Abstract
Background
Interleukin (IL)-6 is a pro-inflammatory cytokine that plays an important role in preterm birth (PTB), Several meta-analyses investigated the association between IL-6 and PTB, but definitive conclusion has not yet been achieved. This updated meta-analysis aimed to ascertain the association between IL-6 and PTB by examining IL-6 levels in both normal birth and PTB groups.
Material and methods
Prospective cohort studies were retrieved in PubMed, Embase, and the Cochrane library from their inception until 18 February 2020. The primary outcome was the association between IL-6 and PTB, and secondary outcomes were the association between IL-6 and spontaneous PTB.
Results
Nine studies involving 1904 patients were included. Overall, IL-6 from different sample types (maternal blood, amniotic fluid and cervicovaginal fluid) was associated with PTB (standard mean difference [SMD]: 0.86, 95% confidence interval [CI]: 0.32 to 1.39, p < 0.001). Furthermore, the association was significant for IL-6 only in amniotic fluid (SMD: 1.87, 95%CI: 0.82 to 2.93, p < 0.001) and cervicovaginal fluid (SMD: 0.46, 95%CI: 0.09 to 0.84, p = 0.022), but not significant in maternal blood (SMD: −0.11, 95%CI: −0.57 to 0.34, p = 0.623). In addition, IL-6 was also associated with spontaneous PTB (SMD: 1.57, 95% CI: 0.18 to 2.95, p < 0.001).
Conclusions
Based on the available evidence, IL-6 in amniotic fluid and cervicovaginal fluid might be useful for predicting preterm birth.
Based on the available evidence
IL-6 in amniotic fluid and cervicovaginal fluid might be useful for predicting preterm birth
KEY MESSAGES
Introduction
Preterm birth (PTB) is defined as the delivery of an infant before completing 37 weeks of gestation [Citation1]. PTB is the most common cause of antenatal hospitalization [Citation2] and is also the leading cause of perinatal morbidity and mortality [Citation2, Citation3]. In the United States, PTB represents 12% of all births [Citation2–4], and about 50% of twin births are PTBs [Citation3, Citation4]. PTB can lead to complications such as prematurity [Citation3, Citation4] and admission to the neonatal intensive care unit (NICU) [Citation4,Citation5]. Premature infants are at higher risk for perinatal complications such as retinopathy of prematurity and apnoea of prematurity, along with associated morbidities. Besides, decreased gestational age amplifies the likelihood of adverse outcomes. The 2013 infant mortality statistics indicated a general infant mortality rate of 6 per 1000 live births in the United States, with preterm-related causes accounting for 36% of all infant deaths [Citation6]. Among PTB subtypes, spontaneous preterm birth refers to cases where the onset of labour and subsequent delivery occurs before 37 weeks of gestation without any medical intervention or indicated cause, such as preterm labour with intact membranes and preterm premature rupture of membranes [Citation7]. It is a leading cause of neonatal morbidity and mortality, and difficult to predict due to multifaceted factors involved in the pathogenesis [Citation7]. Therefore, PTB, as well as spontaneous preterm birth, has always been a major public health problem, and therefore, it is crucial to timely and accurately predict.
Among biomarkers, inflammatory cytokines have been speculated to be associated with preterm [Citation8,Citation9]. Several potential biomarkers of preterm birth (PTB) present in amniotic fluid have been researched. Interleukin (IL)-6, matrix metalloproteinase-8 (MMP-8), and glucose levels stand out as particularly promising candidates [Citation10], but studies about their predictive value yield conflicting results.
