Abstract
Objective
Vector-borne diseases are a growing burden worldwide. In particular, the risks of allergic reactions to bites are associated with growing arthropod populations in contact with the public. The diversity of allergic reactions associated with host and arthropod factors difficult disease diagnosis, prognosis and prevention. Therefore, arthropod-associated allergies are underdiagnosed and require better surveillance of arthropod populations and disease diagnosis and management.
Methods
To face these challenges, in this study, we describe five cases to illustrate arthropod-associated allergies with different symptomatology, including alpha-gal syndrome (AGS) associated with anti-alpha-gal IgE antibody titres. Information on symptoms in response to arthropod bites was collected from patients and medical doctors.
Results
The five cases included patients bitten by a robber fly and different tick species. Cases were in Spain or U.S.A. Two cases were diagnosed with AGS and one case was diagnosed with anaphylaxis in response to tick bite with high anti-alpha-gal IgE levels. The symptoms in response to arthropod bites vary between different cases.
Conclusion
Allergic reactions and symptoms in response to arthropod bites vary in association with host and arthropod factors. Herein we propose recommendations to control allergic symptoms, associated disease risk factors and the way forward to advance in the prevention and control of arthropod-associated allergies.
Introduction
Arthropods produce allergens sensitizing and inducing systemic immunoglobulin E (IgE)-mediated allergic reactions in humans [Citation1]. A hypothesis is that hominids evolved through multiple catastrophic selection events such as losing the capacity to synthesize the glycan alpha-gal or body hear with the trade-off of developing allergy to arthropods [Citation2,Citation3]. Although systemic reactions to flies (insect) and ticks (acari) are considered as rare [Citation4], the risk of arthropod-associated allergies such as alpha-gal syndrome (AGS) is growing in association with the expansion and increase of arthropod and host populations [Citation5]. The AGS is an allergy associated with tick bites and characterized by IgE-mediated hypersensitivity to the oligosaccharide galactose-alpha-1,3-galactose (alpha-gal), present in tick saliva and non-primate mammalian meat and derived products [Citation2,Citation6].
Nevertheless, many of the arthropod-associated allergies are underdiagnosed and require better surveillance of arthropod populations and disease diagnosis and management [Citation7–9]. The AGS is underdiagnosed due to low recognition among primary care doctors and other health care providers. Additionally, the question still to be addressed is why only some individuals and with different timelines and symptoms develop allergic reactions to arthropod bites.
To advance in the prevention of arthropod-associated allergies and diseases, as described in this paper, it is important to inform health care system, society and scientists on these cases and their diversity in clinical presentations and arthropod species involved.
Materials and methods
Patients
Data was collected from patients and medical doctors in five cases who presented allergic reactions in response to arthropod bites. Inclusion criteria were (a) development of allergic reactions in response to arthropod bites from different species (robber fly and various tick species), (b) reported in different regions (located in Spain and U.S.A.), (c) allergic reactions confirmed by pathological data collected at medical centres and/or patient’s provided evidence, and (d) for alpha-gal syndrome (AGS) in support to anti-alpha-gal IgE antibody titres. Cases were excluded if arthropod bites were not detected or presented other allergic diseases. General information collected about cases included aga, gender, and location. The use of samples and individual data was approved by the Ethical and Scientific Committees (University Hospital of Ciudad Real C-352 and SESCAM C-73). Consent was obtained from all participants in this study.
Classification of arthropod species
Arthropod genera and species were determined morphologically following reference indicators. A molecular barcoding analysis was conducted to confirm robber fly species for which limited information is available [Citation10–14]. Fly DNA was extracted using TRI Reagent (Sigma-Aldrich, St. Louis, USA) and following manufacturer’s protocol. The fragment of cytochrome c oxidase subunit I (COI) was amplified by polymerase chain reaction (PCR), yielding a product of 650 bp. Primers used were forward LCO1490 (5′-GGT CAA centers ATC ATA AAG ATA TTG G-3′) and reverse HCO2198 (5′-TAA ACT TCA GGG TGA CCA AAA AAT CA-3′) (Integrated DNA Technologies, Coralville, USA) [Citation14]. For a total volume of 25 µl, the PCR mixture contained 12.5 µl of Promega Master Mix (Promega Corporation, Fitchburg, USA), 1 µl (10 μM) of each primer, 1.5 µl of sample DNA and 9 µl nuclease-free water. PCR protocol consisted of an initial denaturation of 95 °C for 9 min, followed by 41 cycles of 94 °C for 30 sec, annealing at 50 °C for 45 sec, extension at 72 °C for 45 sec and a final extension at 72 °C for 10 min. To verify PCR products, 6 µl of the reaction mixture was used in a 1.5% agarose-gel electrophoresis. PCR amplicons were purified and subjected to Sanger sequencing at Secugen S.I. (Madrid, Spain). Low-quality sequenced areas were removed from both ends using Chromas software version 2.6.6, allowing the submission of the COI sequence to GenBank under the accession number OR355674.
