Abstract
Background:
Second primary malignancies (SPMs) account for an increasing proportion of human malignancies. We estimated the incidence, risk factors and outcomes in lymphoma survivors with SPMs.
Methods:
Patients diagnosed with SPMs after primary lymphoma from 2010 to 2021 were included in this study. The incidence, mortality and clinical characteristics of SPMs in our center and Surveillance, Epidemiology, and End Results database were delineated and analyzed. Standardized incidence ratio quantified second cancer risk.
Results:
A total of 2912 patients of lymphoma were included, 63 cases of SPM met the inclusion criteria, with the prevalence of SPMs after lymphoma was 2.16%. The male-to-female ratio of 2.32:1. The majority of these patients were older (≥60 years old, 61.90%) and previously treated with chemotherapy (68.25%). The common types among SPMs were digestive system tumors (42.86%), respiratory system tumors (20.63%) and urinary system tumors (12.70%). Additionally, cancer risks were significantly elevated after specific lymphoma though calculating the expected incidence. In terms of mortality, the diagnosis of SPMs was significantly associated with an increased risk of death over time. Moreover, although the outcome was favorable in some SPM subtypes (thyroid and breast cancer), other SPMs such as stomach and lung tumors had a dismal prognosis.
Conclusion:
With the improvement of medical standards, the survival of lymphoma patients has been prolonged. However, the incidence of SPM is increasing, particularly among men and older lymphoma survivors. Therefore, more attention should be invested in the SPM to further improve the prognosis of these patients.
KEY MESSAGES
Patterns of SPM incidence varied between China and Northern America.
The incidence of SPM was higher among men and older lymphoma survivors.
Patients with SPM are divided into low-risk and high-risk according to survival analysis.
Introduction
Lymphoma is a heterogeneous group of lymphoproliferative diseases, traditionally divided into Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL) [Citation1]. NHL is the most common hematological malignancy (2.4% of total cancer cases) and HL accounts for approximately 0.4% [Citation2]. Several advancements have been made in the management of lymphoma in recent years. Given the long-term treatment and supportive care, patients with lymphoma are living longer under better disease control [Citation3]. It is known that the second primary malignancies (SPMs) are one of the most common causes of death among long-term survivors of several cancers, now accounting for approximately 16% of all cancer diagnoses [Citation4]. Thus, further investigations on the characteristics of SPM after lymphoma will improve the survival of lymphoma patients.
The SPM was defined as the first subsequent primary cancer occurring at least 2 months after the first cancer diagnosis [Citation5]. The risk of post-lymphoma SPM is significantly elevated in multiple lymphoma subtypes, including chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL) [Citation6]. However, the incidences and risks of SPM for other lymphoma subtypes are still unclear. A variety of factors have been postulated as risk factors for the occurrence of SPMs in lymphoma patients, in addition to the effects of shared environment, genetic susceptibility and aetiological factors with the primary diseases, treatment strategies have been considered to contribute to the development of the SPMs [Citation4]. Evidence regarding the therapy-related side effects indicates that we need to choose better and safer treatment options for lymphoma patients [Citation7,Citation8]. Exploring the difference in incidence and risk of SPMs could sharpen understanding of second malignancy risks thereby providing clinical benefit. Overall, there is a scarcity of comprehensive studies that evaluate the incidence and causes of SPMs.
In this study, a population-based analysis of the incidence, major cancer type, prognostic factors and survival of second malignancy in lymphoma patients was performed. The heterogeneity of the incidence of SPMs and their corresponding cancer types were analyzed. The results of this study will help clinicians to better understand the characteristics of SPM after lymphoma, thereby establishing effective disease prevention strategies and improving long-term survival for patients.
Methods
Patient selection
In this study, patients with a diagnosis of lymphoma at the Shandong Provincial Hospital (SPH) between 1 January 2010 and 31 December 2021 were identified retrospectively. All patients recruited met the following criteria: (1) pathologically diagnosed as lymphoma, (2) older than 18-year old, (3) no previous chemotherapy or immunotherapy treatment. Exclusion criteria include (1) incomplete clinical data and follow-up information, (2) previous malignancies or major diseases and (3) SPMs were detected within the first 2 months of the lymphoma diagnosis, or if lymphoma was not their initial malignant diagnosis. Covariates of interest included demographic characteristics, clinical characteristics, cancer types and outcome information were collected. Data on treatments such as surgery, radiation and chemotherapy were collected. The majority of patients enrolled in this study received chemotherapy, the first-line regimens were RCHOP-based for DLBCL, FL and marginal zone lymphoma (MZL) patients, fludarabine-based for CLL and ABVD-based for HL. Treatment was with BTK inhibitor in 2 CLL cases and autologous stem cell transplant in 1 HL patient. As a supplement, the information of patient demographics, types of SPMs, date of novel diagnosis of lymphoma and SPMs and survival status were extracted in the Surveillance, Epidemiology, and End Results (SEER)-18 cohort, consisting of patients with lymphoma diagnosed between January 2000 and December 2018 in the SEER 18 cancer registries (SEER Research Data, 18 Registries (excl AK), November 2020 Sub (2000–2018)).
