Abstract
Platelet-rich plasma (PRP) has been widely used in clinical practice. The mechanism by which PRP promotes tissue repair lies in the release of multiple growth factors upon platelet activation, which accelerates the proliferation and differentiation of repair cells and the synthesis of extracellular matrix. In recent years, as extracellular vesicles (EVs) research has increased and intensified, it has been found that EVs also play an important role in tissue repair. This article provides a comprehensive review of the role of PRP and PRP-derived extracellular vesicles (PRP-EVs) in tissue repair. It discusses the biological characteristics, extraction, identification, activation, and preservation of PRP-EVs. It also reviews their applications in orthopedics and wound repair. The article highlights the importance of PRP-EVs in modern medicine and suggests that they could be a promising natural nanocarrier.
PLAIN LANGUAGE SUMMARY
The review discusses extracellular vesicles derived from platelet-rich plasma(PRP-EVs). PRP promotes repair of the body’s tissues and has been used in clinical practice for many years. Extracellular vesicles are granules released by cells that facilitate intercellular communication. Studies in recent years have revealed that PRP can also release extracellular vesicles that participate in the repair process of tissues. Here, we elaborate on PRP-EVs. Regarding PRP-EVs, current studies are limited to the animal level and have not yet been used in the clinical setting.
1. Introduction
Platelet-rich plasma (PRP) is a platelet concentrate gotten from autologous blood by centrifugation and is characterized by a higher proportion of platelets than basal levels [Citation1]. When platelets are activated, the scattered alpha‐granules within them can release a variety of growth factors, including platelet-derived growth factor (PDGF), transforming growth factor beta (TGF-β), vascular endothelial growth factor (VEGF), and basic fibroblast growth factor (BFGF), etc [Citation2]. These high concentrations of growth factors act synergistically to accelerate tissue cell proliferation and differentiation and extracellular matrix synthesis, thus promoting the repair of body tissues [Citation3]. PRP is used in various clinical areas, such as orthopaedics [Citation4,Citation5], oral and maxillofacial [Citation6], plastic surgery [Citation7], and dermatology [Citation8]. Although PRP is widely used in clinical practice because of its effectiveness, safety, and simplicity of production, the specific mechanism of tissue repair has not been fully elucidated, mainly because of the complex composition of PRP [Citation9]. In essence, there is a lack of unified standards for PRP preparation, classification and clinical application [Citation10,Citation11].
In recent years, it has been found that platelets can secrete a large number of extracellular vesicles (EVs) that participate in the regulation of tissue repair, in addition to releasing a large number of growth factors to mediate tissue repair [Citation12]. In healthy individuals, blood is one of the most abundant and accessible sources of EV, and platelet-derived EV accounts for the majority of blood EV (up to 70–90%) [Citation13]. More recently, more accurate assays have also shown that nearly 50% of blood EV comes from platelets or megakaryocytes.
The functions and potential mechanisms of extracellular vesicles originating from nucleated cells, such as cancer cells, immune cells, and stem cells, have been extensively studied [Citation14,Citation15]. Nowadays, more and more attention tends to be paid to the study of PRP-derived extracellular vesicles (PRP-EVs). This article provides a comprehensive review of the role of PRP and PRP-EVs in tissue repair. It discusses the biological characteristics, extraction, identification, activation, and preservation of PRP-EVs. It also reviews their applications in orthopedics and wound repair.
2. Historical background of PRP-EVs
EVs are secreted from almost all cell types (prokaryotes and eu-karyotes) [Citation16]. And they are widely distributed in various body fluids, such as urine [Citation17], blood [Citation18], breast milk [Citation19], saliva [Citation20], cerebrospinal fluid [Citation21]. amniotic fluid [Citation22], and semen [Citation23].The International Society for Extracellular Vesicles (ISEV) defines EV as particles released from cells that are separated by a lipid bilayer without a functional nucleus. EVs can be classified into different subtypes based on their biological origin, size and morphology, and mode of collection.EVs have mainly 2 different types of membrane vesicles: microvesicles (MVs,100 nm ∼ 1 mm) and exosomes(40 ∼ 100 nm), similar in size to the internal vesicles in polyvesicular bodies and alpha-granules [Citation24]. Exosomes are small membrane nanoparticles formed by luminal outgrowth of late endosomal membranes and secreted from the plasma membrane [Citation25]. MVs are effluxed directly from the plasma membrane by extracellular action. ISEV’s statement in the 2018 Minimum Information on Extracellular Vesicle Research (MISEV2018) recommends the use of ‘EVs’ as a generic term [Citation26]. EVs carry proteins, lipids, lipoproteins, messenger RNA(mRNA), micro RNA (miRNA), and possibly DNA [Citation27,Citation28]. This information transport facilitates non-contact intercellular communication, which regulates the behavior of distant cells [Citation29]. EVs can release functionally active mRNAs and microRNA in recipient cells and regulate gene expression in recipient cells by, for example, retranslating mRNAs [Citation30]. EVs can also stimulate specific signaling pathways through the release of proteins and thus have some effect on receptor cells [Citation31]. Therefore, EVs are clinically important for biological signaling and are a promising natural nanocarrier [Citation32]. With the continuous development of EVs research, the potential of EVs in modern medicine has attracted more and more attention [Citation33].