Determination of anti-alpha-gal IgE antibody titres
Anti-alpha-gal IgE levels were determined using the ImmunoCAP Phadia 250 automated platform (Thermo Fisher Scientific, Uppsala, Sweden) with the commercial ImmunoCap α-Gal bovine Thyroglobulin kit according to the manufacturer’s instructions. Positive results were considered for anti-alpha-gal IgE levels ≥ 0.1 kU/L [Citation4].
Results: Case presentation
Case 1. Fly induced allergy
A 61-year-old man living in Toledo, Spain, was bitten by a robber fly, Dasypogon diadema (Fabricius, 1781) or Dasypogon iberus [Citation10], developing an anaphylactic reaction that required 3 doses of Adrenaline, Urbason and Polaramine for recovery. The patient was bitten on the left ear, where he experienced a strong pain together with discomfort, dizziness, dyspnoea and dysphagia with uvula edoema. On the next day, he presented a residual ovula edoema and suffered a biphasic reaction that required corticosteroids. Cutaneous injuries were not observed. Serum anti-alpha-gal IgE of 0.03 kU/L, total IgE of 29.6 kU/L and tryptase (7.58 µg/L) levels were normal. The patient never experienced before any allergic reactions to other insects or tick bites or mammalian meat consumption.
Fly Dasypogon genus was determined morphologically according to reference indicators [Citation10–13] (). Fly species was confirmed by COI DNA molecular barcoding [Citation14]. Identified species showed a 94% BLAST nucleotide sequence identity with two D. diadema sequences deposited in NCBI GenBank (accession numbers NC_045239 and KT733402). However, considering that Dasypogon species are not present in the GenBank taxonomy database [Citation15] and that D. diadema current distribution is limited to Central and Western Europe (GBIF Backbone Taxonomy. Accessed 30 July 2023; https://www.gbif.org/dataset/d7dddbf4-2cf0-4f39-9b2a-bb099caae36c), it is possible that the specimen analysed is the closely related Dasypogon iberus [Citation10], which is the most common and widespread Dasypogoninae specie on the Spanish plateau [Citation16,Citation17].
Case 2. Tick induced allergy
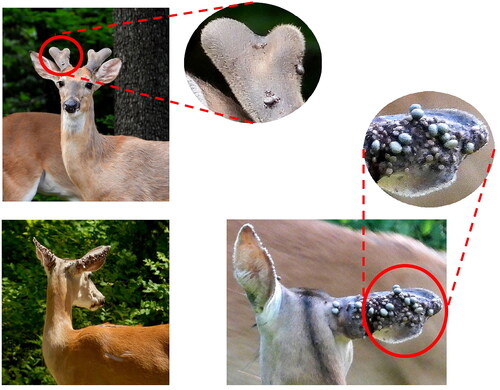
A 47-year-old man living in New York, U.S.A., developed anaphylactic allergic reactions to black-legged deer tick, Ixodes scapularis (). Tick species was determined morphologically (). After tick bite, he started vomiting and had a severe headache, the most painful event of his life. The tick was removed after a few hours, and the pain stopped very quickly. Over several days, the tick bite site presented pruritus, erythema, induration and exudation (). The patient experienced multiple deer tick bites and did not have any allergic reactions to mammalian meat that he consumes frequently. The AGS was discarded as serum anti-alpha-gal IgE levels (0.05 kU/L) were normal. He reported that he could rapidly noticed and removed most of his tick bites. If not, it took approximately a day for the body’s reaction to become apparent, as the spot began to form a papule with central ulceration, accompanied by serous exudate. The affected region undergone induration and pruritus, persisting for several weeks.
Figure 2. Skin reactions to different deer tick bites in case 2. Photos courtesy of participants in case 2.