The follow-up period extends from the date of lymphoma diagnosis to the date of death or the date of the last follow-up. For the SEER database, the last follow-up date is 31 December 2018, while for the SPH database, it is 31 March 2022. Whichever of these dates comes first will be considered as the end of the follow-up period.
Statistical analysis
The relative risk of SPMs relative to the U.S. general population was estimated as the standardized incidence ratio (SIR) which is the ratio of observed (O) number of second malignancies to expected (E) malignancies. SIR is an index to measure the strength of the correlation between first and second cancer and the statistical significance was assessed based on the exact 95% confidence interval (CI). The excess absolute risk (EAR) is a precise measure of the additional burden of cancer occurrences in a specific population. It is calculated by determining the excess (observed to expected) number of SPMs per 10,000 person-years (SEER*Stat software, version 8.3.9.2).
We evaluate whether SIRs varied across strata of patient characteristics by using multivariate Poisson regression models. Multivariable analysis was conducted by Cox proportional hazard model and variables with significance levels on univariate analysis were entered into the multivariate analysis. The two-sided p < .05 was regarded as statistically significant. We performed the Kaplan–Meier test and used the log-rank test to compare the overall survival (OS) of patients with or without SPMs to evaluate the association between OS and history of SPMs.
Results
Patient and disease characteristics between SPH and SEER databases
Among the 2,912 hospitalized lymphoma cases in the SPH database, 63 patients (2.16%) had SPMs, including 3 patients with two separate primary cancers diagnosed during the follow-up. Of these patients, 44 (69.84%) were male and 19 (30.16%) were female, with a male to female ratio of 2.32:1. The median age was 61-year old (range 35–81). The majority of patients diagnosed with SPMs were older (≥60-year old, 61.90%, ). The majority of patients with SPMs were previously treated with chemotherapy for primary lymphoma (n = 43, 68.25%). Moreover, the median follow-up duration for all patients was 4.92 years (range, 0–10 ). The mean interval between the diagnosis of primary lymphoma and SPMs was 3.14 years (range 0–9). It was 3.37 years for digestive system tumors (4.42 years for stomach cancer, 2.8 years for liver cancer and 2.67 for colorectal cancer), 2.92 years for respiratory system tumors, 3.16 years for urinary system tumors (2.33 years for kidney cancer and 3 years for prostate cancer), 2.2 years for breast tumor and 3.25 years for other SPMs. In the SPH database, we have found that the digestive (27 patients, 42.86%), respiratory (13 patients, 20.63%) and urinary (8 patients, 12.70%) systems tumors are particularly vulnerable to the development of SPMs. Among them, the lung (19.6%), stomach (10.7%) and liver (8.9%) are the most frequently affected sites ().
Figure 1. Gender and age disaggregated SPMs after lymphoma incidence rates and 10 leading SPM types between SPH database, 2010–2021 and SEER databases, 2010–2018. Gender distribution of SPMs from (a) SPH database and (d) SEER database. Age distribution of SPMs from (b) SPH database and (e) SEER database. Ten leading SPM types after lymphoma in (c) SPH database and (f) SEER database. Abbreviations: SPMs: second primary malignancies; SPH: Shandong Provincial Hospital; SEER: Surveillance, Epidemiology, and End Results; HL: Hodgkin lymphoma; NHL: non-Hodgkin lymphoma; B-NHL: B-cell non-Hodgkin lymphoma; T-NHL: T-cell non-Hodgkin lymphoma.