IL-6 is a pro-inflammatory cytokine, playing an important role in fever and acute phase response [Citation11]. IL-6 can be secreted by macrophages in response to pathogen-associated molecular patterns and by adipocytes, participating in the chronic low-grade inflammatory state observed in obesity [Citation12]. Among the most promising biomarkers, IL-6 especially raised attention because of the possibility of targeting its receptor to inhibit PTB induced by an inflammatory insult [Citation13]. Nevertheless, the studies of IL-6 in humans showed inconsistent results [Citation10,Citation11,Citation13–22]. Although, meta-analyses of the association between IL-6 and PTB are currently available, some of the outcomes of interest are still controversial. For example, Wu et al. [Citation23] discovered the population-dependent pattern of the associations between IL-6 polymorphisms and PTB; however, Conde-Agudelo et al. [Citation24] examined 30 biomarkers for PTB and concluded that none of them, including IL-6, met the criteria to be considered clinically useful. In consideration of the evolving landscape of research and the potential advancements in our understanding of the association between IL-6 and PTB, conducting an updated meta-analysis becomes essential to solve the inconsistencies in previous findings.
Therefore, we aimed to comprehensively evaluate the differences in IL-6 levels between normal birth and PTB groups, and assess the current state of knowledge regarding the relationship between IL-6 and PTB.
Methods
This meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guidelines [Citation25]. Since no original clinical raw data was collected or used, ethical approval was not requested.
Cohort selection
Three recognized electronic databases, including PubMed, Embase, and the Cochrane library, were searched for relevant studies published up to 18 February 2020, using the MeSH terms of ‘preterm birth’, ‘foetal membranes, premature rupture’, and ‘Interleukin-6’, combined with their analogs. Restriction on publication language was imposed in the electronic literature retrieval. In addition, the references of previous meta-analyses and the included studies were also screened for identifying those studies missing from the electronic literature search. Detailed search strategies of target databases are documented in Supplementary Table 1.
Eligibility criteria
Eligible studies were selected by two independent authors (Ying Chang and Wen Li) following the eligibility criteria as follows: (1) patients: pregnant females undergoing preterm birth and singleton pregnancies; (2) comparison: pregnant females undergoing healthy and term birth (gestational age from 37 weeks, 0 days to 41 weeks, 6 days); (3) outcomes: studies evaluated the difference in the level of IL-6 between term birth and PTB; (4) study type: prospective cohort study; and (5) full-text published in English. Any disagreements about study selection between the two authors (Ying Chang and Wen Li) were resolved through consulting a third senior author (Xu Chen).
Outcomes
The primary outcome was the association between IL-6 and PTB. The secondary outcomes were the association between IL-6 and spontaneous PTB. In the current updated meta-analysis, the association between IL-6 and PTB was clarified through estimating the difference in the level of IL-6 between PTB and term birth. Specifically, it indicated that IL-6 would be associated with the occurrence of PTB if the level of IL-6 in PTB group was higher than that in the term birth group.
Data extraction and quality assessment
Two authors (Yongmei Shen and Shanshan Li) used the pre-designed standard data collection form to independently extract the essential data, including authors, year of publication, country, study design, sample size, age, definition of PTB, specimen/sample source, sample collection time, measure method, IL-6 levels, and outcomes. The quality of the included studies were evaluated using the Newcastle-Ottawa scale (NOS) [Citation26].
Statistical analysis
All analyses were performed using STATA MP 14.0 (StataCorp, College Station, Texas, USA). Standardized mean difference (SMD) with 95% confidence interval (CI) was used to express the estimates of the difference in the level of IL-6 between the term birth and PTB. Statistical heterogeneity among studies was assessed using the Cochran’s Q and the I2 index. An I2>50% and p < 0.10 indicated significant statistical heterogeneity, and the random-effects model was used for meta-analysis; otherwise, the fixed-effects model was applied. Subgroup analyses were conducted according to the sources of IL-6 (maternal blood vs. amniotic fluid vs. cervicovaginal fluid) and the types of PTB (spontaneous PTB vs. general PTB). Sensitivity analysis was conducted to examine the robustness of the pooled results using the leave-one-out strategy. Potential publication bias was assessed by using Egger’s and Begg tests [Citation27]. P-value <0.05 was considered statistically significant.