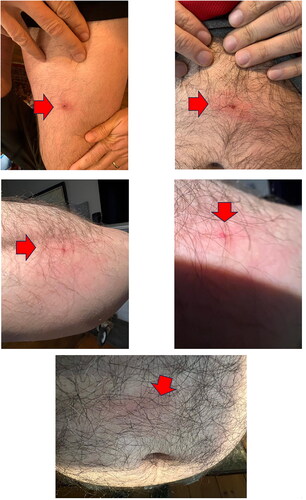
Figure 3. Images of collected adult female and male deer ticks in case 2. Photos courtesy of participants in case 2.

On the most recent tick bite, the patient was standing in the kitchen when he experienced localized pruritus on the abdominal region. Intermittent episodes of pruritus were noted, and shirt was pulled up to remove the tick before attachment to skin. Almost instantly the bite led to pruritus, and shortly afterward, it started exudating and became erythematous, indurated, and warm to the touch. Then, it was noticed that there were two other spots, likely associated with tick bites. This was the third or fourth time where the patient experienced pruritus, along with episodes of vomiting and blinding headaches. It appears that the patient developed protective mechanisms (e.g. antibody response) to prevent tick attachment, however the bite spot persisted and remained uncomfortable for up to a year.
Case 3. Tick associated anaphylaxis with asymptomatic high anti-alpha-gal IgE antibody titres
A 69-year-old man living in Ciudad Real, Spain, developed allergic reactions to a tick bite in the right costal region. Three-to-four hours after tick bite, he experienced generalized pruritus, malaise, dizziness, dyspnoea, and hypotension. Local reactions in the site of tick bite were not observed. The tick was not collected and thus species was not identified but based on location and season, it was probably the castor bean tick Ixodes ricinus or Hyalomma marginatum with high alpha-gal content and associated with the AGS [Citation18,Citation19]. He was diagnosed with anaphylaxis in response to tick bite with anti-alpha-gal IgE levels of 61.9 kU/L and total IgE of 497 kU/L (blood type AB Rh positive). High-sensitivity C-reactive protein (hsCRP) levels were 0.54 mg/dL, and thus higher than normal levels (0.0 − 0.50 mg/dL) with associated inflammation and risks for myocardial infarction, peripheral arterial disease, stroke, and sudden cardiac death. Other serum biomarkers showed normal levels. A year before, the patient experienced a tick bite with only a local reaction. Therefore, this is a case who developed asymptomatic anti-alpha-ga IgE antibodies without the AGS but with the tick-associated anaphylaxis.
Case 4. Tick-associated progressive alpha gal syndrome
A 69-year-old man living in Oklahoma, U.S.A., developed the AGS with progressive allergic symptoms over several years and culminated in full anaphylactic reactions. The patient had had multiple exposures to tick bites primarily by lone star tick, Amblyomma americanum (Linnaeus) (), and I. scapularis over several years prior to the first symptoms. The first symptoms were noticed about 20-23 years ago. In the morning there was a fading rash in the mid body region without noticeable itching while sleeping and at the time were attributed to an insect bite or toxic plant interaction. The rashes were later determined to be hives as the symptoms worsened. This reaction occurred several times over a year. The subsequent symptoms began during early morning. The patient would awaken with a strong feeling of ‘anxiety’ and laboured breathing. Deep breathing eventually alleviated the symptoms which were retrospectively determined to be respiratory constriction following an allergic reaction. There were several of these events over time, but the cause remained unknown. Since then, it was determined that each event followed a meal with mammalian meat. After a long international flight and an evening meal of minced beef the patient suffered a full anaphylactic shock and loss of consciousness. At the time it was attributed to jet lag and fatigue from the trip as nothing was known of AGS. Subsequently, on a business road trip, he stopped for a single hamburger and several hours later experienced urticaria and hives with respiratory constriction. This established a connection between the previous reactions and meat consumption. There was one other anaphylactic episode in 2010 (also mammalian meat mediated) and the patient ceased eating any mammalian meat or products. An immunologist determined that the patient had an allergy to beef, pork and lamb and was subsequently found to have high anti-alpha-gal IgE antibody titres (8.0 kU/L) associated with AGS. Since abstaining from eating mammalian meat products in 2010 there have not been any symptoms or allergic reactions. The last evaluation of anti-alpha-gal IgE antibody titres was conducted five years ago and presented 8.6 kU/L.
