Abstract
The vascular and morphological features of tumors are important predictors of the nature, grade, and stage of various cancers. However, this association has not been tested in bladder cancer. The aim of our study was to investigate the correlation between the morphological characteristics of tumor vessels and the nature, stage and grade of bladder cancer. Between November 2021 and March 2023, we prospectively collated clinical information and cystoscopy information from a series of patients with bladder cancer. Univariate and multivariate logistic regression analysis were used to identify independent risk factors for the nature, grade and stage of bladder cancer. Our analysis showed that cauliflower-like tumors, dotted vessels, and circumferential vessels were independent risk factors for bladder cancer. Reticular vessels were an independent risk factor for high-grade bladder cancer. Thick branching vessels in bladder tumors, along with a wide base, were independent risk factors for the invasion of bladder cancer into the lamina propria. Primary diagnosis, lesion location (beside the left ureteral orifice) and obscure lesion boundaries were all identified as independent risk factors for muscle invasive bladder cancer.
Introduction
Bladder cancer (BC) is one of the most common malignant tumors of the urinary system [Citation1]. The pathological grade and stage of BC have important significance for the choice of treatment and the estimation of survival [Citation2,Citation3]. Tumor size and morphology under white light imaging (WLI) have been reported to be correlated with the nature and stage of BC [Citation4,Citation5]; however, few studies have investigated the correlation between the vascular characteristics of tumors under narrow-band imaging (NBI) and the nature, grading, and staging of BC.
As an enhanced imaging technique, NBI is mainly composed of blue and green light, which can be absorbed by hemoglobin, thereby enhancing the visualization of the blood vessels [Citation6,Citation7]. Bladder tumors are rich in vessels, and the enhanced visualization of vessels increases the contrast between a tumor and the surrounding normal mucosal tissue. Thus, NBI has been widely used in the field of medical imaging diagnosis and plays an important role in the screening of many diseases, including those of the bladder, oropharynx, esophagus, gastrointestinal system and other organs [Citation8–11].
In our study, we analyzed the correlation between vascular and morphological characteristics under NBI and the nature, grading and staging of BC. Our goal was to identify predictive factors for the grading and staging of BC.
Methods
This was a prospective study including several centers which adhered to the Declaration of Helsinki and were approved by the hospital ethics committee and institutional review board. All patients were informed of the purpose and specific objectives of the study before examination and provided written and informed consent. The trial registration number for this study is NCT05611762. The 2004/2016 WHO grading system was used for the histological grading of BC in this study.
Patients
The inclusion criteria were as follows: [1] patients aged between 18 and 75 years; [2] patients with symptoms of hematuria or additional test results suggesting the existence of a bladder mass that required cystoscopy for a definitive diagnosis; or patients with a postoperative review of urothelial tumors. The exclusion criteria were as follows: [1] patients with coagulation dysfunction; [2] patients with a combination of acute urinary tract infection or the instillation of Bacillus Calmette-Guerin for no more than one week; [3] patients with urethral stricture or a bladder volume that was too small for cystoscopy; [4] patients who could not tolerate local anesthesia; [5] women who were menstruating or pregnant for over three months; and [6] reasons deemed by the investigator to be inappropriate for participation in this clinical study.
All patients were examined with an Olympus CYF-VHA flexible cystoscope. The cystoscopy examination included WLI and NBI modality.
Classification of the general and vascular morphology of bladder lesions
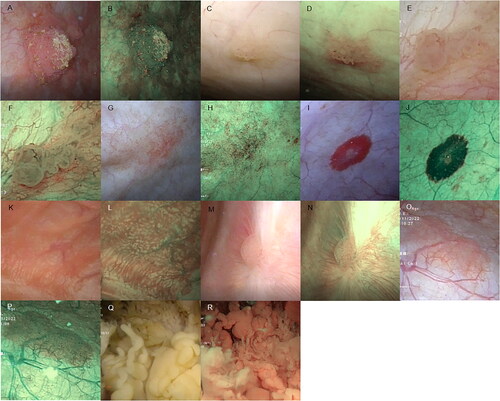
We classified bladder tumors into the several different categories based on their morphological features. Representative morphological images of the different classifications of bladder tumors are shown in .