Results
Included studies
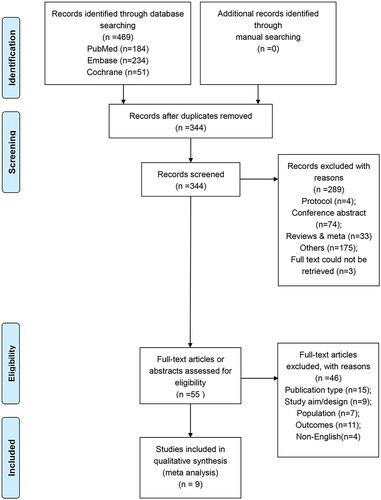
presents the process of study selection. Specifically, electronic literature search retrieved 469 records, of these, 125 duplicates were removed. Then, a total of 344 records were retained for further screening. After screening the title and abstract, 289 unrelated studies were excluded further. From the 55 remaining studies, 46 were excluded because of ineligible publication type (n = 11), ineligible study aims or design (n = 9), ineligible population (n = 7), lack of outcomes (n = 11), and ineligible languages (n = 4). Finally, nine studies were included for meta-analysis [Citation14–21,Citation28]. Besides, the definition of PTB (gestational age <37 weeks) and term birth (gestational age within 37 weeks, 0 days and 41 weeks, 6 days) were consistent across the nine studies.
In total, 1904 patients were accumulated in these nine eligible studies, with 250 in PTB group and 1654 in term birth group. Five studies examined IL-6 in amniotic fluid [Citation14,Citation16,Citation18,Citation20,Citation21], three in maternal blood [Citation14,Citation18,Citation19], and four in cervical/cervicovaginal fluid [Citation15,Citation17,Citation19,Citation28]. The cut-off values for IL-6 positivity ranged from 0.1 to 50 pg/mL across the included studies [Citation14–21,Citation28]. Compared to reported detection limit of IL-6 (5 pg/mL) [Citation29], the findings indicated a moderate to high sensitivity of detection. The details of other characteristics of all studies are summarized in . Besides, As shown in , the GRADE quality assessment for both the PTB and term-birth groups revealed a moderate quality level. This suggested that the actual effect was likely to closely align with the estimated effect, but there remained a possibility of substantial deviation. Detailed methodological assessment results are summarized in Supplementary Table 1.
Table 1. General characteristics of included studies.
Table 2. Grading of recommendations, assessment, development, and evaluations (GRADE) summary of outcomes.
Association between IL-6 and PTB
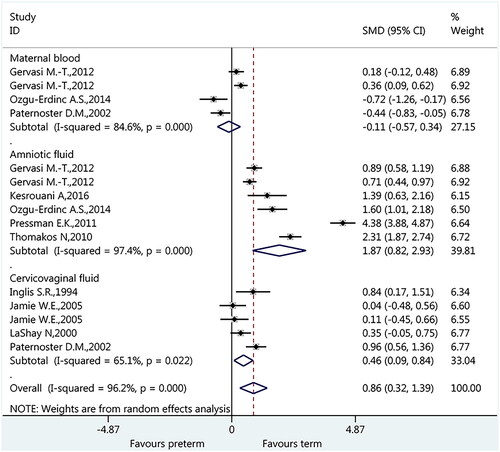
As described previously, IL-6 in the maternal blood, amniotic fluid, and cervicovaginal fluid were identified in the included nine studies. Therefore, we conducted subgroup analysis to clarify the association between IL-6 and PTB according to the sources of IL-6. As shown in ., the overall pooled result suggested a significant association between IL-6 and PTB (SMD: 0.86, 95% CI: 0.32 to 1.39, p < 0.001). Furthermore, subgroup analyses indicated that IL-6 in maternal blood was not associated with PTB (SMD: −0.11, 95%CI: −0.57 to 0.34, p = 0.623, p < 0.001); however, IL-6 in amniotic fluid (SMD: 1.87, 95%CI: 0.82 to 2.93, p < 0.001, p < 0.001) and cervicovaginal fluid was associated with PTB (SMD: 0.46, 95%CI: 0.09 to 0.84, p = 0.016).