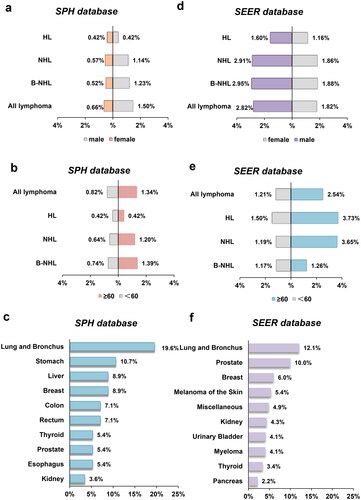
Additionally, in the SEER cohort with 284,178 NHL and 20,518 HL patients from 2010 through 2018, a total of 14,236 (4.67%) patients were diagnosed with SPMs. The median age at diagnosis was 67 years, ranging from 18 to 85 years, and there was a slight male predominance (60.8%, ). The mean interval between the diagnosis of primary lymphoma and SPMs was 2.43 years (range 0–8). Specifically, the mean intervals were 2.45 years for lung and bronchus cancer, 2.69 years for prostate cancer, 2.76 years for breast cancer, 1.81 years for kidney cancer, 2.63 years for urinary bladder cancer, 1.37 years for thyroid cancer and 3.02 years for acute myeloid leukemia (AML). The most commonly diagnosed SPMs were lung and bronchus cancer (12.0%), prostate cancer (9.9%) and breast cancer (6.0%) in the SEER database ().
The overall occurrence of subsequent malignancies following lymphoma is found to be higher in the SEER database compared to the SPH databases. Notably, these SPM cases predominantly involved male individuals of advanced age in both databases. Furthermore, the SPH database exhibited a longer interval between the diagnosis of SPM and lymphoma in comparison to the SEER database. Moreover, the analysis also revealed that lung and bronchus cancer constituted the most prevalent type of SPM in both databases, while the incidence of secondary digestive tumors, such as stomach and colorectal cancer, among lymphoma patients in the SPH cohort was higher than that observed in the SEER cohort.
SPMs after specific lymphoma subtypes
To analyze the features of specific lymphoma with SPM, several common lymphomas were selected for further analysis. In SPH database, histopathologic subtypes of primary lymphoma were as follows: HL (n = 2; 3.17%), NHL (n = 51; 80.96%), including 19 with CLL/SLL, 18 with DLBCL, 4 with FL, 5 with MZL and 5 NHL, not specified subtype and unknown type of lymphoma (n = 10; 15.87%). Further analysis of the first primary lymphoma included patients diagnosed with the five common first primary lymphoma subtypes in the SPH database: DLBCL (n = 1,317), CLL/SLL (n = 279), HL (n = 237), FL (n = 218) and MZL (n = 132). A male predominance was observed in all subtypes of lymphoma, with a male-to-female ratio of 2.07 for CLL/SLL, 1.46 for HL, 1.27 for DLBCL and 1.04 for FL and MZL. The median age at novel diagnosis for different lymphoma subtypes ranged from 35 to 63 years, with CLL at 63 years, DLBCL and MZL at 59 years, FL at 49 years and HL at 35 years. Extracted initial therapy varied substantially by lymphoma subtype. The majority of CLL/SLL (59.4%) patients were untreated, whereas chemotherapy was used to treat other subtypes: HL (81.9%), FL (69.8%), DLBCL (48.9%) and MZL (31.7%) (). Supporting Information Figure S1 displays the distribution of SPM sites for the specific lymphoma evaluated. The most commonly diagnosed SPM was lung cancer in SPH database (21.05% with CLL/SLL, 27.78% with DLBCL, 25.00% with FL, 66.67% with HL and 40.00% with MZL).
Table 1. Selected characteristics of patients surviving after an initial diagnosis of CLL/SLL, DLBCL, FL, HL and MZL with and without SPMs in Shandong province hospital, 2010–2021.
We obtained the data from the SEER database between 2000 and 2018. displays the incidence of the SPM in terms of case counts. To assess the disparity among eight distinct primary lymphomas, a survival curve was constructed (). However, due to the extensive volume of data and the presence of overlapping curves, definitive conclusions cannot be drawn. Nevertheless, a favorable prognosis was observed for HL survivors with SPMs. In order to facilitate a more comprehensive comparison with the SPH database, we retrospectively incorporated specific lymphoid neoplasms patients: DLBCL (n = 60,383), CLL/SLL (n = 50,324), FL (n = 28,968), HL (n = 20,518) and MZL (n = 17,474) in the SEER cohort (Supporting Information Table S1). Similarly, a male predominance was observed for most subtypes other than MZL (46.7%). The age at which individuals are typically diagnosed with their first primary malignancy is usually between 64 and 74 years, with the exception of HL (which occurs between 15 and 54 years). Additionally, more than 80% of these individuals are of white race. For these patients, the range of follow-up time was 0–107 months from the initial diagnosis of lymphoma. The most common SPM was lung and bronchus in the SEER cohort (15.8% with CLL/SLL, 14.8% with DLBCL, 17.3%with FL, 13.1% with HL and 14.1% with MZL) (Supporting Information Figure S1).
