW, Tangen CM, Daneshmand S, et al. A randomized phase II study of coexpression extrapolation (COXEN) with neoadjuvant chemotherapy for bladder cancer (SWOG S1314; NCT02177695). Clin Cancer Res. 2021;27(9):2435–2441. doi: 10.1158/1078-0432.CCR-20-2409.
- Sonpavde GP, Mariani L, Lo Vullo S, et al. Impact of the number of cycles of platinum based first line chemotherapy for advanced urothelial carcinoma. J Urol. 2018;200(6):1207–1214. doi: 10.1016/j.juro.2018.07.035.
- Kato M, Kobayashi T, Matsui Y, et al. Impact of the objective response to and number of cycles of platinum-based first-line chemotherapy for metastatic urothelial carcinoma on overall survival of patients treated with pembrolizumab. Int J Urol. 2021;28(12):1261–1267. doi: 10.1111/iju.14686.
- Griffiths G, Hall R, Sylvester R, et al. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol. 2011;29(16):2171–2177. doi: 10.1200/JCO.2010.32.3139.
- Song Y, Qin C, Zhang C, et al. GNRH family genes contributed to gender-specific disparity of bladder cancer prognosis through exerting opposite regulatory roles between males and females. J Cancer Res Clin Oncol. 2023;149(10):6827–6840. doi: 10.1007/s00432-023-04640-2.
- Ikarashi D, Kitano S, Tsuyukubo T, et al. Pretreatment tumour immune microenvironment predicts clinical response and prognosis of muscle-invasive bladder cancer in the neoadjuvant chemotherapy setting. Br J Cancer. 2022;126(4):606–614. doi: 10.1038/s41416-021-01628-y.
- Luo H, Liu GL, Jian D, et al. Neoadjuvant chemotherapy improves the immunosuppressive microenvironment of bladder cancer and increases the sensitivity to immune checkpoint blockade. J Immunol Res. 2022;2022:9962397.
- Hu J, Chen J, Ou Z, et al. Neoadjuvant immunotherapy, chemotherapy, and combination therapy in muscle-invasive bladder cancer: a multi-center real-world retrospective study. Cell Rep Med. 2022;3(11):100785. doi: 10.1016/j.xcrm.2022.100785.
- Li H, Chen J, Li Z, et al. S100A5 attenuates efficiency of anti-PD-L1/PD-1 immunotherapy by inhibiting CD8(+) T cell-mediated anti-cancer immunity in bladder carcinoma. Adv Sci. 2023;10:e2300110.
- Cai Z, Chen J, Yu Z, et al. BCAT2 shapes a noninflamed tumor microenvironment and induces resistance to anti-PD-1/PD-L1 immunotherapy by negatively regulating proinflammatory chemokines and anticancer immunity. Adv Sci . 2023;10:e2207155.
- Black AJ, Zargar H, Zargar-Shoshtari K, et al. The prognostic value of the neutrophil-to-lymphocyte ratio in patients with muscle-invasive bladder cancer treated with neoadjuvant chemotherapy and radical cystectomy. Urol Oncol. 2020;38(1):3.e17-13–e27. doi: 10.1016/j.urolonc.2019.09.023.
- Teo MY, Mota JM, Whiting KA, et al. Fibroblast growth factor receptor 3 alteration status is associated with differential sensitivity to platinum-based chemotherapy in locally advanced and metastatic urothelial carcinoma. Eur Urol. 2020;78(6):907–915. doi: 10.1016/j.eururo.2020.07.018.
- Ecke TH, Voß PC, Schlomm T, et al. Prediction of response to cisplatin-based neoadjuvant chemotherapy of muscle-invasive bladder cancer patients by molecular subtyping including KRT and FGFR target gene assessment. Int J Mol Sci. 2022;23(14):23. doi: 10.3390/ijms23147898.
- Gil-Jimenez A, van Dorp J, Contreras-Sanz A, et al. Assessment of predictive genomic biomarkers for response to cisplatin-based neoadjuvant chemotherapy in bladder cancer. Eur Urol. 2023;83(4):313–317. doi: 10.1016/j.eururo.2022.07.023.
- Vollmer T, Schlickeiser S, Amini L, et al. The intratumoral CXCR3 chemokine system is predictive of chemotherapy response in human bladder cancer. Sci Transl Med. 2021;13(576):eabb3735. doi: 10.1126/scitranslmed.abb3735.
- Rubino S, Kim Y, Zhou J, et al. Positive Ki-67 and PD-L1 expression in post-neoadjuvant chemotherapy muscle-invasive bladder cancer is associated with shorter overall survival: a retrospective study. World J Urol. 2021;39(5):1539–1547. doi: 10.1007/s00345-020-03342-5.
- Sjödahl G, Abrahamsson J, Holmsten K, et al. Different responses to neoadjuvant chemotherapy in urothelial carcinoma molecular subtypes. Eur Urol. 2022;81(5):523–532. doi: 10.1016/j.eururo.2021.10.035.
- Hu J, Yu A, Othmane B, et al. Siglec15 shapes a non-inflamed tumor microenvironment and predicts the molecular subtype in bladder cancer. Theranostics. 2021;11(7):3089–3108. doi: 10.7150/thno.53649.