Abstract
Colorectal cancer (CRC) is one of the most common cancers worldwide and it involves various biomolecular and cellular levels. CRC has possibly happened due to aging, urbanization, and diet. Different foods have varying effects on the gastrointestinal cells, that’s why additional research is necessary to create effective medical interventions. This review aimed to evaluate the correlation between dietary and nutritional status on the outcome of CRC patients. Study results showed that a healthy diet such as fruit and vegetables is the best diet for improving colorectal cancer outcomes. Moreover, nutritional status affected CRC patients’ outcomes, where high BMI increases the risk of having CRC. However, low BMI was associated with CRC progression and poor quality of life.
Introduction
Colorectal cancer (CRC) is characterized as a precancerous lesion from the normal colonic and rectal epithelium [Citation1]. CRC is the third most frequent malignancy and the fourth leading cause of cancer mortality. The majority of CRC cases are found in Western nations, and its prevalence is growing annually. The chance of developing colorectal cancer is about 4%–5%, and the risk depends on certain personal characteristics or behaviors such as age, history of chronic diseases and lifestyle [Citation2,Citation3].
The incidence of this disease in the United States of America in 2019 was over 1.5 million people of 776,120 men and 768,650 women. Approximately one-third (35%) of these individuals were diagnosed within five years. More than half (56%) are aged 65–84 years, although the incidence in the young population is increasing annually [Citation4–6]. CRCs are often preceded by a noncancerous proliferation of mucosal epithelial cells. These polyps can develop slowly for ten to twenty years before becoming malignant. The most prevalent histological pattern in advanced stages of cancer is differentiated adenocarcinoma, which accounts for 53.52% of all stages described as grade III or IV [Citation7].
Human development levels differ by tenfold in colorectal cancer incidence and death rates of CRC. This suggests that inequities are expanding, and the burden on nations in transition increases [Citation8]. In 2020, there were 17,368 cases of CRC in Indonesia, with 9444 deaths [Citation9]. Meanwhile, the survival rate for CRC is concerning 64% cases in five years but only 12% for poor grade CRC cases. More study is still needed to develop sensible approaches for medical preventive and rehabilitative development [Citation10]. A country’s quality of care and the availability of a population screening program both have an impact on the stage distribution at diagnosis, which in turn affects mortality rates [Citation11].
CRC may develop through various mechanisms, including chromosomal instability (CIN), microsatellite instability (MSI), and inflammatory pathways [Citation12]. A study by Yusuf et al. found that CRC was associated with a mutation on chromosome 15 in the Indonesian population [Citation13]. The CRCs that develop into the colon or rectum wall can penetrate blood or lymphatic vessels, permitting metastasis to remote organs thru the blood or close by lymph nodes [Citation14].
CRC was possibly due to aging, urbanization, increased alcohol consumption, obesity, smoking, and suboptimal diet [Citation15]. Diet may have a role in the development of CRC either negatively or positively, since it substantially influences the colon’s microbiome, with various foods having varying impacts on the microflora population and intestinal inflammation [Citation14]. Inappropriate dietary patterns reported could be increase CRC risk [Citation16].
Supplements that give a greater level of individual nutrient intake than the diet provide an alternate option for the nutritional prevention of colorectal cancer (chemoprevention) [Citation17]. Protective factors associated with a decrease in the incidence of CRC include regular physical activity, a diet rich in fruits and vegetables, garlic, high fiber diet, folate-rich diet, Calcium, Dairy products, Vitamin D, and Vitamin B6, magnesium intake, and fish consumption. However, increasing red or processed meat consumption may raise the chance to become CRC [Citation18–21]. Numerous studies are required to conclusively establish the advantages and hazards of folate on colorectal cancer incidence and death rates [Citation22]. The data on body size (weight, height, and BMI) indicated that higher weight (women only) and height (both genders) were related to an increased risk of colorectal cancer, but not BMI [Citation23].
This review purposed to evaluate the relevance between dietary and nutritional status to colorectal cancer patients and provide recommendations for doctors and patients to carry out preventive and curative diet and nutrition programs in CRC patients.
Method
Literature search
A literature search undertaken from multiple databases, including PUBMED, MEDLINE, and EMBASE, for this systematic review. This will be conducted using the keywords of (((colorectal cancer) OR (bowel cancer) OR (colon cancer) OR (rectal cancer) OR (colorectal carcinoma) OR (bowel carcinoma) OR (rectal carcinoma)) AND ((nutrition) OR (body mass index) OR (nourishing) OR (nourishment))). Furthermore, numerous valid research from outside the database will be added when the conditions are met.