Figure 2. The incidence and survival rate of SPMs in SEER database, 2000–2018. The crude incidence rate of specific lymphoma in SPH database, 2010–2021 and SEER databases, 2010–2018. (a) Heatmaps of the lymphoma survivors with SPMs and (b) Kaplan–Meier analysis for OS of SPMs after lymphoma in SEER database. (c) The comparison of the crude incidence rate of SPMs after specific lymphoma between two databases. Abbreviations: SPMs: second primary malignancies; SPH: Shandong Provincial Hospital; SEER: Surveillance, Epidemiology, and End Results; OS: overall survival; HL: Hodgkin’s lymphoma; CLL: chronic lymphocytic leukemia; FL: follicular lymphoma; DLBCL: diffuse large B-cell lymphoma; MZL: marginal zone lymphoma; BL: Burkitt lymphoma; TCL: T-cell lymphoma; MCL: mantle cell lymphoma.
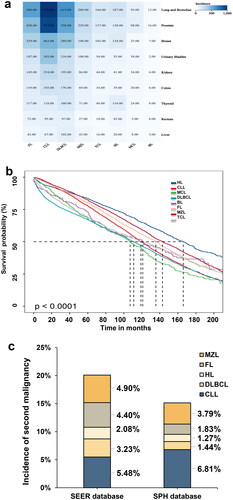
The most common type of SPMs observed in our study was lung and bronchus, which aligns with our findings. However, our results differ from those obtained from the SEER database. Our analysis indicates that the incidence rate of SPMs after specific lymphoma is generally lower in the SPH database, except for CLL ().
SIR analyses of SPMs in the SEER database
Additionally, we analyzed the incidence of specific lymphoma during 2010–2018, and the cumulative incidence of SPMs in different lymphomas in the SEER database, and compared the SPMs after specific lymphoid neoplasms between the SEER database and the SPH database. Because the crude incidence rate of SPM was not adjusted for the expected number of lymphoma or the number of high-risk patients in a reference population, the SIR and EAR of SPM were calculated in further analysis (). Among patients at lymphoma diagnosis, overall malignancy risk was significantly higher than expected in the U.S. general population after FL (SIR = 2.01; 95%CI, 1.93–2.09), MZL (SIR = 1.78; 95%CI, 1.68–1.88), HL (SIR = 1.76; 95%CI, 1.62–1.91), CLL/SLL (SIR = 1.51; 95%CI, 1.46–1.57) and DLBCL (SIR = 1.45; 95%CI, 1.39–1.50).
Table 2. Second primary malignancy risks among patients surviving after an initial diagnosis of CLL/SLL, DLBCL, FL, HL and MZL with and without SPMs in 18 SEER Program Registries, 2010–2018.
Of the solid organ second malignancies, risks for thyroid and kidney cancer and cutaneous melanoma were elevated after the lymphoma, especially FL and MZL. Moreover, for solid tumors, most of the excess risk was posed by lung and bronchus (EAR: CLL/SLL = 13.42, 95%CI, 1.41–1.68; DLBCL = 7.30, 95%CI, 1.23–1.54; FL = 12.04, 95%CI, 1.44–1.85; MZL = 8.83, 95%CI, 1.20–1.68; HL = 2.79, 95%CI, 1.15–1.99), followed by thyroid (EAR: CLL/SLL = 2.67, 95%CI, 1.74–2.89; DLBCL = 6.92, 95%CI, 3.53–5.18; FL = 7.02, 95%CI, 3.16–5.03; MZL = 6.69, 95%CI, 2.83–5.26; HL = 5, 95%CI, 3–5.55). Nevertheless, the second malignancy risk was different among these lymphoma subtypes. For example, the risk of secondary liver cancer is more evident in survivors of HL, MZL and DLBCL (SIR = 2.04, 95%CI, 1.05–2.56; SIR = 1.71, 95%CI, 1.06–2.61; SIR = 1.65, 95%CI, 1.24–2.16) than FL and CLL/SLL (SIR = 1.04, 95%CI, 0.64–1.59; SIR = 0.75, 95%CI, 0.51–1.06). Risks for Kaposi sarcoma followed a pattern similar to liver cancer, overly limited more aggressive lymphoma, DLBCL (SIR = 8.19, 95%CI, 3.54–16.14) but excluded indolent lymphomas, CLL/SLL (SIR = 0), FL (SIR = 3.79, 95%CI, 0.46–13.68) and MZL (SIR = 0).
