Abstract
Purpose
Individual genetic background can play an essential role in determining the development of esophageal squamous cell carcinoma (ESCC). PTPN13 and CHEK2 play important roles in the pathogenesis of ESCC. This case-control study aimed to analyze the association between gene polymorphisms and ESCC susceptibility.
Methods
DNA was extracted from the peripheral blood of patients. The Agena MassARRAY platform was used for the genotyping. Statistical analysis was conducted using the chi-squared test or Fisher’s exact test, logistic regression analysis, and stratification analysis.
Results
The ‘G’ allele of rs989902 (PTPN13) and the ‘T’ allele of rs738722 (CHEK2) were both associated with an increased risk of ESCC (rs989902: OR = 1.23, 95% CI = 1.02–1.47, p = 0.028; rs738722: OR = 1.28, 95% CI = 1.06–1.55, p = 0.011). Stratification analysis showed that SNPs (rs989902 and rs738722) were notably correlated with an increased risk of ESCC after stratification for age, sex, smoking, and drinking status. In addition, rs738722 might be associated with lower stage, while rs989902 had a lower risk of metastasis.
Conclusion
Our findings display that PTPN13 rs989902 and CHEK2 rs738722 are associated with an increased risk of ESCC in the Chinese Han population.
1. Introduction
Esophageal cancer (EC) is a common malignant tumor of the digestive system. The incidence and mortality of EC in China account for about half of the world [Citation1]. EC mainly includes two subtypes: esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC) [Citation2]. Squamous cell carcinoma is the most common pathological type of esophageal cancer in China [Citation3]. At present, the international treatment methods for esophageal cancer are mainly for ECA [Citation1], while the treatment and prognosis of ESCC patients are still poor. Studies have shown that the formation of ESCC is a multi-factor and multi-stage process, which is the result of the interaction of environmental risk factors and individual genetic factors [Citation4,Citation5]. Different individuals have different genetic susceptibility to esophageal cancer, and a family history of ESCC has a higher incidence [Citation6]. There are differences in the incidence of ESCC even when exposed to the same risk factors [Citation7]. The above studies indicate that genetic factors play a crucial role in the occurrence and development of ESCC [Citation8]. Some single nucleotide polymorphisms (SNPs) have been reported to play an important role in the progression and prognosis of ESCC, but the pathogenesis of ESCC is still not fully understood [Citation9–11]. Screening for genetic polymorphisms significantly associated with ESCC susceptibility in a specific population will help to screen high-risk individuals with genetic susceptibility in the clinic, and then take targeted preventive measures.
Protein tyrosine phosphatase non-receptor type 13 (PTPN13), located on chromosome 4q21.3, encodes a non-receptor protein phosphate with the highest molecular weight (270 kDa; 2466 amino acids) [Citation12]. PTPN13 is not only a carcinogenic protein but also a tumor suppressor [Citation13]. Most PTPN family members are associated with digestive tract cancers. Aberrant activation of the protein tyrosine kinase (PTK) receptor signaling can contribute to various types of human cancers, including ESCC [Citation14]. Inhibiting the expression of PTPN13 in esophageal adenocarcinoma cell lines might promote proliferation and migration and increase phosphorylation of the EGFR/Src pathway [Citation15]. Previous studies have shown that rs989902 in PTPN1 is significantly associated with the risk of colorectal cancer (CRC) [Citation16], breast cancer (BC) [Citation17], and squamous cell carcinoma of the head and neck [Citation18]. However, an association between PTPN13-rs989902 and susceptibility to ESCC has not yet been reported.
Cell cycle checkpoint kinase 2 (CHEK2) is an established BC susceptibility gene. Its protein plays an important role in cell cycle regulation and DNA damage repair [Citation19]. Studies have shown that women carrying the pathogenic CHEK2 variant are approximately three times more likely to develop BC [Citation20]. CHEK2 is a predisposing factor for many types of cancers [Citation21]. The results of whole-genome sequencing showed that there was a mutation in the CHEK2 gene in esophageal squamous cell carcinoma [Citation22]. Previous results regarding the correlation between CHEK2 rs738722 and ESCC risk were inconsistent; one relevant study reported a risk-increasing association, whereas another showed no correlation [Citation23,Citation24].