Case 5. Tick associated alpha gal syndrome with urologic symptoms
A 77-year-old woman living in Oklahoma, U.S.A., with a long history of exposure to tick bites primarily A. americanum () and I. scapularis provided serum samples in 2012 for a study on the AGS. She tested positive with a high IgE antibody titres to alpha-gal (7.2 kU/L) comparable to patients with anaphylactic reactions to mammalian meat consumption. Several years later in 2018, serum sample was submitted again for another ongoing study with the same results of high antibody titres to alpha-gal (7.4 kU/L). Rather than presenting typical symptoms of anaphylaxis, this individual encountered gastro-intestinal and urologic symptoms several hours after ingesting pork-containing food. These symptoms included waking up with abdominal pain and cramping and acute onset of urologic symptoms consistent with a urinary tract infection but short-lived and without bacteraemia. Alpha-gal associated gastro-intestinal symptoms were recently reported [Citation20,Citation21], but urologic symptoms have not been recognized to be associated with AGS. Retrospectively and after testing positive for IgE anti-alpha-gal antibodies, the individual recounted two previous episodes several hours after ingesting pork products with the same symptoms. After serologic confirmation of the AGS, the patient eliminated mammalian meat products from her diet without additional allergic reactions.
Discussion
The diversity of symptoms associated with arthropod-borne allergies supports the complexity of the diagnosis and treatment of these diseases [Citation22]. An effective surveillance of allergic reactions in response to arthropods under the One Health concept when human, animal and environmental factors and variables are considered is fundamental to informing the general population and medical services on the incidence and risks for these diseases, the use of pharmaceuticals containing alpha-gal, and to facilitate disease prevention and control [Citation23,Citation24]. The common thread associated with these allergies is the development of pathologies and comorbidities that not only affect health but may also condition response to different compounds such as food and pharmaceuticals with various symptoms that are difficult to diagnose and treat. Recent research has advanced the identification of arthropod allergens affecting human health, but new studies are required to characterize the functional role of these molecules and develop diagnostic, prognostic and control interventions [Citation1,Citation25,Citation26]. Anti-tick vaccines have been registered and commercialized for the control of cattle tick infestations, but ongoing and future research will result in more effective arthropod control interventions by combining community-based tick management with innovative biotechnological approaches [Citation27,Citation28]. Based on available information and cases described here, we consider recommendations to control allergic symptoms, determine risk factors, and prevent and control arthropod-associated allergies.
Recommendations to control allergic reactions include and are not limited to prevention of arthropod bites by using protective clothing, repellents and insecticides, and avoiding unnecessary contact with farm and wild animals [Citation29]. For diagnosis of AGS, patient relevant history and positive serum IgE levels to alpha-gal are required [Citation7]. If diagnosed with AGS, the patient should avoid consuming mammalian meat, its derivatives and drugs such as cetuximab and infliximab containing alpha-gal. Patients can take a daily dose of 20 mg ebastine (e.g. Ebastel Forte) for the symptomatic treatment of allergic conditions such as pruritus or urticaria after arthropod bite. To carry two intramuscular auto-injection ampoules of Adrenaline (Epinephrine) when moving into zones with a high presence of ticks, robber flies or other arthropod causing allergies. In case of an adverse reaction in the form of hoarseness, choking, digestive symptoms or dizziness, the pre-filled dose should be administered on the lateral aspect of the thigh (following the packaging instructions), and seek immediate medical attention at a healthcare facility. If the symptoms do not subside, a repeat dose of Adrenaline should be administered after 5–10 min.
Risk factors of allergic reactions to arthropod bites are associated with contact with growing populations of arthropods and hosts involved in their life cycle, exposure to arthropod bites, abundance of arthropod species, changes in wild host populations promoting higher arthropod abundance, leisure and high-risk occupations in agriculture, forestry, hunting, and nursing among other, and incidence of other diseases with increasing risk capacity of allergic reactions [Citation30].
The way forward to advance in the prevention and control of arthropod-associated allergies and vector-borne diseases includes multidisciplinary and interdisciplinary research to determine the reasons behind the high variability of disease symptoms and the actual distribution and origin of circulating arthropods, identification of arthropod-derived proteins and biological mechanisms triggering allergic reactions in some individuals, communication to stakeholders and citizens who need to be aware of the situation regarding these diseases on each region, and public health activities. Although the risk is considered low, hospital doctors and general practitioners should be alert of the appearance of new cases, given the possibility of high allergenicity in some individuals. A detailed medical history of the patient, including travel history and possible risk factors, is critical for rapid diagnosis and implementing appropriate therapeutic measures to effectively manage the symptoms.