Hematological malignancies contributed more to the SPMs than solid-organ cancers among these subtypes. Among hematological second malignancies, risks for HL were elevated after the other four lymphoma subtypes. There was still an overall increased risk of SPMs of AML after DLBCL (SIR = 7.07, 95%CI, 7.88–41.11), HL (SIR = 6.33, 95%CI, 3.75–10.00), FL (SIR = 4.08, 95%CI, 2.93–5.54), CLL/SLL (SIR = 3.23, 95%CI, 2.52–4.06) and MZL (SIR = 1.23, 95%CI, 0.53–2.42).
Risk of SPMs
In a multivariate analysis, most patients with second malignancies were diagnosed at younger age (<55 years) and found to be other (Asian or Pacific Islander, American Indian/Alaska Native) races (SIR: CLL/SLL = 1.89; DLBCL = 1.74; FL = 2.85; MZL = 2.87; HL = 3.80, Supporting Information Table S2). Although only a subset of lymphoma patients in the SEER database had staging data, most of these patients who received the SPM diagnosis had advanced stages. Meanwhile, we showed that the advanced clinical stage appeared to elevate the second malignancy risks after most lymphoma, but risks were not increased in MZL. In addition, the second malignancies risk was higher during the early follow-up period (latency ≤ 5).
Survival analysis
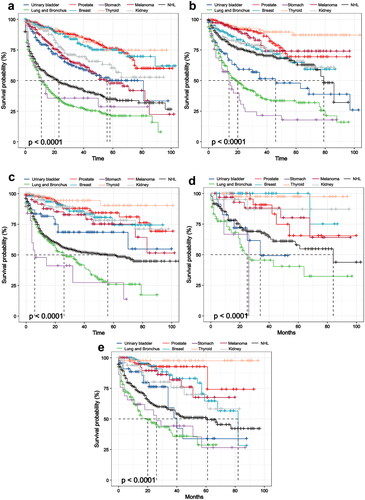
The multivariable Cox proportionate hazards analysis was performed for looking at the predictive risk factors of SPMs in lymphoma survivors. In the multivariate analysis, a higher mortality rate is associated with advanced age, there was a progressively increasing hazard of SPMs noted within the months from primary lymphoma diagnosis (Supporting Information Table S3, p < .01). It is an interesting finding that the survival in lymphoma with SPMs except for DLBCL was better until nearly 30 months after the diagnosis of SPMs compared to lymphoma without SPMs (, p < .01). In further analysis, we compared the survival of the most common SPMs in people who were diagnosed with specific lymphoma. The results showed that lymphoma patients with NHL, urinary bladder, stomach, lung and bronchus cancer experienced worse survival compared with other sites (such as melanoma, thyroid, kidney, breast and prostate cancer , p < .01).
Figure 3. Kaplan–Meier curves of survival among lymphoma stratified by the occurrence of SPMs in SEER databases, 2010–2018. Kaplan–Meier for OS in groups with SPMs and groups without SPMs in (a) CLL, (b) DLBCL, (c) FL, (d) HL and (e) MZL patients. Abbreviations: SPMs: second primary malignancies; SEER: Surveillance, Epidemiology, and End Results; OS: overall survival; HL: Hodgkin’s lymphoma; CLL: chronic lymphocytic leukemia; FL: follicular lymphoma; DLBCL: diffuse large B-cell lymphoma; MZL: marginal zone lymphoma.
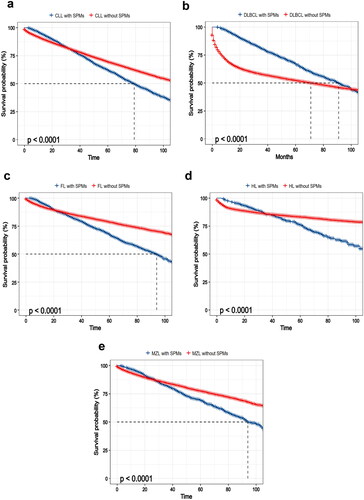
Figure 4. The survival of most common SPMs after specific lymphoma in SEER databases, 2010–2018. Kaplan–Meier survival curves for (a) CLL, (b) DLBCL, (c) FL, (d) HL and (e) MZL survivors with SPMs divided into two prognostic groups based on survival of the types of SPMs. Abbreviations: SPMs: second primary malignancies; SEER: Surveillance, Epidemiology, and End Results; OS: overall survival; HL: Hodgkin’s lymphoma; CLL: chronic lymphocytic leukemia; FL: follicular lymphoma; DLBCL: diffuse large B-cell lymphoma; MZL: marginal zone lymphoma.