Therefore, we analyzed the relationship between PTPN1 rs989902 (G/T) and CHEK2 rs738722 (T/C) and ESCC risk in the Chinese Han population. We further performed stratification analysis by age, sex, BMI, smoking, and drinking to evaluate the impact of epidemiological features on genetic association. In addition, the relationship between candidate SNPs and stage and metastasis in patients with ESCC was explored.
2. Materials and methods
2.1. Subject recruitment and ethics committee statement
A total of 506 primary ESCC patients and 507 healthy controls were enrolled in this study (). All participants were recruited from Hainan Cancer Hospital. Patients with ESCC are diagnosed and histologically confirmed by ultrasound, magnetic resonance imaging (MRI), or computed tomography (CT) according to specific histopathological or morphological criteria. Patients with distant esophageal tumor metastasis, history of other malignant tumors, and receiving radiotherapy or chemotherapy were also excluded. Healthy controls had no history of chronic or severe endocrine, nutritional, metabolic, cancer, or other diseases.
Table 1. The characteristics of case and control.
Both the control and case groups were informed of the procedures and objectives of this study, both written and oral, and informed consent was obtained. The use and protocol of human tissue in this study strictly followed the principles of the Declaration of Helsinki and was approved by the Ethics Committee of Hainan Cancer Hospital. All subsequent research analyses were conducted by the approved guidelines.
2.2. SNP selection and genotyping
Peripheral blood (5 mL) was collected in ethylenediaminetetraacetic acid (EDTA)-coated tubes. Genomic DNA was extracted using a Goldmag-Mini Purification Kit (GoldMag Co. Ltd., Xi’an, China) according to the manufacturer’s instructions. A NanoDrop 2000 (Thermo Fisher Scientific, Waltham, Massachusetts, USA) was used to measure the concentration and purity of DNA. In the 1000 Genomes Project (http://www.internationalgenome.org/), two SNPs (rs989902 G/T and rs738722 T/C) with a minor allele frequency (MAF) > 5% were selected. Genotyping was performed using the MassARRAY platform (Agena Bioscience, San Diego, CA). USA). It is mainly completed using matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF), measuring the quality of the extension primers, and then identifying the SNP alleles. The genotyping step and PCR conditions can be found in the MassARRAY ® -IPLEX SNP Genotyping System Guide (http://www.agenabio.com) [Citation25]. Specific amplification and extension primers were designed using MassARRAY Assay Design software (https://support.agenabio.com/s/online-tools). The primer sequences are displayed in Supplemental Table 1.
2.3. Statistical analyses
Pearson’s χ2 test and independent sample Student’s t-test were used to assess differences in the distribution of demographic characteristics. The Hardy-Weinberg equilibrium (HWE) test in controls was performed by comparing the observed and expected genotype frequencies. All statistical analyses were performed using the SPSS software (version 19.0; SPSS, Chicago, IL) and PLINK software. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to estimate the association between candidate SNPs and ESCC risk using unconditional logistic regression analysis [Citation26]. The genotype model (co-dominant, dominant, recessive, and log-additive) was used to evaluate the genetic relationship between the candidate SNPs and ESCC susceptibility. In addition, we used FPRP (positive report probability (FPRP) analysis to detect whether the positive result observed in this association study is a noteworthy finding (FPRP threshold is 0.2, prior probability level is 0.25) [Citation27]. All p values of the statistical tests were two-sided, and p < 0.05.
2.4. Functional annotation based on the GTEx databases
The Genotype-Tissue Expression (GTEx) database (https://www.gtexportal.org) was used to predict the difference in candidate gene expression in esophageal tissues under different genotypes of candidate SNPs, and then evaluate whether SNP can affect gene expression.
3. Results
3.1. Characteristics of the participants
A total of 1013 subjects were interviewed, including 506 patients (374 males and 132 females; mean age:63.69 ± 9.26 years) and 507 healthy control subjects (375 males and 132 females; mean age:63.51 ±7.76 years). The clinical characteristics of the patients and controls are shown in . There were no significant differences in sex or age distribution between patients with ESCC and healthy controls (p = 0.521 and p = 0.448, respectively).