Association between IL-6 and spontaneous PTB
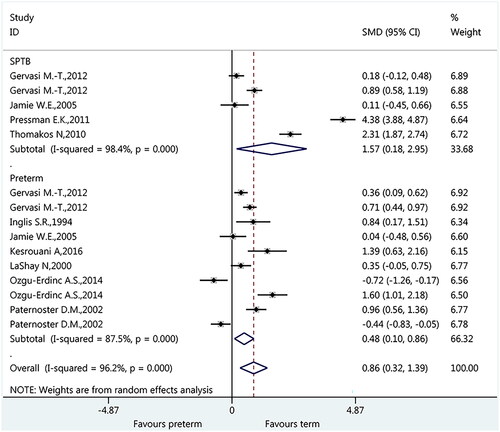
Among the included nine studies, five studies reported PTB as the spontaneous PTB. Therefore, subgroup analysis was also conducted to investigate the association between IL-6 and spontaneous PTB. As shown in , meta-analysis indicated a significant association between IL-6 and spontaneous PTB (SMD: 1.57, 95%CI: 0.18 to 2.95, p = 0.026). Meanwhile, a significant association was also present for general PTB (SMD: 0.48, 95%CI: 0.10 to 0.86, p = 0.013).
Sensitivity analysis
As shown in , all pooled results were not significantly changed after omitting one study at one time, indicating the robustness of the pooled results.
Publication bias
For the meta-analysis of the overall association between IL-6 and PTB, Begg’s (p = 0.235) and Egger’s (p = 0.421) tests suggested no publication bias among the included studies. Publication bias examination for subgroup analyses was not conducted due to the limited number of eligible studies.
Discussion
Preterm birth is a major cause of neonatal morbidity and mortality around the world, and it consumes a large portion of healthcare resources. Early and accurate identification of high-risk patients plays a critical role in effectively treating this condition. Research has unveiled diverse roles for IL-6, encompassing pro-inflammatory cytokine and anti-inflammatory myokine functions [Citation30]. The potential role of IL-6 in predicting PTB has been debated, with varying findings from primary studies and subsequent meta-analyses [Citation10,Citation11,Citation13–22]. This has led to uncertainty about predictive utility of IL-6 for PTB. Thus, an essential task is to elucidate the association between IL-6 and PTB via an updated meta-analysis.
In the currently updated meta-analysis, a total of nine nine studies involving 1904 patients were included for data analysis, and the pooled results suggest that IL-6 in amniotic fluid and cervicovaginal fluid might be useful for predicting PTB. Furthermore, the current meta-analysis also showed that IL-6 in maternal blood could not predict the occurrence of PTB. Finally, the pooled results indicated that the predictive value of IL-6 on PTB could not be negatively compromised by the types of PTB.
Currently, several meta-analyses have attempted to investigate the association between IL-6 and PTB. The meta-analysis by Conde-Agudelo et al. [Citation24] examined 30 biomarkers for association with PTB and observed that none were associated with PTB in any clinically relevant manner, but that IL-6 in amniotic fluid had a moderate predictive accuracy for PTB. Liu et al. [Citation10] also conducted a meta-analysis to address this issue and found that IL-6 in amniotic fluid IL-6 were associated with PTB. Furthermore, the meta-analysis by Wei et al. [Citation22] showed that IL-6 in cervicovaginal fluid was associated with PTB, but not IL-6 in maternal blood, consistent with the findings in the present meta-analysis. In addition, compared to previous meta-analysis, the current meta-analysis only included prospective cohort studies to significantly increase the reliability of the pooled results. Furthermore, the current meta-analysis clarified the association between IL-6 and PTB through calculating the difference in the level of IL-6 between PTB and term birth groups rather than calculating the risk ratio, which can provide the specific value of IL-6 for estimating the risk of PTB.
Conflicting findings on the association between IL-6 and PTB in the available meta-analyses could be attributed to the intricate nature of PTB, stemming from various mechanisms—such as inflammation, infection, uteroplacental ischaemia, haemorrhage, uterine overdistension, and stress—amid a web of environmental and genetic influences [Citation31]. Therefore, PTB is a highly heterogeneous condition among women. Numerous immune cytokines are involved in PTB, and PTB is considered as an altered immune state [Citation32]. Maternal tolerance is a prerequisite to successful term pregnancy, and immune maladaptation may induce an immune response that could cause miscarriage or precipitate delivery [Citation33,Citation34]. Increase levels of pro-inflammatory cytokines IL-1, IL-2, IL-6, IL-8, and TNF-α, precede labour [Citation35,Citation36]. Subclinical uterine inflammation might induce contractions [Citation37]. Therefore, elevated levels of pro-inflammatory cytokines might be associated with a higher risk of PTB.