Discussion
With the growing advancement of diagnostic technology and the updating of treatment strategies, the survival rate for lymphoma patients has risen steadily over the last few decades [Citation9]. The risk of SPMs could be significant in the long-term survival of lymphoma patients. Our study comprehensively analyzed the latest epidemiology of SPMs in lymphoma survivors in a large population-based cohort study, demonstrated the incidence of SPMs in different races, identified the second malignancy risks for specific lymphoma subtypes, and further explored the clinical outcomes of different SPMs.
We noted the incidence rate of SPMs among lymphoma patients in the U.S. population is higher compared with our center. The following reasons may somewhat explain this phenomenon, (1) we found that the survival rate was higher in the U.S. with lymphoid malignancies which were mainly due to the diligent observation and improved adherence to screening procedures [Citation10]. The association between longer life expectancy and a higher incidence of SPMs has been observed. (2) Additionally, the differences in outcomes could be attributed to the heterogeneity of patient demographics, the longer follow-up period in the United States, and the absence of comprehensive clinical data and smaller study populations in our single center. Consistent with prior reports, we also found a high incidence of secondary lung cancer among lymphoma survivors. The current lymphoma survivorship consensus in China emphasizes the importance of regular follow-up, we observed increasing risk for SPMs among lymphoma survivors. Therefore, we recommend cancer screening for early diagnosis. According to early screening guidelines, it is necessary to conduct endoscopic screening for individuals over 45-year old to detect secondary digestive system tumors, with a low risk of overdiagnosis. Additionally, it is recommended that individuals in high-risk groups participate in annual low dose CT screening between the ages of 50 and 74 years [Citation11,Citation12].
Conversely, lymphoma is a heterogeneous malignant disease originating from lymphoid tissue, ranging from indolent to aggressive [Citation13]. Our study revealed different patterns of second malignancy after specific lymphoid neoplasms. Patients with indolent lymphoma experience long-term immune dysfunction due to the characteristics of the disease and treatment [Citation14,Citation15]. We observed the risk of SPMs was higher after CLL/SLL compared to other subtypes, which aligns with the previous studies [Citation16]. While causes for this phenomenon appear multifactorial, impaired immune surveillance, genetic risk factors and chemoimmunotherapy may explain at least some of it [Citation17,Citation18]. Our study also confirmed the elevated excess risk of SPM in AML, consistent with previous research [Citation19]. However, the risk of SPMs among lymphoma survivors varies, due to the heterogeneity of disease characteristics, treatment methods and survival. Second malignancy risks were lower in patients with aggressive lymphoma compared with indolent lymphoma. Therefore, this research enhanced our comprehension of SPMs in lymphoma survivors by emphasizing the elevated risk and illustrating the heterogeneity observed among patients with different types of lymphoma.
In our analysis of survival rates, we observed that the majority of lymphoma survivors with SPMs did not experience a lower survival rate compared to those without SPMs within 30 months. This finding aligns with a previous study which indicated that the negative impact on survival due to SPMs in NHL shows from year 6 onwards [Citation20]. The following reasons may explained some of the phenomena, in the management of lymphoma, targeted therapies, cellular therapies and others have shown potential effects [Citation21,Citation22], thus, prolonged survival is associated with the incidence of SPMs [Citation23]. Additionally, patients with SPMs may benefit from active early detection, diagnosis and treatment, leading to improved survival. For instance, the annual computed tomographic screening could diagnose lung cancer at earlier stages, while personalized early detection and strategies for breast cancer are crucial in enhancing survival [Citation24,Citation25]. The tumor microenvironment (TME) plays an important role in lymphoma, although the specific mechanism remains unclear. Some studies have suggested that previous lymphoma or treatment may impact the unique genetic composition of tumor cells and their interaction with TME even reshaping TME [Citation26–28]. Further investigations are necessary to explore the potential explanations for the observed consequences. Moreover, we analyzed survival rates in common types of second primary cancers to examine the hypothesis that the type of SPMs is critical for survival. In summary, we should improve our awareness of how patients can benefit from early treatment and the utilization of susceptibility screening tools, consequently improving outcomes for lymphoma survivors.
The primary strength was that we utilized a large cohort of lymphoma survivors, allowing us to accurately calculate risks. Additionally, our near-complete follow-up data helped eliminate the effects of selection bias commonly seen in hospital-based series. We also compared the incidence of SPMs in our SPH database with the SEER database, highlighting their similarities and heterogeneity. Another strength was our ability to assess the risks of second malignancies based on specific lymphoma subtypes, as well as testing survival in two prognostic groups. However, some limitations may attenuate our results when interpreting our findings. Firstly, we lacked detailed information on the therapy strategies received by lymphoma survivors, which prevented us from analyzing associations between specific treatments and SPM risk. Moreover, this single-institution study is limited by a small sample size, which may limit our ability to accurately estimate the true risk of second cancer.