Figure 1. Bladder tumor morphology under WLI and NBI modality. (A and B) Cauliflower-like lesions. These resembled a cauliflower and were primarily spherical or ellipsoidal, usually with a tip. (C and D) Papillary lesions. These were shorter cylindrical mucosal protrusions with a broad base that resembled a papilla. These may be present singly or as multiple short cylinders closely adjacent to each other in a cluster. For these lesions, there was a lack of a three-dimensional spherical structure when compared with cauliflower swellings. (E and F) Follicular lesions. Follicular lesions which were mainly light red or white vesicular structures under WLI, with thin and transparent walls and sparse vascularity visible within the vesicular structures. (G and H) Patchy lesions. Patchy lesions: slightly elevated or non-elevated lesion with an irregular distribution pattern and interspersed presence of lesion and normal mucosa. (I and J) Round lesions. Round lesions which were slightly elevated or non-elevated lesions with a broad base and a regular shape approximating a circle. (K and L) Villous lesions. Villous lesions featuring a thin cylindrical swelling that was slightly longer in length than the papillary lesions which were arranged in a column or a cluster and floated downstream under the influence of water, resembling a villus. (M and N) Flat bulges. Flat bulges which represented minor hemispherical swellings with a broad base, without a tip, and were elevated on the mucosal surface. These may be present singly or diffusely distributed in the bladder wall. (O and P) Mossy lesions. Mossy lesions which were slightly elevated lesions with a uniform thickness, the majority of which exhibit a lamellar or large lamellar distribution, resembling moss, with clear demarcation around the lesion and normal mucosa. These can be differentiated from patchy masses by the absence of regular mucosal interlacing within the area of the lesion. (Q and R) Algae-like lesions. Algae-like lesions which featured long thick stripes or long columnar swellings with tips. These can float downstream under the influence of water, resembling kelp, or with thick branches in a coral-like manner.
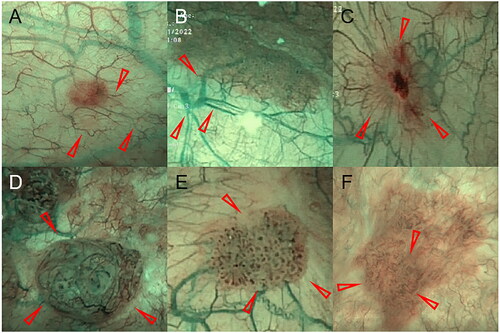
In addition, we classified blood vessels according to their different morphological characteristics under NBI. First, fine branching vessels which were indistinguishable under WLI and clear in the NBI modality. These vessels are small, hair-like, brown-green in color, and the exhibit bifurcation in a ‘dendritic’ pattern. Second, thick branching vessels which were thicker red vessels under WLI. The color of the vessels was often green or brown-green under NBI modality, and the vessels showed bifurcation in a ‘dendritic’ pattern. Third, reticulated vessels in which the distribution of vessels resembled a ‘spider web’, gathering toward the center of the lesion or a longitudinal/horizontal parallel arrangement similar to a ‘fishing net’. Fourth, disorganized vessels which were disorganized, with different directions and no regularity. Occasionally, a red vascular mass was observed under WLI due to vascular exudation. Fifth, dotted vessels in which vessels appeared dot-shaped. Sixth, circumferential vessels which were tortuous and arranged in rings or semi-rings, without vascular branches. Representative morphological images of the system used to classify blood vessels are given in .
Figure 2. Illustration of the vascular morphology of the bladder mucosa by NBI modality. (A) Fine branching vessels. (B) Thick branching vessels. (C) Reticulated vessels. (D) Disorganized vessels. (E) Dotted vessels. (F) Circumferential vessels. (Red arrow indicates vessel location)
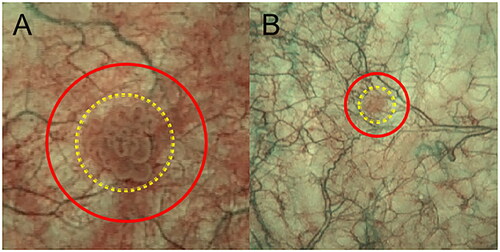
With regards to the bladder lesion area, the mucosal area was divided into two categories: [1] the area of the lesion, which represented the area occupied by the lesion mucosa, and [2] the surrounding ring area, referred to as the basal mucosal area ().