Study eligibility and screening criteria
The study criteria for inclusion in this systematic review are (1) The design is an observational study; (2) The language used is Indonesian or English; (3) Nutritional status, dietary intake, or supplementation as a source of exposure; (4) Evaluated Incidence, recurrence, complication mortality, and quality of life in CRC patients. The following research requirements were be disregarded: (1) No abstract; (2) Unavailable access for Full-text; and (3) Incorrect article type. Studies of the literature that meet the eligibility requirements were included, while the others were eliminated with further explanation. The conflicts arising from combining the research were considered together until a conclusion is reached. The study was conducted according to PRISMA guidelines.
Data collection
Data grouping were conducted for (1) Main author; (2) Year of publication; (3) Research location; (4) Design of the study; (5) Sample sizes; (6) Mean/Range age; (7) Exposure Type; (8) Outcome. Data collection was conducted by RM, AB, AC, SW, H, NI and then cross-checked by TH, HR, AT, AIA, FNH, AMA, and IC. The author is contacted when included research has missing data; however, the research is eliminated when answers are not given.
Quality assessment
Five reviewers consisting of AT, AM, IC, SW, and AC assessed the quality of the observational research using the Newcastle-Ottawa Scale (NOS) [Citation24].
In a cross-sectional and case-control study, an evaluation became completed on 3 major aspects: selection, comparability, and exposure. Meanwhile, in a cohort study, the exposure issue became changed with an evaluation of the study result. The evaluation is conducted through a point-by-point discussion. The Cochrane risk of bias tools examined the randomized controlled trial [Citation25] across seven domains. (1) Random Sequence Generation; (2) Allocation concealment; (3) Blinding of contributors and personnel; (4) Blinding of final results assessment; (5) Incomplete final results statistics (6) Selective reporting (7) Additional biases. Each domain will be assigned a low, high, and unclear risk score. In addition, the results of quality assessments from RCT studies are reported in graphical form.
Results
Literature search and screening
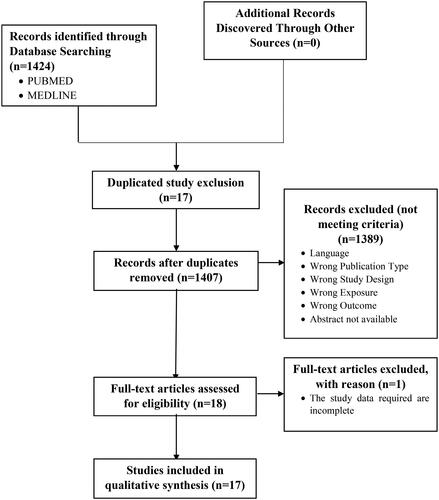
In this systematic review, the keywords obtained from 1424 studies were filtered using predetermined criteria after searching PUBMED and MEDLINE. Prior to the screening, 17 duplicated studies were removed. In addition, 1407 research papers’ titles and abstracts were evaluated by three independent reviewers (RM, SW, IC). A total of 1389 studies were removed because they did not meet predefined criteria. The next 18 were screened by thoroughly reading the text. As a result, one study was removed due to an incomplete result, leaving 17 studies with a total sample size of 30.482 that met the criteria and were considered for qualitative analysis. depicts the entire set of search and filter results.
Characteristics of the eligible studies
The 17-research considered were primary investigations done in 13 different countries and included a total sample size of 30,482 participants. Ten of the seventeen studies used a cohort design, and three employed a case-control design, one employed a cross-sectional design, and three employed an intervention design. Furthermore, seven research looked at the influence of nutritional status on colorectal cancer, while 12 looked at the effect of food on colorectal cancer. summarizes the features of the included studies in detail.
Table 1. Characteristics of inclusion studies.
Quality assessment result
The quality of the 14 included observational studies was represented by the total number of stars obtained for each study ranging from 0 to 9. For example, the lowest score in one cross-sectional study was four stars; the average score in six studies was six stars; and the highest score in two studies was eight stars. contains the exact data from the quality assessment.
Table 2. Quality assessment result using Newcastle-Ottawa Scale.