Conclusions
Our study illustrated the incidence, mortality and related risk factors of SPMs in survivors of specific lymphoma. The findings emphasize the importance of physicians remaining vigilant regarding the potential development of SPMs in lymphoma survivors. It is crucial to implement focused cancer screening strategies for early detection and interventions, particularly for high-incidence SPMs. For instance, given the lung and bronchus are the most prone sites for SPM development and are associated with poor prognosis, it is strongly recommended to promote smoking cessation and encourage early screening. While our current understanding of the pathogenesis of SPM is still limited, awareness of the predisposing population could permit early detection of SPMs, thereby reducing the mortality burden associated with lymphoma and other SPMs.
Authors contributions
Y L. and X.Z. designed the study. X.G., M.D., and P.L. collected the data. Y.L., Y.C., and J.L. analyzed the data. Y.L. drafted the manuscript. X.Z. and X.W. reviewed and revised the manuscript. X.W. provided direction and guidance throughout the preparation of the manuscript. All authors contributed-the interpretation of data and approved the final manuscript.
| Abbreviations | ||
| 95% CI | = | 95% confidence interval |
| AML | = | acute myeloid leukemia |
| CLL/SLL | = | chronic lymphocytic leukemia/small lymphocytic lymphoma |
| DLBCL | = | diffuse large B-cell lymphoma |
| EAR | = | excess absolute risk |
| FL | = | follicular lymphoma |
| HL | = | Hodgkin lymphoma |
| MZL | = | marginal zone lymphoma |
| NHL | = | non-Hodgkin lymphoma |
| OS | = | overall survival |
| SEER | = | Surveillance, Epidemiology, and End Results |
| SIR | = | standardized incidence ratio |
| SPH | = | Shandong Provincial Hospital |
| SPMs | = | second primary malignancies |
| TME | = | tumor microenvironment |
Supplemental Material
Download PDF (201.9 KB)Acknowledgement
We would like to thank the researchers and study participants for their contributions and part of the material has been used in the conference ‘64th ASH Annual Meeting’.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability
The data underlying this article are available in SEER 18 Database: Incidence-Based Mortality—SEER Research Data, 18 Registries (excl AK), November 2020 Sub (2000–2018) at https://seer.cancer.gov/data/access.html, and can be accessed with Surveillance Research Program, National Cancer Institute SEER*Stat software (seer.cancer.gov/seerstat).
Additional information
Funding
References
- Armitage J, Gascoyne R, Lunning M, et al. Non-Hodgkin lymphoma. Lancet. 2017;390(10091):1–13. doi: 10.1016/s0140-6736(16)32407-2.
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492.
- Ayyappan S, Maddocks K. Novel and emerging therapies for B cell lymphoma. J Hematol Oncol. 2019;12(1):82. doi: 10.1186/s13045-019-0752-3.
- Travis L, Demark Wahnefried W, Allan J, et al. Aetiology, genetics and prevention of secondary neoplasms in adult cancer survivors. Nat Rev Clin Oncol. 2013;10(5):289–301. doi: 10.1038/nrclinonc.2013.41.
- Kumar V, Ailawadhi S, Bojanini L, et al. Trends in the risk of second primary malignancies among survivors of chronic lymphocytic leukemia. Blood Cancer J. 2019;9(10):75. doi: 10.1038/s41408-019-0237-1.
- Morton L, Curtis R, Linet M, et al. Second malignancy risks after non-Hodgkin’s lymphoma and chronic lymphocytic leukemia: differences by lymphoma subtype. J Clin Oncol. 2010;28(33):4935–4944. doi: 10.1200/jco.2010.29.1112.
- Furman RR, Byrd JC, Owen RG, et al. Pooled analysis of safety data from clinical trials evaluating acalabrutinib monotherapy in mature B-cell malignancies. Leukemia. 2021;35(11):3201–3211. doi: 10.1038/s41375-021-01252-y.
- Franklin J, Eichenauer D, Becker I, et al. Optimisation of chemotherapy and radiotherapy for untreated Hodgkin lymphoma patients with respect to second malignant neoplasms, overall and progression-free survival: individual participant data analysis. Cochrane Database Syst Rev. 2017;9(9):CD008814. doi: 10.1002/14651858.CD008814.pub2.
- Molina A. A decade of rituximab: improving survival outcomes in non-Hodgkin’s lymphoma. Annu Rev Med. 2008;59(1):237–250. doi: 10.1146/annurev.med.59.060906.220345.
- Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–1075. doi: 10.1016/s0140-6736(17)33326-3.
- Zhou Q, Fan Y, Wang Y, et al. China national lung cancer screening guideline with low-dose computed tomography (2018 version). Zhongguo Fei Ai Za Zhi. 2018;21(2):67–75. doi: 10.3779/j.issn.1009-3419.2018.02.01.
- Brenner H, Altenhofen L, Stock C, et al. Prevention, early detection, and overdiagnosis of colorectal cancer within 10 years of screening colonoscopy in Germany. Clin Gastroenterol Hepatol. 2015;13(4):717–723. doi: 10.1016/j.cgh.2014.08.036.
- Bauer AT, Gorzelanny C, Gebhardt C, et al. Interplay between coagulation and inflammation in cancer: limitations and therapeutic opportunities. Cancer Treat Rev. 2022;102:102322. doi: 10.1016/j.ctrv.2021.102322.
- Bond D, Huang Y, Fisher J, et al. Second cancer incidence in CLL patients receiving BTK inhibitors. Leukemia. 2020;34(12):3197–3205. doi: 10.1038/s41375-020-0987-6.
- Leslie L. Novel therapies for follicular lymphoma and other indolent Non-Hodgkin lymphomas. Curr Treat Options Oncol. 2021;22(12):111. doi: 10.1007/s11864-021-00909-1.
- Beiggi S, Johnston J, Seftel M, et al. Increased risk of second malignancies in chronic lymphocytic leukaemia patients as compared with follicular lymphoma patients: a Canadian population-based study. Br J Cancer. 2013;109(5):1287–1290. doi: 10.1038/bjc.2013.381.
- Fang J, Seki T, Maeda H. Therapeutic strategies by modulating oxygen stress in cancer and inflammation. Adv Drug Deliv Rev. 2009;61(4):290–302. doi: 10.1016/j.addr.2009.02.005.
- Benjamini O, Jain P, Trinh L, et al. Second cancers in patients with chronic lymphocytic leukemia who received frontline fludarabine, cyclophosphamide and rituximab therapy: distribution and clinical outcomes. Leuk Lymphoma. 2015;56(6):1643–1650. doi: 10.3109/10428194.2014.957203.
- Joelsson J, Wästerlid T, Rosenquist R, et al. Incidence and time trends of second primary malignancies after non-Hodgkin lymphoma: a Swedish population-based study. Blood Adv. 2022;6(8):2657–2666. doi: 10.1182/bloodadvances.2021006369.
- Chattopadhyay S, Zheng G, Sud A, et al. Second primary cancers in non-Hodgkin lymphoma: family history and survival. Int J Cancer. 2020;146(4):970–976. doi: 10.1002/ijc.32391.
- Hu S, Ren S, Cai Y, et al. Glycoprotein PTGDS promotes tumorigenesis of diffuse large B-cell lymphoma by MYH9-mediated regulation of wnt-β-catenin-STAT3 signaling. Cell Death Differ. 2022;29(3):642–656. doi: 10.1038/s41418-021-00880-2.
- Zhou X, Zhan L, Huang K, et al. The functions and clinical significance of circRNAs in hematological malignancies. J Hematol Oncol. 2020;13(1):138. doi: 10.1186/s13045-020-00976-1.
- Miller K, Nogueira L, Mariotto A, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69(5):363–385. doi: 10.3322/caac.21565.
- Sands J, Tammemägi M, Couraud S, et al. Lung screening benefits and challenges: a review of the data and outline for implementation. J Thorac Oncol. 2021;16(1):37–53. doi: 10.1016/j.jtho.2020.10.127.
- Pashayan N, Antoniou A, Ivanus U, et al. Personalized early detection and prevention of breast cancer: ENVISION consensus statement. Nat Rev Clin Oncol. 2020;17(11):687–705. doi: 10.1038/s41571-020-0388-9.
- Nakasone E, Askautrud H, Kees T, et al. Imaging tumor-stroma interactions during chemotherapy reveals contributions of the microenvironment to resistance. Cancer Cell. 2012;21(4):488–503. doi: 10.1016/j.ccr.2012.02.017.
- Liu Y, Zhou X, Wang X. Targeting the tumor microenvironment in B-cell lymphoma: challenges and opportunities. J Hematol Oncol. 2021;14(1):125. doi: 10.1186/s13045-021-01134-x.
- Pesce S, Greppi M, Grossi F, et al. PD/1-PD-Ls checkpoint: insight on the potential role of NK cells. Front Immunol. 2019;10:1242. doi: 10.3389/fimmu.2019.01242.