Figure 3. Delineation of the mucosal and basal mucosal areas of the lesions under NBI modality. (A) The yellow area represents the mucosal region of a cauliflower-like lesion with dotted vessels visible under the mucosa. Between the red and yellow areas lies the basal mucosal area, with a sparse distribution of thick and fine branching vessels and continuous vascular pathways. (B) The yellow area is the round lesion mucosal area, and between the red and yellow areas is the basal mucosal area, with sparse, thick visible branching vessels and continuous vascular alignment.
Statistical methods
Continuous data are expressed as mean ± standard deviation or interquartile range (IQR) while count data are expressed as percentages. Univariate and multivariate analyses were performed using R version 4.2.2 software (The R Foundation for Statistical Computing, Vienna, Austria) and p < 0.05 was considered statistically significant. Significant variables (p < 0.05) in univariate logistic regression analysis were included in multivariate regression analysis, while significant variables (p < 0.05) in multivariate logistic regression analysis were regarded as independent risk factors.
Results
We prospectively collected information relating to 275 patients who underwent preoperative cystoscopy. We examined the data and excluded 22 patients, including three patients who were >75 years-of-age, two patients with prostate cancer, two patients with gastric metastases, one patient with rectal cancer metastases, two patients with BC of unknown invasiveness, and 12 patients with incomplete information. Ultimately, 253 patients were included in our analysis, of which 121 patients had benign lesions (including papilloma, inflammation, epithelial hyperplasia) and 132 patients had BC. Patient characteristics are given in .
Table 1. Patient characteristics.
Cauliflower-like tumors, dotted vessels, and circumferential vessels were identified as independent risk factors for BC
After univariate analysis, it was evident that primary diagnosis (bladder tumor; p = 0.002), gross hematuria (p < 0.001), tumor location beside the right ureteral orifice (p = 0.019), tumor location in the left lateral wall (p = 0.017), tumor location in the neck of the bladder (p = 0.029), a clear tumor boundary (p = 0.008), cauliflower-like lesions (p < 0.001), rich vessels in the area of the lesion (p < 0.001), thick branching vessels under NBI (p < 0.001), dotted vessels under NBI (p < 0.001), circumferential vessels under NBI (p < 0.001), rich vessels in the basal area under NBI (p = 0.024), and thick branching vessels under NBI (p = 0.003) were all factors related to BC. Primary diagnosis (postoperative re-examination; p = 0.008), wide-base tumors (p < 0.001), patchy lesions (p < 0.001), flat bulges (p < 0.001), mossy lesions (p = 0.001), fine branching vessels under NBI (p < 0.001), reticulated vessels under NBI (p = 0.046), disorganized vessels under NBI (0.013), and fine branching vessels under NBI (p = 0.004) were all factors related to benign lesions of the bladder. Further multivariate logistic regression analysis showed that gross hematuria (p = 0.003), cauliflower-like lesions (p < 0.001), dotted vessels (p = 0.008), and circumferential vessels (p = 0.002) in the lesion area under NBI were all independent risk factors for BC. These results are summarized in Supplementary Table 1.
Reticulated vessels as an independent predictor for high-grade BC
In our study, we identified 38 patients with low-grade BC and 79 patients with high-grade BC. After univariate analysis, we found that painful micturition (p = 0.033), wide-base tumors (p = 0.016), mossy lesions (p = 0.046), and reticular vessels in the lesion area under NBI (p = 0.013) were all factors related to high-grade BC. Further multivariate logistic regression analysis showed that reticular vessels in the lesion area under NBI (p = 0.028) were an independent risk factor for high-grade BC. These results are summarized in Supplementary Table 2.
Thick branching vessels and bladder tumors with a wide base were identified as independent risk factors for BC invasion into the lamina propria
BC was classified as invasive (T1 stage and higher, 63 patients) or non-invasive (Ta, 69 patients) according to whether the lamina propria had been invaded. Univariate analysis showed that wide-base tumors (p = 0.006), an obscure boundary around the lesion (p = 0.037), rich vessels in the basal area under NBI (p = 0.041), disorganized vessels in the lesion area (p = 0.042), and thick branching vessels in the basal area under NBI (p = 0.006) were all factors related to invasive BC. Multivariate logistic regression analysis showed that wide-base tumors (p = 0.022) and basal thick branching vessels under NBI (p = 0.021) were independent risk factors for invasive BC. These results are summarized in Supplementary Table 3.