Variations in IL-6 levels across gestational age exhibited disparities in different fluid compartments among women with term births. Notably, IL-6 concentration in maternal blood increased from the first to the third trimester [Citation38]. Conversely, IL-6 levels in amniotic fluid remained independent of gestational age, demonstrating consistency throughout pregnancy [Citation39]. In our study, five investigations assessed IL-6 levels during mid-trimester amniocentesis, while two employed different timing protocols, and two did not report timing information. Importantly, when compared the IL-6 level of certain sample type between term birth and preterm birth groups, the difference of sampling time was subtle, which well controlled the potential impacts of gestational age on IL-6 levels and ensured the reasonability of our findings. Besides, the optimal gestational age for IL-6 testing as a predictor of preterm birth can vary among different studies. Consequently, the decision to employ IL-6 as a predictive tool should be guided by clinical judgement, individual medical history, and the presence of other risk factors. Nevertheless, our research underscored the predictive potential of detecting IL-6 in amniotic and cervicovaginal fluid during the second trimester.
The results must be considered with caution because of the following limitations of the present meta-analysis. First, only nine prospective cohort studies are included in the current meta-analysis. Since randomized trials are, of course, impossible for the outcome analyzed here, prospective cohort studies are selected to decrease bias. On the other hand, it left out a number of retrospective studies. Second, heterogeneity was high among the included studies, possibly affecting the results. Importantly, Wu et al. [Citation23] suggested that the association between IL-6 polymorphisms and PTB is population-dependent and that the CC genotype of rs1800795 is associated with PTB only in women of European descent. IL-6 polymorphisms refer to genetic variations within the IL-6 gene, which can influence the production, secretion, and activity [Citation40]. Since IL-6 polymorphisms have been associated with altered susceptibility to PTB, and are highly variable among populations and affect its biological function, it is possible that the association between IL-6 and PTB varies among different populations [Citation41–43]. Of note, seven of the nine studies were done in countries of European descent, and two were in Middle East countries. No included study was done in Asian populations. This issue should be examined in future studies. Thirdly, biomarkers of PTB are influenced by the exact moment they are assessed in relation to delivery [Citation44]. On the other hand, Liu et al. [Citation10] showed that both early- and mid-trimester IL-6 levels were associated with PTB. In the present study, five studies examined IL-6 levels at mid-trimester amniocentesis, while two had different timings, and two did not report the timing. Future studies should also examine this issue. Fourthly, the formal analysis encompassed exclusively maternal blood, amniotic fluid, and cervicovaginal fluid. Omission of tissue-based studies, such as those involving the placenta or foetal membrane, restricted the exploration of underlying mechanisms of preterm birth and the role of IL-6. In future research, expanding the scope to include tissue-based studies could provide a more comprehensive understanding of the complex interactions between IL-6 and preterm birth. Fifthly, our study mainly concentrated on preterm birth as the main outcome, with limited emphasis on associated complications and direct experiences of patients. Therefore, it might not completely align with the CROWN initiative’s goals of establishing tandardized and harmonised outcome reporting across diverse women’s and newborn health studies [Citation45]. Finally, the present study only examined IL-6. The other cytokines, pro-inflammatory and anti-inflammatory, should be examined in future analyses. It was worth noting that due to the multifactorial aetiology of PTB, the adoption of multi-marker screening emerges as a plausible strategy to augment the accuracy and reliability of risk assessment [Citation46]. The integration of a spectrum of biomarkers, including IL-6, could facilitate a more holistic insight into PTB aetiology.