3.2. Associations between candidate SNPs and ESCC risk
The allele frequencies and basic information for these SNPs are shown in . All SNPs were in the HWE in the control group (p = 0.693 and p = 0.396, respectively). The chi-squared test was used to estimate the genetic association of candidate SNPs with ESCC risk in the allele model. The frequency of the ‘G’ allele of rs989902 was significantly higher in ESCC cases than in controls (39.0% vs 34.3%), which suggested that the ‘G’ allele of rs989902 could contribute to an increased ESCC risk (OR = 1.23, 95% CI = 1.02–1.47, p = 0.028). The frequency of the ‘T’ allele of rs738722 was also significantly much higher in ESCC cases than in controls (33.4% vs 28.1%), which suggested that the ‘T’ allele of rs738722 was a risk factor for increased ESCC risk (OR = 1.28, 95% CI = 1.06–1.55, p = 0.011).
Table 2. Basic information of candidate SNPs and minor allele frequency between cases and controls.
Next, we examined the genetic relationship between candidate SNPs and ESCC susceptibility in the genetic model (.). Our analyses showed that rs989902 was associated with a 1.34-fold risk in the genotype model (95% CI = 1.02-1.74, p = 0.033), a 1.35-fold risk in the dominant model (95% CI = 1.05–1.74, p = 0.019), and a 1.23-fold risk in the log-additive model (95% CI = 1.02–1.48, p = 0.026). Furthermore, rs738722 was associated with a 1.33-fold and 1.56-fold risk in the genotype model (95% CI = 1.02–1.72 for ‘C/T’ genotype; 95% CI = 1.01–2.43, p = 0.034 for ‘TT’ genotype), 1.37-fold risk in the dominant model (95% CI = 1.07–1.75, p = 0.013), and 1.28-fold risk in the log-additive model (95% CI = 1.06–1.55, p = 0.011).
Table 3. Association between candidate SNPs and the risk of ESCC.
3.3. Associations between candidate SNPs and ESCC risk in stratified analysis
Sex: The results of the stratified analysis showed that rs989902 (GT vs. TT: OR = 1.90, p = 0.017; GT-GG vs. TT: OR = 1.85, p = 0.017) and rs738722 (OR = 1.46, p = 0.046) were associated with an increased risk of ESCC in females. There was no candidate genetic locus that had an association with susceptibility to ESCC in males. The details can be seen in .
Table 4. The association between candidate SNPs and ESCC risk in the sex, age and BMI stratification.
Age: A significant association was observed between rs738722 and increased risk of ESCC in subjects aged < 64 years (CT vs. CC: OR = 1.50, p = 0.037; TC-TT vs. CC: OR = 1.51, p = 0.025; and log-additive: OR = 1.33, p = 0.040), whereas rs989902 (OR = 1.44, p = 0.045) was associated with an increased risk of ESCC in subjects aged ≥ 64 years (.).
BMI: There was a significant association between rs738722 and an increased risk of ESCC in the subjects with BMI ≥ 24 kg/m2 (allele: OR = 1.71, p = 0.017; genotype: OR = 1.98, p = 0.045, and OR = 2.02, p = 0.032; dominant: OR = 2.14, p = 0.018; log-additive: OR = 1.98, p = 0.009). . showed that there was no candidate genetic locus associated with susceptibility to ESCC among subjects with BMI < 24 kg/m2.
Smoking status: Rs738722 may confer an increased risk of ESCC among non-smokers under the allele (OR = 1.51, p = 0.002), genotype (OR = 1.69, p = 0.006; OR = 1.88, p = 0.036), dominant (OR = 1.72, p = 0.002), and log-additive (OR = 1.47, p = 0.004) models. Rs989902 had no association with susceptibility to ESCC whether in smoking or non-smoking subjects. The details can be found in .
Table 5. The association between candidate SNPs and ESCC risk in stratified analysis (smoking, drinking, stage, and metastasis).
Drinking status: An association between rs738722 and increased risk of ESCC among drinking subjects has been found (T vs. C: OR = 1.40, p = 0.016; TT vs. CC: OR = 2.17, p = 0.023; log-additive: OR = 1.41, p = 0.016). There was no evidence indicating that rs989902 was associated with susceptibility to ESCC among drinking or non-drinking subjects ().
Stage: We divided case groups according to the stage of ESCC patients for association analysis (). The results showed that rs738722 was associated with higher-stage ESCC patients (OR = 0.58, p = 0.019). We found no evidence that rs989902 was associated with ESCC susceptibility in stratified analysis by stage.
Metastasis: We also divided case groups according to whether ESCC patients developed metastasis or not for stratified analysis (). We found evidence that rs989902 was strongly associated with ESCC patients with metastasis (GT vs. TT: OR = 0.61, p = 0.036; GT-GG vs. TT: OR = 0.64, p = 0.046). Rs738722 had no association with metastasis of ESCC.