As early as 1967, Peter Wolf described the release of tiny lipid-rich particulate material from platelets that could be separated by ultracentrifugation, were distinguishable from intact platelets and showed coagulant properties, and were referred to as ‘platelet dust’ [Citation34]. Later, future studies again identified platelet-released particles that were observed in electron microscopy samples. This further characterization and description allows renaming ‘platelet dust’ to a more precise term: microparticles [Citation35]. In addition, particle release was observed in many other cell types, so that the particles were attached to what is now called EV [Citation26]. The aggregation of different historical names under a common label for EVs aims to lead to a more comprehensive and accurate report of EV activity and function, and thus to a consensus among different disciplines.
When PRP-EVs were first discovered, their primary function was thought to be the transport of procoagulant substances, performing most of the same functions as platelets [Citation36]. As interest in PRP-EVs continues to grow, a growing number of additional features are being demonstrated. They have also been shown to be involved in hemostasis, vascular integrity, immune regulation, and inflammatory regulation. In addition, platelet-derived EVs have been reported to be associated with pathological processes in certain diseases, such as rheumatoid arthritis [Citation37], cancer [Citation38], cardiovascular disease [Citation39] and neurogenerative diseases [Citation40,Citation41]. More importantly, a recent study found that PRP-EVs have great potential in the field of tissue repair and regeneration [Citation42].
In the treatment of chronic wounds, PRP has shown promising experimental and clinical results. Platelets are cytoplasmic fragments that form in the bone marrow and are approximately 2 μm in diameter. The regenerative potential of PRP is often thought to be attributable to supraphysiological concentrations of growth factors released by activated platelets, including PDGF, TGF-β1, and VEGF, etc. These growth factors have been demonstrated in numerous studies to play a crucial role in tissue regeneration and wound healing, including neovascularization [Citation43]. Despite these benefits, one drawback that limits the use of PRP in the clinical setting is the need for autologous platelets. In addition to these growth factors, stimulation of platelets causes them to secrete many EVs. In recent years, great attention has been paid to PRP-EVs, which have similar biological functions to platelets and are considered as potential effector molecules of platelets. In contrast to platelets, PRP-EVs have nanoscale size and local release capability to cross tissue barriers and accomplish cellular communication and material transport [Citation44]. One study conducted protein profiling of PRP and found extracellular vesicles as its major component, thus speculating that PRP-EVs may be the main pathway through which PRP exerts its biological functions [Citation45]. In addition, compared with platelets, PRP-EVs have low immunogenicity and are less likely to cause adverse reactions such as immune rejection and fever during treatment; they are also more stable, protect the contents from degradation enzymes or chemicals, and are easy to store and transport; they have no species differences and are safer for mass production and clinical treatment, making them an ideal alternative to platelets and PRP.
3. Extraction and identification of PRP-EVs
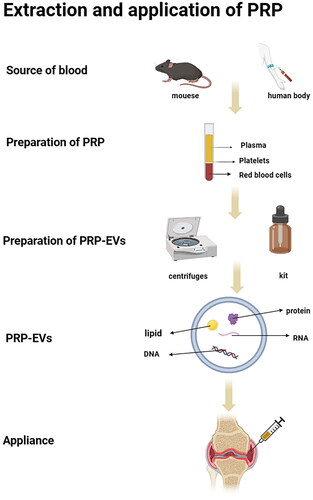
In the study of EVs, it is important to improve the purity of EVs and exclude the effects of cellular debris, impurity proteins and other nano-sized extracellular structures to ensure the accuracy of physiological properties and functional analysis. The extraction of PRP-EVs can be divided into 2 steps. Firstly, the preparation of PRP needs to be completed, and the method of PRP extraction is relatively mature. In 1977, Harke et al. first used a Haemonetics blood processor to isolate and prepare PRP from whole blood of 17 patients and applied it in clinical surgery, reducing intraoperative and postoperative bleeding in patients [Citation46]. Some studies have found that PRP contains a variety of growth factors that promote tissue repair. Since then, the application of PRP has expanded to many clinical areas [Citation47].With the continuous development of PRP preparation technology, most of the current studies opt for 2 times centrifugation [Citation9, Citation48]. In a study conducted by Perez et al. [Citation49], blood was added to a blood collection tube with anticoagulant and first centrifuged at 100–300 g for 5–10 min to separate platelets from erythrocytes and leukocytes in the plasma, then the separated platelet-containing plasma was transferred to a new centrifuge tube and centrifuged again at 400–700 g for 10–17 min to discard most of the supernatant plasma, and then platelet precipitate was resuspended in the residual plasma to obtain platelet-rich plasma. The second step needs to be completed with the extraction of extracellular vesicles. Extraction methods for PRP-EVs have not yet been reported in targeted studies, and can be referred to the extraction methods of other EVs, which often include ultra-high-speed centrifugation and kit methods. The ultracentrifugation method is widely used and is considered to be the ‘gold standard’ [Citation50]. The ultracentrifugation method, which separates particles with different densities by different centrifugal