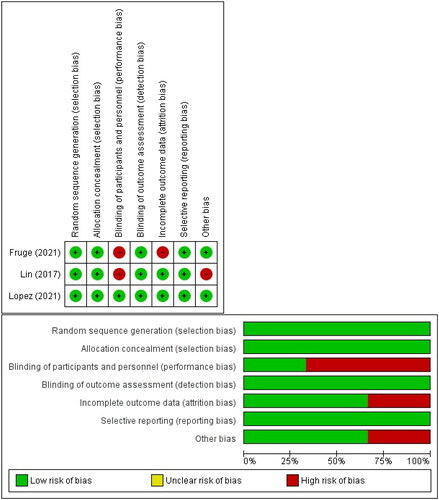
The quality of three randomized controlled trials was assessed using graphs and a risk of bias summary. In terms of participant blinding, two studies had a significant risk of bias. This is due to the fact that patients are aware of the intervention delivered, and the outcome measured is subjective; hence, there is a significant possibility of bias. One additional research, however, found a low risk of bias over the whole domain, and displays the complete breadth of the quality evaluation.
Discussion
Nutritional status has a significant impact on the occurrence and progression of colorectal cancer. Ten studies assess the role of nutritional status in colorectal cancer for various outcomes [Citation26–33]. Five studies evaluate the nutritional status from the body mass index (BMI) aspect [Citation26–31]. Studies conducted by Guinter et al. and Feliciano et al. assessed the impact of BMI on colorectal cancer incidence [Citation26,Citation27]. Those studies showed that increased BMI was strongly associated with colorectal cancer incidence. The increase in BMI indicates excess body fat, which can impair the body metabolic system and be colorectal cancer. The Feliciano et al. study also assessed the impact of BMI on colorectal cancer mortality. This study reported that BMI ≥ 35 kg/m2 was associated with higher cancer mortality rates with HR 1.40 and 95%CI 1.04–1.88. Meanwhile, a study conducted by Kurk et al. reported that the loss in BMI was associated with early colorectal cancer progression but was not associated with late disease progression [Citation28]. The study examines the incidence, course of illness and the effect of BMI on the quality of life of colorectal cancer patients. Furthermore, Formica et al. reported that BMI was strongly associated with the quality of life of colorectal cancer patients, where BMI < 23 kg/m2 was associated with a poor score of health-related quality of life (HRQoL) [Citation29].
The study conducted by Lee et al. assessed the Impact of BMI on colorectal cancer patient recurrence and survival. This study showed that BMI or even body weight was not associated with colorectal cancer recurrence and survival. A large cohort study is recommended to validate these findings because the results of Lee et al. conflict with previous conclusions that BMI or body weight was connected with colorectal cancer recurrence and survival [Citation30]. This systematic review also includes research that looked at the effect of BMI on the chance of developing advanced colorectal neoplasia (AN), which is a combination of colorectal cancer and advanced precancerous polyps. The study conducted by Gathirua-Mwangi et al. showed that obese BMI (≥30 kg/m2) was associated with AN (OR 1.87 and 95%CI 1.08–3.23). However, this study found that waist circumference (WC) rather than BMI was superior for predicting AN risk, where participants with increased WC (OR 1.44 and 95%CI 1.08–3.23) or maintained a high risk of WC (≥35 inches for females and ≥40 inches for males; OR 2.5 and 95% CI 1.08–3.23) had an increased risk of AN. According to the findings of the Gathirua-Mwangi et al. study, having an unhealthy BMI and WC may increase the likelihood of having AN [Citation31].
Three studies in this systematic review also assessed the nutritional status from different aspects together with BMI [Citation28,Citation32,Citation33]. The study conducted by Kurk et al. also assessed the nutritional status from the skeletal muscle index (SMI) aspect and its impact on the disease progression. SMI loss can occur without weight loss and may be key in identifying early protein-energy malnutrition in colorectal cancer. This study reported that the loss in SMI was associated with late colorectal cancer progression. Another study conducted by López-Rodríguez-Arias et al. reported that poor body composition of low SMI and high BMI ≥ 35 kg/m2 was strongly associated with significant postoperative colorectal cancer complications and the increase in length of hospital stay for 3.6 d [Citation32]. The last study conducted by Baar et al. assessed the nutritional status from SMI, subcutaneous and visceral adipose tissue (SAT and VAT, respectively) aspects and its impact on colorectal cancer mortality. SMI and mortality were strongly associated in men, whereas lower SMI level was associated with higher mortality. Lower SAT level, on the other hand, was substantially connected with increased mortality in both men and women, although VAT level was not significantly associated with mortality. There are still many limitations, one of which is that there are several essential outcomes affected by nutritional status in colorectal cancer like the stadium and functional capacity yet to be investigated. Therefore, further future research to assess the various outcomes affected by the nutritional status of colorectal cancer is still needed [Citation33].