3.5. FPRP analysis
FPRP analysis showed that (Supplemental Table 2) the association between CHEK2-rs738722 and ESCC in participants with BMI ≥24 kg/m2 may be not noteworthy at the prior probability level of 0.25 and FPRP threshold of 0.2. However, the positive results observed in this study are noteworthy.
3.6. Functional annotation
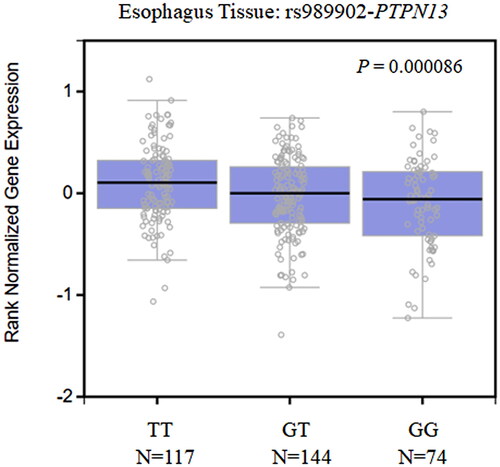
We evaluated the relationship between rs989902 in PTPN13 and rs738722 in CHEK2 and the expression level of genes using the expression quantitative trait loci (eQTL) browser of the GTEx database. As shown in , a significant association was observed between the expression level of PTPN13 and the different genotypes of rs989902 (p = 8.6E-5) in normal esophageal tissues. Specifically, the relative expression of PTPN13 was significantly lower in subjects with the GT/GG genotype than in those with the TT genotype. No data on the association between the rs738722 genotypes and the expression level of CHEK2 were found in the GTEx database.
4. Discussion
In this study, we investigated the association between PTPN13 rs989902 and CHEK2 rs738722 and ESCC risk in the Chinese Han population. Our results showed that rs989902 and rs738722 are associated with an increased risk of ESCC. In particular, confounding factors, such as age, sex, smoking, and drinking status, may contribute to the association of these SNPs with ESCC susceptibility. These results suggest that PTPN13 and CHEK2 polymorphisms may play catalytic roles in the development of ESCC in the Chinese Han population.
PTPN13 has been mapped to the human chromosomal locus 4q21. Studies have suggested that PTPN13 polymorphisms are associated with cancer susceptibility [Citation14]. Niu et al. hypothesized that the genetic polymorphisms of PTPN13 may be a biomarker for susceptibility to squamous cell carcinoma of the head and neck (SCCHN) in populations in the USA [Citation18]. Laczmanska et al. found that PTPN13 (rs989902) was significantly associated with an increased risk of CRC in the Polish population [Citation16]. A case-control study in the Japanese population showed that PTPN13 SNPs increased the risk of cancers other than ESCC [Citation28]. Currently, there are no reports on the relationship between PTPN13 rs989902 and ESCC risk. We are the first to find that PTPN13-rs989902 is associated with an increased risk of EC, indicating that PTPN13-rs989902 may be a biomarker for susceptibility to EC. PTPN13 rs989902 (c.6241 T > G Y2081D) is a functional polymorphism in the PTPN13 coding regions. The GTEx database predicted that PTPN13 has lower expression in normal esophageal tissues with the mutant allele (GG) compared to other genotypes, suggesting that rs989902 is associated with the level of PTPN13 mRNA. Combined with the results of this study, we speculated that PTPN13-rs989902 affected susceptibility to ESCC by affecting the expression level of PTPN13. Based on the above, it is necessary to further design a functional study of PTPN13-rs989902 and explore the specific mechanism by PTPN13 rs989902 affects ESCC susceptibility. To our knowledge, this study is the first to show that PTPN13-rs989902 is associated with susceptibility to ESCC, which will provide new insights into the pathological mechanism of EC.