Primary diagnosis, lesion location (beside the left ureteral orifice) and an obscure boundary around a tumor were identified as independent risk factors for muscle-invasive BC (MIBC)
We identified 19 patients with MIBC and 113 patients with non-muscle invasive BC (NMIBC). Univariate analysis showed that primary diagnosis (postoperative re-examination; p = 0.028) was a key factor related to NMIBC. Lesion location (beside the orifice of the left ureter, p = 0.015), an obscure boundary around the lesion (p = 0.015), follicular lesions (p = 0.015), and fine branching vessels in the lesion area under NBI (p = 0.048) were all associated with MIBC. Following multivariate analysis, primary diagnosis (postoperative re-examination, p = 0.028), left ureteral peritubular lesions (p = 0.022) and an obscure boundary around the lesion (p = 0.013) were independent risk factors for MIBC. These results are summarized in Supplementary Table 4.
Discussion
Cystoscopy has been widely applied for the early screening of BC and the monitoring of recurrence after treatment. The application of NBI technology can facilitate tumor detection by providing clearer imaging of vessels in the bladder. In this study, we analyzed the general and vascular morphological characteristics under NBI, and investigated related factors from NBI findings to the nature, pathological grade and stage of BC.
The results of our study demonstrated that gross hematuria was an independent risk factor for BC; this finding was similar to the conclusions of previous studies [Citation12]. In addition, we found that painful micturition was associated with high-grade BC, but could not be used as an independent risk factor for high-grade BC. This suggests that the symptoms of lower urinary tract irritation may not be specific for diagnosing and determining the pathological features of BC [Citation13].
Correlations between the morphological characters of tumors and the nature and pathological features of BC have been reported in many studies using WLI cystoscopy [Citation14,Citation15]. In general, pedunculated tumors (narrow-base tumors), tumors with a large diameter, and bladder tumors with solid components tend to be associated with a greater possibility of malignancy; these previous results are similar to our present findings in that cauliflower-like tumors can be used as an independent risk factor for BC, and that patchy lesions, flat bulges, and mossy lesions are associated with benign bladder lesions. This is because in our classification of tumor morphology, the latter three morphological types were flat and lacked solid components, whereas cauliflower-like tumors are defined as tumors clearly protruding from the bladder mucosa with a larger diameter and tumor parenchyma. Notably, cauliflower-like tumors were not classified as having a high pathological grade and greater levels of aggressiveness. Previous researchers reported that flat and wide-base tumors tend to be associated with a high pathological grade and an advanced stage of cancer [Citation16,Citation17]. We also found that mossy lesions and wide-base tumors were associated with high-grade BC and tumor invasion of the lamina propria. Wide-base tumors were identified as an independent risk factor for the invasion of BC into the lamina propria. In addition, we found that obscure lesion boundaries under NBI modality indicated an increased risk of both lamina propria invasion and muscle invasion; these findings were consistent with those of previous studies derived from WLI cystoscopy [Citation18].
In addition, we found that the location of tumors beside the left ureteral orifice was an independent risk factor for MIBC. Furthermore, different tumor locations were associated with different risks of BC. Although previous studies reported that tumor location was related to biological differences in bladder tumors [Citation19], it is difficult to provide a reasonable explanation for this result; the differences in tumor properties caused by tumor location need to be investigated further. A primary diagnosis before cystoscopy represents an independent risk factor for MIBC. Our results showed that patients with postoperative follow-up had a lower risk of BC and MIBC; this may be related to intravesical therapy and regular cystoscopy after surgery. Intravesical therapy has been shown to delay the recurrence and progression of BC while regular cystoscopy has proven helpful for the early detection and treatment of BC recurrence [Citation20,Citation21].
In this study, we investigated the correlation between vascular morphological characteristics and the nature, grade and stage of BC. The value of these characters as predictors, along with their potential for clinical application, has been demonstrated in the diagnosis and treatment of tumors of the digestive system [Citation22,Citation23]. NBI provides a clear visualization of blood vessels in the bladder. Due to their rapid growth and proliferation, malignant tumor cells require a greater nutrition supply; therefore, the density of vessels in the tumor tissue increases, and the vessels change in shape and arrange themselves in an irregular manner [Citation24].
In our study, we similarly found that vascular rich areas within lesion and basal areas were associated with BC. The significance of our study is that we identified independent risk factors related to tumor nature, pathological grade and stage. For example, dotted vessels and circumferential vessels in the area of lesions were independent risk factors for BC and that reticulated vessels in the lesion area were an independent risk factor for high-grade BC. We also found that thick branching vessels in the basal area represented an independent risk factor for invasion of the lamina propria by BC. The specific mechanisms that influence differences in the angiogenesis and distribution of blood vessles in bladder tumors remain unclear. This may be related to the heterogeneity of the tumors themselves or differences in their growth environment [Citation25].