Conclusion
The updated meta-analysis established that IL-6 in amniotic and cervicovaginal fluids could hold potential for PTB prediction. In clinical application, amniotic fluid cytokine testing lacks justification in asymptomatic women due to amniocentesis risks. In contrast, acquiring cervicovaginal fluid is less risky and more feasible. Elevated cervicovaginal IL-6 could signal possible PTB, prompting additional testing and vigilant follow-up. Notably, the association of IL-6 with spontaneous PTB suggested its potential value for testing in asymptomatic women.
Authors’ contributions
YC and XC: study design, data collection and analysis, statistical analysis, and manuscript drafting. WL: study design, and data collection and analysis. YS and SL: data collection. YC: manuscript revision. XC: study design and critical revision of the manuscript. WL: study design and manuscript revision. YC and WL contributed equally to this study. All authors have read and approved the manuscript.
Supplemental Material
Download Zip (170.6 KB)Acknowledgements
Not applicable.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article.
Additional information
Funding
References
- Goldenberg RL, Culhane JF, Iams JD, et al. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):1–10. doi: 10.1016/S0140-6736(08)60074-4.
- American College of O, Gynecologists’ Committee on Practice B-O. Practice bulletin no. 171: management of preterm labor. Obstet Gynecol. 2016; 128(4):e155-64.
- Iams JD. Prevention of preterm parturition. N Engl J Med. 2014; 370(3):254–261. doi: 10.1056/NEJMcp1103640.
- Rundell K, Panchal B. Preterm labor: prevention and management. Am Fam Physician. 2017;95(6):366–372.
- American College of O, Gynecologists, Society for Maternal-Fetal M. Obstetric care consensus no. 6: periviable birth. Obstet Gynecol. 2017;130(4):e187–e199.
- Matthews TJ, MacDorman MF, Thoma ME. Infant mortality statistics from the 2013 period linked birth/infant death data set. National vital statistics reports: from the centers for disease control and prevention, national center for health statistics. Natl Vital Stat Rep. 2015;64(9):1–30.
- Vidal MS, Jr., Lintao RCV, Severino MEL, et al. Spontaneous preterm birth: involvement of multiple feto-maternal tissues and organ systems, differing mechanisms, and pathways. Front Endocrinol. 2022;13:1015622. doi: 10.3389/fendo.2022.1015622.
- Lucaroni F, Morciano L, Rizzo G, et al. Biomarkers for predicting spontaneous preterm birth: an umbrella systematic review. J Matern-FetalNeonatal Med 2018;31(6):726–734. doi: 10.1080/14767058.2017.1297404.
- Polettini J, Cobo T, Kacerovsky M, et al. Biomarkers of spontaneous preterm birth: a systematic review of studies using multiplex analysis. J Perinat Med. 2017;45(1):71–84. doi: 10.1515/jpm-2016-0097.
- Liu Y, Liu Y, Zhang R, et al. Early- or mid-trimester amniocentesis biomarkers for predicting preterm delivery: a meta-analysis. Ann Med. 2017;49(1):1–10. doi: 10.1080/07853890.2016.1211789.
- Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6(10):a016295–a016295. doi: 10.1101/cshperspect.a016295.
- Bastard JP, Jardel C, Delattre J, et al. Evidence for a link between adipose tissue interleukin-6 content and serum C-reactive protein concentrations in obese subjects. Circulation. 1999;99(16):2221–2222. doi: 10.1161/circ.99.16.2219/c.
- Wakabayashi A, Sawada K, Nakayama M, et al. Targeting interleukin-6 receptor inhibits preterm delivery induced by inflammation. Mol Hum Reprod. 2013;19(11):718–726. doi: 10.1093/molehr/gat057.
- Gervasi MT, Romero R, Bracalente G, et al. Midtrimester amniotic fluid concentrations of interleukin-6 and interferon-gamma-inducible protein-10: evidence for heterogeneity of intra-amniotic inflammation and associations with spontaneous early (<32 weeks) and late (>32 weeks) preterm delivery. J Perinat Med. 2012;40(4):329–343. doi: 10.1515/jpm-2012-0034.