CHEK2 is located on chromosome 22q12.1. A previous study has shown that CHEK2 mutations are associated with an increased risk of cancer. However, the effects of the genetic mutations of this gene on different cancers are different, which shows that, to some extent, there is scope for dispute regarding these studies. For example, a study reported that genetic mutations in CHEK2 were not associated with susceptibility to BC in the Iranian population [Citation29]. Lawrenson et al. showed that CHEK2 may be the strongest risk factor for epithelial ovarian cancer [Citation30]. Furthermore, CHEK2 has also been linked to the genetic susceptibilities of prostate cancer [Citation31], thyroid cancer [Citation32], and lung cancer [Citation33]. Gu et al. found that rs738722 on the CHEK2 gene was not correlated with the risk of ESCC based on an associated study of 380 cases and 380 controls [Citation34]. However, Xiaobin J et al. reported that rs738722 may be used as a genetic biomarker for increasing ESCC susceptibility [Citation35], which is inconsistent with previous results. The dissimilarity in these reports may be due to the complexity of gene and environmental interactions or the small sample size. The results of this study showed that CHEK2 rs738722 was associated with increased ESCC risk in the Chinese Han population.
An epidemiological study on esophageal squamous cell carcinoma has shown that the occurrence of ESCC is affected by smoking/drinking status, gender, genetic factors, et al. the etiology of ESCC is multifactorial [Citation36]. Previous studies have found that the occurrence of ESCC in many countries is attributable to the prevalence of smoking and alcohol abuse [Citation37]. A gender difference in the prevalence of ESCC was also found [Citation38]. Studies have shown that ESCC is associated with increased BMI [Citation36]. Accordingly, we have also divided the subjects according to BMI, age, sex, smoking/drinking status, and performed a stratified analysis. Stratified analysis showed that PTPN13 rs989902 was significantly associated with an increased risk of ESCC in females and subjects aged ≥ 64 years. And rs989902 was also observed to be associated with ESCC metastasis. We have also found that the risk associated with CHEK2 variant genotypes differed by age, sex, BMI, smoking and drinking status, and stage. Combined with the results of previous studies and this study, it can be further demonstrated that ESCC is the result of environmental and genetic interaction. To our knowledge, there have been no reports on the correlation between PTPN13 and CHEK2 variants and ESCC susceptibility in Chinese Han population. The candidate PTPN13 and CHEK2 variants in this study are expected to be new targets for individualized prevention and treatment of ESCC.
Taken together, our study showed that PTPN13 and CHEK2 variants were associated with the risk of ESCC in the Chinese Han population, which sheds light on the new candidate genes and new perspectives for the study of the subsequent occurrence mechanism of EC. Some limitations of our study should be considered when the results are interpreted. Our sample size was small, and the population was single; therefore, we needed to expand the sample size. Moreover, the lack of information on environmental factors is one of the most important limitations of this study. We can expand this range when we study haplotypes to obtain more findings. For the most basic reason of our current research, it is necessary to acquire further functional studies to understand the in-depth genetic factors underlying ESCC.
Conclusion
Our findings showed that PTPN13 rs989902 and CHEK2 rs738722 are associated with an increased risk of ESCC in the Chinese Han population. Our study provides accumulating evidence for an association between PTPN13 and CHEK2 polymorphisms and susceptibility to EC.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Hainan Cancer Hospital. This study conformed to the ethical principles for medical research involving humans outlined in the World Medical Association Declaration of Helsinki. All participants signed an informed consent form before participating in the study.
Consent to publication
All authors agreed to publish the manuscript.
Authors’ contributions
Conceptualization, Jian Song; methodology, Dunjing Zhong; Ping Li and Yongyu Li, software; Zhuang Chen and Feixiang Hu; data curation, Guihong Yuan, Zhaowei Chen, and Shuyong Yu; writing, review, and editing; Ruisha Tu. All authors have read and approved the manuscript.
Supplemental Material
Download MS Word (19.1 KB)Acknowledgments
We thank all patients and individuals for their participation, as well as clinicians and other hospital staff. We are also grateful to the editors and reviewers for their patience and valuable comments on this work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and analyzed in the current study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):1–10. doi: 10.3322/caac.21492.
- Harada K, Rogers JE, Iwatsuki M, et al. Recent advances in treating oesophageal cancer. F1000Res. 2020;9:1189. doi: 10.12688/f1000research.22926.1.
- Niu C, Liu Y, Wang J, et al. Risk factors for esophageal squamous cell carcinoma and its histological precursor lesions in China: a multicenter cross-sectional study. BMC Cancer. 2021;21(1):1034. doi: 10.1186/s12885-021-08764-x.
- Li Y, Yang B, Ma Y, et al. Phosphoproteomics reveals therapeutic targets of esophageal squamous cell carcinoma. Signal Transduct Target Ther. 2021;6(1):381. doi: 10.1038/s41392-021-00682-5.