Our findings provide new evidence that may assist clinicians in their decision making at the time of cystoscopy and may contribute to the cystoscopic determination of BC grade and stage. Although mp-MRI has been used for the diagnosis and prediction of MIBC [Citation26], our results provide a new direction for imaging diagnosis to investigate whether the combination of imaging examinations such as CT and mp-MRI, or urine exfoliative cytology and cystoscopy, can achieve a higher prediction accuracy for BC. Our study has several limitations that need to be considered. For example, the sample size of our study was small; this may have affected the significance of the association between some factors and our outcome variables. In addition, our study was an observational study; further experimental validation is required with regards to the correlation between vascular morphology and the nature, grade and stage of BC.
Conclusions
Our study found that the general and vascular morphology of bladder tumors were correlated with the nature, grade and stage of BC. These characteristics can be used as independent predictors for predicting the nature, grade and stage of BC and may help clinicians in their diagnosis and treatment planning.
Ethics approval and Patient consent
Approval was provided by the Ethics Committee of Qilu Hospital of Shandong University (Qingdao). Institutional Review Board Approval Number: KYLL-2021010.
Consent for publication
All participants of our study consented to the publication of this paper. All personal information was anonymized before publication.
Author contributions
HL, HS, QF, TXD, JW, WH, YX, NG, QYY, BKS, and JC: project development; HL, HS, QF, TXD, JW, WH, YX, NG, XWJ, SZC, YL, ZNZ, QYY, BKS, and JC: data collection or management; HL, QYY, and JC: data analysis; HL, QYY, and JC: manuscript writing. LH and JC had full access to the entire dataset and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Supplemental Material
Download MS Word (100.5 KB)Acknowledgments
The authors thank the patients who participated in this study and the personnel at each study site.
Disclosure statement
The authors declare that the study received financial support from Olympus Corporation and Qingdao Science and Technology Demonstration and Guidance Special Fund for the Benefit of the People, which may be considered a potential conflict of interest. The sponsor has not been specifically involved in the research. The authors declare that they have no competing interests.
Data availability statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Richters A, Aben KKH, Kiemeney LALM. The global burden of urinary bladder cancer: an update. World J Urol. 2020;38(8):1–8. doi:10.1007/s00345-019-02984-4.
- Jin Y-H, Zeng X-T, Liu T-Z, et al. Treatment and surveillance for non-muscle-invasive bladder cancer: a clinical practice guideline (2021 edition). Mil Med Res. 2022;9(1):44. doi:10.1186/s40779-022-00406-y.
- Witjes JA, Bruins HM, Cathomas R, et al. European association of urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2020 guidelines. Eur Urol. 2021;79(1):82–104. doi:10.1016/j.eururo.2020.03.055.
- Holmang S, Johansson SL. Stage Ta-T1 bladder cancer: the relationship between findings at first followup cystoscopy and subsequent recurrence and progression. J Urol. 2002;167(4):1634–1637. doi:10.1016/S0022-5347(05)65168-3.
- Mariappan P, Lavin V, Phua CQ, et al. Predicting grade and stage at cystoscopy in newly presenting bladder cancers-a prospective double-blind clinical study. Urology. 2017;109:134–139. doi:10.1016/j.urology.2017.08.007.
- Cauberg ECC, de Bruin DM, Faber DJ, et al. A new generation of optical diagnostics for bladder cancer: technology, diagnostic accuracy, and future applications. Eur Urol. 2009;56(2):287–296. doi:10.1016/j.eururo.2009.02.033.
- Zheng C, Lv Y, Zhong Q, et al. Narrow band imaging diagnosis of bladder cancer: systematic review and meta-analysis. BJU Int. 2012;110(11 Pt B):E680–E687. doi:10.1111/j.1464-410X.2012.11500.x.
- Giulianelli R, Gentile BC, Mirabile G, et al. Narrow band imaging reduces persistence of cancer in patients with pT1 high grade bladder cancer. Eur J Surg Oncol. 2019;45(3):466–470. doi:10.1016/j.ejso.2018.06.004.