Furthermore, dietary exposure also plays an essential role in the development of colorectal cancer. Several studies have indicated that various diets affect colorectal cancer. Patients with a healthy diet can prevent the progression of colorectal cancer [Citation34]. This is supported by the study of Guinter et al. and Lin et al. where the deterioration of colorectal cancer can be prevented with a healthy diet by controlling glucose hemostasis to maintain the patient’s energy balance as well as increase body weight, albumin and prealbumin [Citation35].
Healthy diets that improve colorectal cancer outcomes are rich in fruits, vegetables, fish, poultry, and whole-grain products [Citation36,Citation37]. These results are consistent with the findings of Gigic et al. who focused on the use of fruits and vegetable diets to improve the quality of life of patients with colorectal cancer [Citation38]. In another finding by Fruge et al. a randomized controlled trial was used to determine the effects of green leafy vegetables including spinach, kale, collards, mustard greens, and turnip greens in colorectal cancer patients. The result showed the ability to repair DNA damage as evidenced by decreased TNFα and increased vitamin K from plasma and fecal 8OHdG samples [Citation39]. Therefore, the fruit and vegetable diet is the most favorable candidate for a healthy diet in this finding. A diet rich in fruits and vegetables has been shown to prevent and slow the growth of colorectal cancer because it is rich in fiber, which is considered a protective agent in preventing constipation [Citation38]. Green leafy vegetables also inhibit cytotoxic and carcinogenic activities in heme [Citation39]. Additional diet using supplements such as periOlimel N4-E also reported improved patient outcomes, particularly in postoperative and length of hospital stay [Citation32]. Therefore, a healthy diet such as fruits & vegetables and supplementation is a type of diet that is beneficial for colorectal cancer patients. In the future, it is also possible that the combination of the two can provide a better outcome, so more research is needed.
Some pro-inflammatory diets include more red and processed meat, extra sugar and refined carbohydrates, a bread and butter diet, and milk/dairy products. Dietary fat cheeses were reported to increase the risk of colorectal cancer in the studies of Mehta et al.; Gigic et al.; Wesselink et al. and Alegria et al. [Citation36–38,Citation40,Citation41]. The Western diet is poor in fiber and contributes to the carcinogenic knockdown pathway, which is defined by particular molecular modifications including CIMP-high, MSI-high, and BRAF mutations [Citation36]. The bread and butter diet is related to continuing appetite loss, and new findings indicate that appetite reduction at the start of follow-up is strongly associated with lower survival [Citation38]. Milk and dairy products are heavy in saturated fat, and some studies indicate that consuming a high-fat diet increases bile acid excretion. An increase in bile acid concentrations above physiological levels has promoted colorectal cancer [Citation37]. However, Wesselink et al. did not show a relationship between an anti-inflammatory diet and the progression of colorectal cancer, as was predicted according to the short duration of patient follow-up of 2.8 years compared to another study from 5 years to 12 years [Citation42].
Conclusion
A healthy diet such as fruit and vegetable improve colorectal cancer outcomes. Meanwhile, other dietary patterns, such as the western diet, bread and butter, and milk/dairy products, have increased colon cancer risk. Nutritional status affects some colorectal cancer outcomes, where a high BMI increases the risk of having colorectal cancer, and a low BMI is associated with disease progression and poor quality of life. Poor body composition raises the likelihood of significant postoperative colorectal cancer complications and duration of stay. SMI and SAT levels are both linked to colorectal cancer mortality.
Authors contributions
Study design: RM, TH, HR, IC; Data acquisition: RM, AB, AT, AM, AC, SW, H, NI; Drafting the manuscript: RM, AC, H, NI, SW; Reviewing and editing: TH, HR, AB, FNH, AT, AM, IC; Final approval: all authors (RM, TH, HR, AB, IC, AIA, FNH, AT, AM, AC, H, NI, SW).