- Ko KP, Huang Y, Zhang S, et al. Key genetic determinants driving esophageal squamous cell carcinoma initiation and immune evasion. Gastroenterology. 2023;165(3):613–628.e20. doi: 10.1053/j.gastro.2023.05.030.
- Chen T, Cheng H, Chen X, et al. Family history of esophageal cancer increases the risk of esophageal squamous cell carcinoma. Sci Rep. 2015;5(1):16038. doi: 10.1038/srep16038.
- Dong J, Buas MF, Gharahkhani P, et al. Determining risk of Barrett’s esophagus and esophageal adenocarcinoma based on epidemiologic factors and genetic variants. Gastroenterology. 2018;154(5):1273–1281.e3. doi: 10.1053/j.gastro.2017.12.003.
- Jajosky A, Fels Elliott DR. Esophageal cancer genetics and clinical translation. Thorac Surg Clin. 2022;32(4):425–435. doi: 10.1016/j.thorsurg.2022.06.002.
- Mahmood MQ, Shukla SD, Dua K, et al. The role of epidermal growth factor receptor in the management of gastrointestinal carcinomas: present status and future perspectives. Curr Pharm Des. 2017;23(16):2314–2320. doi: 10.2174/1381612823666170124115159.
- Shen Y, Shao Y, Ruan X, et al. Genetic variant in miR-17-92 cluster binding sites is associated with esophageal squamous cell carcinoma risk in Chinese population. BMC Cancer. 2022;22(1):1253. doi: 10.1186/s12885-022-10360-6.
- Xu X, Sun Z, Rong L, et al. Genetic variant of ADH1C for predicting survival in esophageal squamous cell cancer patients who underwent postoperative radiotherapy. Front Genet. 2022;13:988433. doi: 10.3389/fgene.2022.988433.
- van den Maagdenberg AM, Olde Weghuis D, Rijss J, et al. The gene (PTPN13) encoding the protein tyrosine phosphatase PTP-BL/PTP-BAS is located in mouse chromosome region 5E/F and human chromosome region 4q21. Cytogenet Cell Genet. 1996;74(1–2):153–155. doi: 10.1159/000134405.
- McHeik S, Aptecar L, Coopman P. et al. Dual Role of the PTPN13 Tyrosine Phosphatase in Cancer. Biomolecules. 2020;10(12):1659.
- Freiss G, Chalbos D. PTPN13/PTPL1: an important regulator of tumor aggressiveness. Anticancer Agents Med Chem. 2011;11(1):78–88. doi: 10.2174/187152011794941262.
- Yu M, Maden SK, Stachler M, et al. Subtypes of barrett’s oesophagus and oesophageal adenocarcinoma based on genome-wide methylation analysis. Gut. 2019;68(3):389–399. doi: 10.1136/gutjnl-2017-314544.
- Laczmanska I, Karpinski P, Gil J, et al. The PTPN13 Y2081D (T > G) (rs989902) polymorphism is associated with an increased risk of sporadic colorectal cancer. Colorectal Dis. 2017;19(7):O272–O278. O272-o8. doi: 10.1111/codi.13727.
- Wei W, Jiang M, Luo L, et al. Colorectal cancer susceptibility variants alter risk of breast cancer in a chinese han population. Genet Mol Res. 2013;12(4):6268–6274. doi: 10.4238/2013.December.4.14.
- Niu J, Huang YJ, Wang LE, et al. Genetic polymorphisms in the PTPN13 gene and risk of squamous cell carcinoma of head and neck. Carcinogenesis. 2009;30(12):2053–2058. doi: 10.1093/carcin/bgp265.
- Shieh SY, Ahn J, Tamai K, et al. The human homologs of checkpoint kinases Chk1 and Cds1 (Chk2) phosphorylate p53 at multiple DNA damage-inducible sites. Genes Dev. 2000;14(3):289–300. doi: 10.1101/gad.14.3.289.
- Nguyen-Dumont T, Dowty JG, Steen JA, et al. Population-based estimates of the age-specific cumulative risk of breast cancer for pathogenic variants in CHEK2: findings from the Australian breast cancer family registry. Cancers . 2021;13(6):1378. doi: 10.3390/cancers13061378.