- Jamie WE, Edwards RK, Ferguson RJ, et al. The interleukin-6–174 single nucleotide polymorphism: cervical protein production and the risk of preterm delivery. Am J Obstet Gynecol. 2005;192(4):1023–1027. doi: 10.1016/j.ajog.2005.01.035.
- Kesrouani A, Chalhoub E, El Rassy E, et al. Prediction of preterm delivery by second trimester inflammatory biomarkers in the amniotic fluid. Cytokine. 2016;85:67–70. doi: 10.1016/j.cyto.2016.06.008.
- LaShay N, Gilson G, Joffe G, et al. Will cervicovaginal interleukin-6 combined with fetal fibronectin testing improve the prediction of preterm delivery? J Matern Fetal Med. 2000;9(6):336–341. doi: 10.1002/1520-6661(200011/12)9:6<336::AID-MFM1003>3.0.CO;2-F.
- Ozgu-Erdinc AS, Cavkaytar S, Aktulay A, et al. Mid-trimester maternal serum and amniotic fluid biomarkers for the prediction of preterm delivery and intrauterine growth retardation. J Obstet Gynaecol Res. 2014;40(6):1540–1546. doi: 10.1111/jog.12371.
- Paternoster DM, Stella A, Gerace P, et al. Biochemical markers for the prediction of spontaneous pre-term birth. Int J Gynaecol Obstet. 2002;79(2):123–129. Novdoi: 10.1016/s0020-7292(02)00243-6.
- Pressman EK, Thornburg LL, Glantz JC, et al. Inflammatory cytokines and antioxidants in midtrimester amniotic fluid: correlation with pregnancy outcome. Am J Obstet Gynecol. 2011;204(2):155 e1–7. doi: 10.1016/j.ajog.2010.08.064.
- Thomakos N, Daskalakis G, Papapanagiotou A, et al. Amniotic fluid interleukin-6 and tumor necrosis factor-alpha at mid-trimester genetic amniocentesis: relationship to intra-amniotic microbial invasion and preterm delivery. Eur J Obstet Gynecol Reprod Biol. 2010;148(2):147–151. doi: 10.1016/j.ejogrb.2009.10.027.
- Wei SQ, Fraser W, Luo ZC. Inflammatory cytokines and spontaneous preterm birth in asymptomatic women: a systematic review. Obstet Gynecol. 2010;116(2 Pt 1):393–401. doi: 10.1097/AOG.0b013e3181e6dbc0.
- Wu W, Clark EA, Stoddard GJ, et al. Effect of interleukin-6 polymorphism on risk of preterm birth within population strata: a meta-analysis. BMC Genet. 2013;14(1):30. doi: 10.1186/1471-2156-14-30.
- Conde-Agudelo A, Papageorghiou AT, Kennedy SH, et al. Novel biomarkers for the prediction of the spontaneous preterm birth phenotype: a systematic review and meta-analysis. BJOG. 2011;118(9):1042–1054. doi: 10.1111/j.1471-0528.2011.02923.x.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339(1):b2535–b2535. doi: 10.1136/bmj.b2535.
- Lo CK, Mertz D, Loeb M. Newcastle-Ottawa scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. 2014;14(1):45. doi: 10.1186/1471-2288-14-45.
- Higgins J, Thomas J. Cochrane handbook for systematic reviews of interventions (version 6). London: Cochrane Collaboration; 2019.
- Inglis SR, Jeremias J, Kuno K, et al. Detection of tumor necrosis factor-alpha, interleukin-6, and fetal fibronectin in the lower genital tract during pregnancy: relation to outcome. Am J Obstet Gynecol. 1994;171(1):5–10. doi: 10.1016/s0002-9378(94)70069-9.
- Rahbar M, Wu Y, Subramony JA, et al. Sensitive colorimetric detection of interleukin-6 via lateral flow assay incorporated silver amplification method. Front Bioeng Biotechnol. 2021;9:778269. doi: 10.3389/fbioe.2021.778269.
- Scheller J, Chalaris A, Schmidt-Arras D, et al. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813(5):878–888. doi: 10.1016/j.bbamcr.2011.01.034.