To further advance in disease diagnosis, prognosis, and prevention, it is important the characterization of the effect of vector-borne allergens on allergic reactions. Difficulties in the diagnosis of vector-borne diseases require the implementation of new surveillance approaches [Citation31,Citation32]. For example, a recent study suggested that Lyme borreliosis caused by the tick-borne pathogen, Borrelia burgdorferi, and AGS may constitute comorbidities in response to tick bites [Citation8]. In this study, seven cases with a record of tick bites and diagnosed with Lyme borreliosis presented allergic reactions to mammalian meat consumption [Citation8]. Additionally, as shown for COVID-19 [Citation33,Citation34], other factors suppressing or altering the immune system such as pathogen infection and response to vaccination may affect anti-alpha-gal antibody levels and should be considered in cases with allergic reactions to arthropod bites. Questions received in 2020–2021 from affected individuals illustrate these concerns (). The interaction between food and drug-associated allergies is also relevant [Citation35]. Due to antigenic similarity between alpha-gal and blood group antigen B, it is important to avoid its use in non-group B patients with AGS [Citation36]. Research collaborations between medical doctors and scientists and citizen participation are required to develop effective diagnosis and prognosis tools, and preventive vaccine interventions for the control of arthropod populations.
Table 1. Addressing questions received by affected individuals regarding COVID-19 and immune response to the glycan alpha-gal.
Conclusions
As illustrated in these cases, allergic anaphylactic reactions and symptoms in response to arthropod bites vary depending on both host and arthropod factors. The symptoms vary from severe pain accompanied by discomfort, dizziness, dyspnoea, and dysphagia with uvula residual edoema in response to robber fly (Case 1), to reactions with painful localized pruritus, vomiting and blinding headaches after bites by black-legged deer tick (Case 2), to generalized pruritus, malaise, dizziness, dyspnoea, and hypotension with AGS diagnosis after European hard tick bite (Case 3), to progressive AGS (Case 4) and AGS with urologic symptoms (Case 5) likely associated with lone star tick. Information on arthropod-borne allergic reactions and other diseases is key for personalized preventive, diagnostic and treatment interventions.
Ethics approval and consent to participate
Informed consent was obtained from all participants in this study. The use of samples and individual data was approved by the Ethical and Scientific Committees (University Hospital of Ciudad Real C-352 and SESCAM C-73).
Authors’ contributions
R.V.R. performed research and wrote the paper. F.F.B. performed research. R.G.R. performed research. L.M. performed research. J.F. designed research, interpreted data, and wrote the paper.
Acknowledgements
We would like to thank the Diptera specialist Piluca Alvarez Fidalgo for her advice on fly identification.
Disclosure statement
J.F. is section editor at the same journal. The other authors have no conflict of interest.
Data availability statement
The data obtained for the study are included in the article. Further inquiries can be directed to the corresponding author.
Additional information
Funding
References
- Arlian LG. Arthropod allergens and human health. Annu Rev Entomol. 2002;47(1):1–10. doi: 10.1146/annurev.ento.47.091201.145224.
- Galili U. Evolution in primates by ‘catastrophic-selection’ interplay between enveloped virus epidemics, mutated genes of enzymes synthesizing carbohydrate antigens, and natural anti-carbohydrate antibodies. Am J Phys Anthropol. 2019;168(2):352–363. doi: 10.1002/ajpa.23745.
- de la Fuente J, Contreras M. Vaccinomics: a future avenue for vaccine development against emerging pathogens. Expert Rev Vaccines. 2021;20(12):1561–1569. doi: 10.1080/14760584.2021.1987222.
- Bircher AJ. Systemic immediate allergic reactions to arthropod stings and bites. Dermatology. 2005;210(2):119–127. doi: 10.1159/000082567.
- Thompson JM, Carpenter A, Kersh GJ, et al. Geographic distribution of suspected alpha-gal syndrome cases - United States, January 2017–December 2022. MMWR Morb Mortal Wkly Rep. 2023;72(30):815–820. doi: 10.15585/mmwr.mm7230a2.
- Vaz-Rodrigues R, Mazuecos L, de la Fuente J. Current and future strategies for the diagnosis and treatment of the alpha-gal syndrome (AGS). J Asthma Allergy. 2022;15:957–970. doi: 10.2147/JAA.S265660.