- Fei-Fei H, Chang-Long G, Li-Hong L. The effect of CHEK2 variant I157T on cancer susceptibility: evidence from a meta-analysis. DNA Cell Biol. 2013;32(6):329–335. doi: 10.1089/dna.2013.1970.
- Hu N, Kadota M, Liu H, et al. Genomic landscape of somatic alterations in esophageal squamous cell carcinoma and gastric cancer. Cancer Res. 2016;76(7):1714–1723. doi: 10.1158/0008-5472.CAN-15-0338.
- Jia X, Liu P, Zhang M, et al. Genetic variants at 6p21, 10q23, 16q21 and 22q12 are associated with esophageal cancer risk in a Chinese Han population. Int J Clin Exp Med. 2015;8(10):19381–19387.
- Yang J, Wu H, Wei S, et al. HPV seropositivity joints with susceptibility loci identified in GWASs at apoptosis associated genes to increase the risk of esophageal squamous cell carcinoma (ESCC). BMC Cancer. 2014;14(1):501. doi: 10.1186/1471-2407-14-501.
- Gabriel S, Ziaugra L. SNP genotyping using sequenom MassARRAY 7K platform. Curr Protoc Hum Genet. 2004; Chapter 2:Unit 2.12. doi: 10.1002/0471142905.hg0212s42.
- Bland JM, Altman DG. Statistics notes. The odds ratio. BMJ. 2000;320(7247):1468–1468. doi: 10.1136/bmj.320.7247.1468.
- Wacholder S, Chanock S, Garcia-Closas M, et al. Assessing the probability that a positive report is false: an approach for molecular epidemiology studies. J Natl Cancer Inst. 2004;96(6):434–442. doi: 10.1093/jnci/djh075.
- Mita Y, Yasuda Y, Sakai A, et al. Missense polymorphisms of PTPRJ and PTPN13 genes affect susceptibility to a variety of human cancers. J Cancer Res Clin Oncol. 2010;136(2):249–259. doi: 10.1007/s00432-009-0656-7.
- Jalilvand M, Oloomi M, Najafipour R, et al. An association study between CHEK2 gene mutations and susceptibility to breast cancer. Comp Clin Path. 2017;26(4):837–845. doi: 10.1007/s00580-017-2455-x.
- Kate L, Iversen ES, Jonathan T, et al. Common variants at the CHEK2 gene locus and risk of epithelial ovarian cancer. Carcinogenesis. 2015;36(11):1341–1353. doi: 10.1093/carcin/bgv138.
- Wang Y, Dai B, Ye D. CHEK2 mutation and risk of prostate cancer: a systematic review and meta-analysis. Int J Clin Exp Med. 2015;8(9):15708–15715.
- Monika SE, Cezary C, Danuta GSP, et al. CHEK2 mutations and the risk of papillary thyroid cancer. Int J Cancer. 2015;137(3):548–552.
- Xu W, Liu D, Yang Y, et al. Association of CHEK2 polymorphisms with the efficacy of platinum-based chemotherapy for advanced non-small-cell lung cancer in Chinese never-smoking women. J Thorac Dis. 2016;8(9):2519–2529. doi: 10.21037/jtd.2016.08.70.
- Gu H, Qiu W, Wan Y, et al. Variant allele of CHEK2 is associated with a decreased risk of esophageal cancer lymph node metastasis in a Chinese population. Mol Biol Rep. 2012;39(5):5977–5984. doi: 10.1007/s11033-011-1410-1.
- Jia X, Liu P, Zhang M, et al. Genetic variants at 6p21, 10q23, 16q21 and 22q12 are associated with esophageal cancer risk in the Chinese Han population. Int J Clin Exp Med. 2015;8(10):19381.
- Abnet CC, Arnold M, Wei WQ. Epidemiology of esophageal squamous cell carcinoma. Gastroenterology. 2018;154(2):360–373. doi: 10.1053/j.gastro.2017.08.023.
- Chung CS, Lee YC, Wang CP, et al. Secondary prevention of esophageal squamous cell carcinoma in areas where smoking, alcohol, and betel quid chewing are prevalent. J Formos Med Assoc. 2010;109(6):408–421. doi: 10.1016/S0929-6646(10)60072-1.
- Ferndale L, Aldous C, Hift R, et al. Gender differences in oesophageal squamous cell carcinoma in a South African tertiary hospital. Int J Environ Res Public Health. 2020;17(19):7086. doi: 10.3390/ijerph17197086.