- Romero R, Espinoza J, Kusanovic JP, et al. The preterm parturition syndrome. BJOG. 2006;113(Suppl 3):17–42. doi: 10.1111/j.1471-0528.2006.01120.x.
- Makhseed M, Raghupathy R, Azizieh F, et al. Th1 and Th2 cytokine profiles in recurrent aborters with successful pregnancy and with subsequent abortions. Hum Reprod. 2001;16(10):2219–2226. doi: 10.1093/humrep/16.10.2219.
- Poole JA, Claman HN. Immunology of pregnancy. Implications for the mother. Clin Rev Allergy Immunol. 2004;26(3):161–170. doi: 10.1385/CRIAI:26:3:161.
- Makhseed M, Raghupathy R, El-Shazly S, et al. Pro-inflammatory maternal cytokine profile in preterm delivery. Am J Reprod Immunol. 2003;49(5):308–318. doi: 10.1034/j.1600-0897.2003.00038.x.
- Gravett MG, Witkin SS, Haluska GJ, et al. An experimental model for intraamniotic infection and preterm labor in rhesus monkeys. Am J Obstet Gynecol. 1994;171(6):1660–1667. doi: 10.1016/0002-9378(94)90418-9.
- Christiaens I, Zaragoza DB, Guilbert L, et al. Inflammatory processes in preterm and term parturition. J Reprod Immunol. 2008;79(1):50–57. doi: 10.1016/j.jri.2008.04.002.
- Raghupathy R, Al Mutawa E, Makhseed M, et al. Redirection of cytokine production by lymphocytes from women with pre-term delivery by dydrogesterone. Am J Reprod Immunol. 2007;58(1):31–38. doi: 10.1111/j.1600-0897.2007.00488.x.
- Graham AM, Rasmussen JM, Rudolph MD, et al. Maternal systemic interleukin-6 during pregnancy is associated with newborn amygdala phenotypes and subsequent behavior at 2 years of age. Biol Psychiatry. 2018;83(2):109–119. doi: 10.1016/j.biopsych.2017.05.027.
- Del Barco E, Franco-Jarava C, Vargas M, et al. Reference values for interleukin-6 in the amniotic fluid of asymptomatic pregnant women. Acta Obstet Gynecol Scand. 2023;102(4):480–485. doi: 10.1111/aogs.14524.
- Woo P, Humphries SE. IL-6 polymorphisms: a useful genetic tool for inflammation research? J Clin Invest. 2013;123(4):1413–1414. doi: 10.1172/JCI67221.
- Huth C, Illig T, Herder C, et al. Joint analysis of individual participants’ data from 17 studies on the association of the IL6 variant -174G > C with circulating glucose levels, interleukin-6 levels, and body mass index. Ann Med. 2009;41(2):128–138. doi: 10.1080/07853890802337037.
- Prairie E, Cote F, Tsakpinoglou M, et al. The determinant role of IL-6 in the establishment of inflammation leading to spontaneous preterm birth. Cytokine Growth Factor Rev. 2021;59:118–130. doi: 10.1016/j.cytogfr.2020.12.004.
- Smith AJ, Humphries SE. Cytokine and cytokine receptor gene polymorphisms and their functionality. Cytokine Growth Factor Rev. 2009;20(1):43–59. doi: 10.1016/j.cytogfr.2008.11.006.
- Goldenberg RL, Goepfert AR, Ramsey PS. Biochemical markers for the prediction of preterm birth. Am J Obstet Gynecol. 2005;192(5 Suppl):S36–S46. doi: 10.1016/j.ajog.2005.02.015.
- van ‘t Hooft J, Alfirevic Z, Asztalos EV, et al. CROWN initiative and preterm birth prevention: researchers and editors commit to implement core outcome sets. BJOG. 2018;125(1):8–11. doi: 10.1111/1471-0528.14987.
- Della Rosa PA, Miglioli C, Caglioni M, et al. A hierarchical procedure to select intrauterine and extrauterine factors for methodological validation of preterm birth risk estimation. BMC Pregnancy Childbirth. 2021;21(1):306. doi: 10.1186/s12884-021-03654-3.