- Backes Y, Moss A, Reitsma JB, et al. Narrow band imaging, magnifying chromoendoscopy, and gross morphological features for the optical diagnosis of T1 colorectal cancer and deep submucosal invasion: a systematic review and meta-analysis. Am J Gastroenterol. 2017;112(1):54–64. doi:10.1038/ajg.2016.403.
- Mannath J, Subramanian V, Hawkey CJ, et al. Narrow band imaging for characterization of high grade dysplasia and specialized intestinal metaplasia in barrett’s esophagus: a meta-analysis. Endoscopy. 2010;42(5):351–359. doi:10.1055/s-0029-1243949.
- Yang Q, Liu Z, Sun H, et al. A narrative review: narrow-band imaging endoscopic classifications. Quant Imaging Med Surg. 2023;13(2):1138–1163. doi:10.21037/qims-22-728.
- Compérat E, Amin MB, Cathomas R, et al. Current best practice for bladder cancer: a narrative review of diagnostics and treatments. Lancet. 2022;400(10364):1712–1721. doi:10.1016/S0140-6736(22)01188-6.
- Schmidt-Hansen M, Berendse S, Hamilton W. The association between symptoms and bladder or renal tract cancer in primary care: a systematic review. Br J Gen Pract. 2015;65(640):e769–e75. doi:10.3399/bjgp15X687421.
- Dekalo S, Matzkin H, Mabjeesh NJ. Can urologists accurately stage and grade urothelial bladder cancer by assessing endoscopic photographs? J Telemed Telecare. 2018;24(9):603–607. doi:10.1177/1357633X17727773.
- Satoh E, Miyao N, Tachiki H, et al. Prediction of muscle invasion of bladder cancer by cystoscopy. Eur Urol. 2002;41(2):178–181. doi:10.1016/s0302-2838(01)00035-5.
- Czerniak B, Dinney C, McConkey D. Origins of bladder cancer. Annu Rev Pathol. 2016;11(1):149–174. doi:10.1146/annurev-pathol-012513-104703.
- Durdin T, Goh A, Pietzak E. Can an imaging-guided pathway replace the current paradigm for muscle-invasive bladder cancer? Eur Urol. 2021;80(1):16–17. doi:10.1016/j.eururo.2021.03.018.
- Herr HW, Donat SM, Dalbagni G. Correlation of cystoscopy with histology of recurrent papillary tumors of the bladder. J Urol. 2002;168(3):978–980. doi:10.1016/S0022-5347(05)64555-7.
- Wu Q, Cai L, Yuan B, et al. The application value of multi-parameter cystoscope in improving the accuracy of preoperative bladder cancer grading. BMC Urol. 2022;22(1):111. doi:10.1186/s12894-022-01054-z.
- Tran L, Xiao J-F, Agarwal N, et al. Advances in bladder cancer biology and therapy. Nat Rev Cancer. 2021;21(2):104–121. doi:10.1038/s41568-020-00313-1.
- Teoh JY-C, Kamat AM, Black PC, et al. Recurrence mechanisms of non-muscle-invasive bladder cancer - a clinical perspective. Nat Rev Urol. 2022;19(5):280–294. doi:10.1038/s41585-022-00578-1.
- Sato H, Inoue H, Ikeda H, et al. Utility of intrapapillary capillary loops seen on magnifying narrow-band imaging in estimating invasive depth of esophageal squamous cell carcinoma. Endoscopy. 2015;47(2):122–128. doi:10.1055/s-0034-1390858.
- Oka S, Tanaka S, Takata S, et al. Clinical usefulness of narrow band imaging magnifying classification for colorectal tumors based on both surface pattern and microvessel features. Dig Endosc. 2011;23 Suppl 1(s1):101–105. doi:10.1111/j.1443-1661.2011.01108.x.
- Roudnicky F, Poyet C, Buser L, et al. Characterization of tumor blood vasculature expression of human invasive bladder cancer by laser capture microdissection and transcriptional profiling. Am J Pathol. 2020;190(9):1960–1970. doi:10.1016/j.ajpath.2020.05.020.
- Lugano R, Ramachandran M, Dimberg A. Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol Life Sci. 2020;77(9):1745–1770. doi:10.1007/s00018-019-03351-7.
- Pecoraro M, Takeuchi M, Vargas HA, et al. Overview of VI-RADS in bladder cancer. AJR Am J Roentgenol. 2020;214(6):1259–1268. doi:10.2214/AJR.20.22763.