- Carpenter A, Drexler NA, McCormick DW, et al. Health care provider knowledge regarding alpha-gal syndrome - United States, March–May 2022. MMWR Morb Mortal Wkly Rep. 2023;72(30):809–814. doi: 10.15585/mmwr.mm7230a1.
- de la Fuente J, Estrada-Peña A, Gortázar C, et al. Citizen science on lyme borreliosis in Spain reveals disease-associated risk factors and control interventions. Vector Borne Zoonotic Dis. 2023;23(9):441–446. doi: 10.1089/vbz.2023.0016.
- de la Fuente J. The alpha-Gal syndrome is underdiagnosed. Actas Dermosifiliogr. 2023. doi: 10.1016/j.ad.2023.07.018.
- Tomasovic G. Notes sur les asilidae paléarctiques (diptera brachycera) (10 et 11). description et répartition géographique de 2 espèces nouvelles de dasypogon du groupe ‘diadema (FABRICIUS, 1781)’. Bulletin de La Société Royale Belge D’Entomologie. 1999;135:216–221.
- Rikhter VA. Family asilidae. Keys to the insects of the european part of the USSR. In: Bei-Bienko GIA (editor). Program for scientific translations. [available from U.S. Dept. of Commerce, Clearinghouse for Federal Scientific and Technical Information: Springfield, VA] Jerusalem, Israel;1988:778–820.
- Oosterbroek P. The european families of the diptera: identification, diagnosis, biology. Leiden (the Netherlands): KNNV Publishing; 2006.
- van den Broek R, Schulten A. Field guide to the robberflies of The Netherlands and Belgium. Zuid-Holland (Netherlands): Jeugdbondsuitgeverij Publisher; 2017.
- Drukewitz S, Fuhrmann N, Undheim E, et al. A dipteran’s novel sucker punch: evolution of arthropod atypical venom with a neurotoxic component in robber flies (asilidae, diptera). Toxins. 2018;10(1):29. doi: 10.3390/toxins10010029.
- Schoch CL, Ciufo S, Domrachev M, et al. NCBI taxonomy: a comprehensive update on curation, resources and tools. Database: j. Biol. Databases Curation. 2020;2020:baaa062. doi: 10.1093/database/baaa062.
- Álvarez Fidalgo P, van den Broek R. Checklist de fauna ibérica. Familia asilidae (arthropoda: insecta: diptera) en la península ibérica e islas Baleares. In Ramos Sánchez MA, Sánchez Ruiz M, editors, Vol. 2, Documentos fauna ibérica. Madrid (Spain): Museo Nacional de Ciencias Naturales; 2019; p.16.
- van den Broek R, Álvarez Fidalgo P, Pires F. New faunistic data of asilids from Spain and Portugal (dipera: asilidae). Boletín de La Sociedad Entomológica Aragonesa (S.E.A.). 2018;63:95–102.
- Villar M, Pacheco I, Mateos-Hernández L, et al. Characterization of tick salivary gland and saliva alphagalactome reveals candidate alpha-gal syndrome disease biomarkers. Expert Rev Proteomics. 2021;18(12):1099–1116. doi: 10.1080/14789450.2021.2018305.
- ECDC. Mapas actualizados de la distribución de garrapatas en España. Control de plagas 2018; July 10. https://higieneambiental.com/control-de-plagas/mapas-actualizados-de-la-distribucion-de-garrapatas-en-espana.
- Croglio MP, Commins SP, McGill SK. Isolated gastrointestinal alpha-gal meat allergy is a cause for gastrointestinal distress without anaphylaxis. Gastroenterology. 2021;160(6):2178–2180.e1. doi: 10.1053/j.gastro.2021.01.218.
- McGill SK, Hashash JG, Platts-Mills TA. AGA clinical practice update on alpha-gal syndrome for the GI clinician: commentary. Clin Gastroenterol Hepatol. 2023;21(4):891–896. doi: 10.1016/j.cgh.2022.12.035.
- Pacheco I, Fernández de Mera IG, Feo Brito F, et al. Characterization of the anti-α-gal antibody profile in association with Guillain-Barré syndrome, implications for tick-related allergic reactions. Ticks Tick Borne Dis. 2021;12(3):101651. doi: 10.1016/j.ttbdis.2021.101651.
- González Polanco E, Borowitz S. Delayed hypersensitivity reaction to infliximab due to mammalian meat allergy. JPGN Rep. 2023;4(3):e322. doi: 10.1097/PG9.0000000000000322.