Acknowledgment
All authors would like to thank the senior lecturer and staff of the Hematology-Medical Oncolgy Division, Internal Medicine Department, Faculty of Medicine Hasanuddin University for their advices and support. Wahidin Sudirohusodo Hospital and Hasanuddin University Hospital for the facilities and infrastructure that support the writing process of this paper.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article.
Additional information
Funding
References
- Alzahrani S, Al Doghaither H, Al‑Ghafari A. General insight into cancer: an overview of colorectal cancer (review). Mol Clin Oncol. 2021;15(6):1. doi: 10.3892/mco.2021.2433.
- Mármol I, Sánchez-de-Diego C, Dieste AP, et al. Colorectal carcinoma: a general overview and future perspectives in colorectal cancer. Int. J. Mol. Sci. 2017;18:1–9.
- Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009;22(4):191–197. doi: 10.1055/s-0029-1242458.
- American Cancer Society Inc. Colorectal cancer facts and figures 2020–2022. Am Cancer Soc. 2020;66:1–41.
- Abualkhair WH, et al. Trends in incidence of early-Onset colorectal cancer in the United States among those approaching screening age. JAMA Netw Open. 2020;3:1–12.
- Vuik FE, Nieuwenburg SA, Bardou M, et al. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut. 2019;68(10):1820–1826. doi: 10.1136/gutjnl-2018-317592.
- Alhilfi HSQ, Almohammadawi KOM, Alsaad RKA, et al. Colorectal cancer epidemiology and clinical study. Coloproctology. 2019;39(02):159–162. doi: 10.1016/j.jcol.2018.12.001.
- Arnold M, Sierra MS, Laversanne M, et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683–691. doi: 10.1136/gutjnl-2015-310912.
- The Global Cancer Observatory. Cancer incident in Indonesia. vol. 858. International Agency for Research on Cancer; 2020. p. 1–2.
- Xie YH, Chen YX, Fang JY. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct Target Ther. 2020;5:1–20.
- Kuipers EJ, Grady WM, Lieberman D, et al. Colorectal cancer. Nat Rev Dis Prim. 2015;1:1–25.
- Abdullah M, Sudoyo AW, Utomo AR, et al. Molecular profile of colorectal cancer in Indonesia: is there another pathway? Gastroenterol. Gastroenterol Hepatol Bed Bench. 2012;5(2):71–78.
- Yusuf I, Pardamean B, Baurley JW, et al. Genetic risk factors for colorectal cancer in multiethnic indonesians. Sci Rep. 2021;11(1):9988. doi: 10.1038/s41598-021-88805-4.
- Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol. 2019;14(2):89–103. doi: 10.5114/pg.2018.81072.
- Safiri S, Sepanlou SG, Ikuta KS, et al. The global, regional, and national burden of colorectal cancer and its attributable risk factors in 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol Hepatol. 2019;4(12):913–933. doi: 10.1016/S2468-1253(19)30345-0.
- Sawicki T, Ruszkowska M, Danielewicz A, et al. Factors, development, symptoms and diagnosis. Cancers. 2021;13(9):1–23. doi: 10.3390/cancers13092025.
- Hull MA. Conference on diet and digestive disease symposium 4: gi cancers, the role of nutrition in prevention, pathology and management: nutritional prevention of colorectal cancer. Proc Nutr Soc. 2021;80(1):59–64. doi: 10.1017/S0029665120000051.
- Thanikachalam K, Khan G. Colorectal cancer and nutrition. Nutrients. 2019;11(1):164. doi: 10.3390/nu11010164.
- Pan P, Yu J, Wang L-S. Colon cancer: what We eat pan. Physiol Behav. 2016;176:139–148.
- Veettil SK, Wong TY, Loo YS, et al. Role of diet in colorectal cancer incidence: umbrella review of meta-analyses of prospective observational studies. JAMA Netw Open. 2021;4:1–14.
- Gavrilaș LI. Foods and food groups associated with colorectal cancer: a case-control study. Farmacia. 2018;66(5):846–852. doi: 10.31925/farmacia.2018.5.15.
- Zam W, Hasan B. Diet influence on colorectal cancer. Prog Nutr. 2019;21:42–48.
- Keskin H, Wang S-M, Etemadi A, et al. Colorectal cancer in the linxian China nutrition intervention trial: risk factors and intervention results. PLOS One. 2021;16(9):e0255322. doi: 10.1371/journal.pone.0255322.
- Wells G, Shea B, O’Connell J. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute; 2014.
- Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898.