- Cuthbert RN, Darriet F, Chabrerie O, et al. Invasive hematophagous arthropods and associated diseases in a changing world. Parasit Vectors. 2023;16(1):291.
- Contreras M, Vaz-Rodrigues R, Mazuecos L, et al. Allergic reactions to tick saliva components in zebrafish model. Parasit Vectors. 2023;16:242.
- Mateos-Hernández L, Villar M, Moral A, et al. Tick-host conflict: immunoglobulin E antibodies to tick proteins in patients with anaphylaxis to tick bite. Oncotarget. 2017;8(13):20630–20644. doi: 10.18632/oncotarget.15243.
- de la Fuente J, Mazuecos L, Contreras M. Innovative approaches for the control of ticks and tick-borne diseases. Ticks Tick Borne Dis. 2023;14(6):102227. doi: 10.1016/j.ttbdis.2023.102227.
- Schulze TL, Eisen L, Russell K, et al. Community-based integrated tick management programs: cost and feasibility scenarios. J Med Entomol. 2023;60(5):1048–1060. doi: 10.1093/jme/tjad093.
- Platts-Mills TAE, Li RC, Keshavarz B, et al. Diagnosis and management of patients with the α-Gal syndrome. J Allergy Clin Immunol Pract. 2020;8(1):15–23.e1. doi: 10.1016/j.jaip.2019.09.017.
- Fischer J, Lupberger E, Hebsaker J, et al. Prevalence of type I sensitization to alpha-gal in Forest service employees and hunters. Allergy. 2017;72(10):1540–1547. doi: 10.1111/all.13156.
- Maxwell SP, Brooks C, Kim D, et al. Improving surveillance of human tick-borne disease risks: spatial analysis using multimodal databases. JMIR Public Health Surveill. 2023;9:e43790. doi: 10.2196/43790.
- Holcomb KM, Khalil N, Cozens DW, et al. Comparison of acarological risk metrics derived from active and passive surveillance and their concordance with tick-borne disease incidence. Ticks Tick Borne Dis. 2023;14(6):102243. doi: 10.1016/j.ttbdis.2023.102243.
- Urra JM, Ferreras-Colino E, Contreras M, et al. The antibody response to the glycan α-gal correlates with COVID-19 disease symptoms. J Med Virol. 2021;93(4):2065–2075. doi: 10.1002/jmv.26575.
- Vaz-Rodrigues R, Mazuecos L, Villar M, et al. Serum biomarkers for nutritional status as predictors in COVID-19 patients before and after vaccination. J Funct Foods. 2023;101:105412. doi: 10.1016/j.jff.2023.105412.
- Božan M, Vukičević Lazarević V, Marković I, et al. Alpha-gal syndrome-Food or drug allergy: a case report. Clin Case Rep. 2023;11(9):e7830. doi: 10.1002/ccr3.7830.
- Gilstad CW, Conry-Cantilena K, Zarpak R, et al. An outbreak of anaphylactic transfusion reactions to group B plasma and platelets and its possible relationship to alpha-gal syndrome. Transfusion. 2023;63(10):1997–2000. doi: 10.1111/trf.17521.
- Steinke JW, Pochan SL, James HR, et al. Altered metabolic profile in patients with IgE to galactose-alpha-1,3-galactose following in vivo food challenge. J Allergy Clin Immunol. 2016;138(5):1465–1467.e8. doi: 10.1016/j.jaci.2016.05.021.
- Breiman A, Ruvoën-Clouet N, Deleers M, et al. Low levels of natural anti-α-N-acetylgalactosamine (Tn) antibodies are associated with COVID-19. Front Microbiol. 2021;12:641460. doi: 10.3389/fmicb.2021.641460.
- Ridolo E, Pucciarini F, Barone A, et al. Dermatological manifestations during COVID-19 infection: a case series and discussion on the problem of differential diagnosis. Acta Biomed. 2021;92:e2021103.
- Kounis NG, Koniari I, de Gregorio C, et al. Allergic reactions to current available covid-19 vaccinations: pathophysiology, causality, and therapeutic considerations. Vaccines. 2021;9:221. doi: 10.3390/vaccines9030221.
- de la Fuente J, Gortázar C, Cabezas-Cruz A. Immunity to glycan α-gal and possibilities for the control of COVID-19. Immunotherapy. 2021;13(3):185–188. doi: 10.2217/imt-2020-0247.