- Guinter MA, Gapstur SM, McCullough ML, et al. Prospective association of energy balance scores based on metabolic biomarkers with colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2020;29(5):974–981. doi: 10.1158/1055-9965.EPI-19-1382.
- Feliciano EMC, Prentice RL, Kroenke CH, et al. Mortality in the women’s health initiative. 2018;141:2281–2290.
- Kurk SA, Stellato RK, Peeters PHM, et al. Trajectory of body mass and skeletal muscle indices and disease progression in metastatic colorectal cancer patients. Am J Clin Nutr. 2019;110(6):1395–1403. doi: 10.1093/ajcn/nqz209.
- Formica V, Nardecchia A, Morelli C, et al. Health-related quality of life in patients with advanced colorectal cancer: a predictive nomogram including BMI, sex and age. Ann Palliat Med. 2021;10(4):4252–4261. doi: 10.21037/apm-20-2194.
- Lee DW, Cho S, Shin A, et al. Body mass index and body weight change during adjuvant chemotherapy in Colon cancer patients: results from the AVANT trial. Sci Rep. 2020;10(1):19467. doi: 10.1038/s41598-020-76643-9.
- Gathirua-Mwangi WG, Monahan P, Song Y, et al. Changes in adult BMI and waist circumference are associated with increased risk of advanced colorectal neoplasia. Dig Dis Sci. 2017;62(11):3177–3185. doi: 10.1007/s10620-017-4778-5.
- López-Rodríguez-Arias F, Sánchez-Guillén L, Lillo-García C, et al. Assessment of body composition as an indicator of early peripheral parenteral nutrition therapy in patients undergoing colorectal cancer surgery in an enhanced recovery program. Nutrients. 2021;13(9):3245. doi: 10.3390/nu13093245.
- van Baar H, Winkels RM, Brouwer JGM, et al. Associations of abdominal skeletal muscle mass, fat mass, and mortality among men and women with stage I–III colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2021;29(5):956–965. doi: 10.1158/1055-9965.EPI-19-1134.
- Shiao SPK, Grayson J, Lie A, et al. Predictors of the healthy eating index and glycemic index in multi-ethnic colorectal cancer families. Nutrients. 2018;10(6):674. doi: 10.3390/nu10060674.
- Lin JX, Chen XW, Chen ZH, et al. A multidisciplinary team approach for nutritional interventions conducted by specialist nurses in patients with advanced colorectal cancer undergoing chemotherapy: a clinical trial. Medicine. 2017;96:10–13.
- Mehta RS, Song M, Qian ZR, et al. Dietary pattarns and Risk of Colorectal Cancer: Analysis by Tumor Location and Molecular Subtype. Gastroenterology, 2017;152(8):1944–1953.
- Alegria-Lertxundi I, Aguirre C, Bujanda L, et al. Food groups, diet quality and colorectal cancer risk in the Basque Country. World J Gastroenterol. 2020;26(28):4108–4125. doi: 10.3748/wjg.v26.i28.4108.
- Gigic B, Boeing H, Toth R, et al. Associations between dietary patterns and longitudinal quality of life changes in colorectal cancer patients: the colocare study. Nutr Cancer. 2018;70(1):51–60. doi: 10.1080/01635581.2018.1397707.
- Frugé AD, Smith KS, Riviere AJ, et al. A dietary intervention high in green leafy vegetables reduces oxidative DNA damage in adults at increased risk of colorectal cancer: biological outcomes of the randomized controlled meat and three greens (M3G) feasibility trial. Nutrients. 2021;13(4):1220. doi: 10.3390/nu13041220.
- Obón-Santacana M, Romaguera D, Gracia-Lavedan E, et al. Dietary inflammatory index, dietary non-enzymatic antioxidant capacity, and colorectal and breast cancer risk (MCC-Spain study). Nutrients. 2019;11(6):1406. doi: 10.3390/nu11061406.
- Barrubés L, Babio N, Mena-Sánchez G, et al. Dairy product consumption and risk of colorectal cancer in an older mediterranean population at high cardiovascular risk. Int J Cancer. 2018;143(6):1356–1366. doi: 10.1002/ijc.31540.
- Wesselink E, Staritsky LE, van Zutphen M, et al. The association between the adapted dietary inflammatory index and colorectal cancer recurrence and all-cause mortality. Clin Nutr. 2021;40(6):4436–4443. doi: 10.1016/j.clnu.2021.01.004.