forces, is simple to operate, but has many shortcomings such as long running time, expensive equipment and large sample volume requirements, and the obtained EVs may be at risk of contamination with lipoproteins and protein impurities [Citation50,Citation51]. Centrifugation is mainly through two aspects of shear stress, one is centrifugation from the friction of the tube wall to generate shear, the other is the resuspension of the liquid and air mixed with the shear force generated in the shock. Antwi-Baffour et al. [Citation51] suggested that centrifugation generates shear stress that stimulates platelet activation or apoptosis and the release of extracellular vesicles. When platelets are activated, hybrid enzymes, calpain and agglutinin are activated by the release of calcium from the endothelial network, cleavage of long actin filaments and actin-capping proteins., inactivation of translocases, and damage to membrane asymmetry. The protein anchoring on the cytoskeleton is then disrupted, allowing membrane outgrowth, which leads to the formation and release of extracellular vesicles. Štukel et al. [Citation52]. In their experiments, EVs were isolated by repeated centrifugation (up to 17,570 × g) and washing, and counted by flow cytometry.The results showed that the concentration of EVs in the isolates was positively correlated with the work done by the shear force of the sample flowing through the syringe. They concluded that the shear stress generated by centrifugation can cause platelet activation and release EVs into the blood, and that the concentration of released vesicles is positively correlated with the effect of shear force. The method of EV isolation has been reported to affect the biological effects of PRP-EVs and their clinical translation. Specifically, lipoproteins are present together with isolated PRP-EVs (e.g. traditional ultracentrifugation), thus reducing the purity of PRP-EVs because lipoproteins may increase pro-inflammatory effects and thus lead to adverse effects [Citation53]. Ultracentrifugation is the traditional method for extracting Evs, while the kit method can provide a simpler, faster, and more efficient approach to Evs isolation. Cervenakova et al. [Citation54] used ExoQuickTM kit to extract EVs more conveniently, and incubated overnight at 4 °C and centrifuged at 1500 × g for 30 min. The tangential flow filtration (TFF) method, which has become more popular in recent years, is a method for separating EVs based on their diameters and is suitable for obtaining EVs purification products on a large scale, which has a greater potential for clinical application [Citation55].
Regarding the identification of extracellular vesicles, many techniques exist to characterize as well as analyze nanoparticles and nanovacuoles [Citation56]. These include: western blotting, flow cytometry, enzyme-linked immunosorbent assay, dynamic light scattering, transmission electron microscopy methods, and nanoparticle tracking analysis techniques. Western blotting and enzyme-linked immunosorbent assay techniques are used for the analysis of proteins in extracellular vesicles. Flow cytometry techniques can analyze cell surface antigen markers carried by extracellular vesicles. Transmission electron microscopy technique allows analysis of the molecular diameter and morphological structure of extracellular vesicles. The nanoparticle tracking analysis technique allows real-time molecular waveform and concentration measurements of specific extracellular vesicles in the 50–1000 nm diameter range in low concentration extracellular vesicle suspensions. In the study of Xu et al. [Citation57], the obtained PRP-EVs ranged in size from 40 ∼ 100 nm, and most of these particles exhibited cup-shaped or spherical morphological features consistent with EVs. Western blotting analysis showed the presence of exosome markers CD9 and TSG101 in these samples, thus confirming the successful enrichment of exosomes in PRP samples.
4. Effect of different activators on PRP-EVs
Although PRP-EVs have been studied extensively in recent years, few studies have evaluated the effects of different activation modalities on EVs. The reported approach allows the use of agonists, such as thrombin, Ca2+, collagen, epinephrine, ADP and arachidonic acid, alone or in concert, which interact with specific receptors on the platelet membrane and induce their degranulation and EVs release [Citation58].
Platelets can generate a large number of Evs upon activation, and different activation conditions induce different molecular pathways of platelet activation and differences in the biological characteristics of their released EVs. Platelet activation is ultimately dependent on an elevated concentration of intracytoplasmic calcium ions. Therefore, exogenous calcium ion carriers can rapidly elevate the concentration of free intracytoplasmic calcium ions to activate platelets [Citation59]. The results of Miquel et al. [Citation60] showed that Ca2+ was shown to alter the cytokine expression profile of PRP-EVs, with significantly different cytokine expression profiles compared to EVs isolated from non-activated platelets. Western blot analysis showed that Ca2+-activated platelets released more EVs than unactivated platelets. Rui et al. [Citation61] showed that a mixture of calcium gluconate and thrombin was superior to a single activator and could activate PRP to release high concentrations and high quality EVs. In the study by Laffont et al. [Citation62], PRP-EVs may act as intercellular carriers of functional Ago2-microRNA complexes and may act as heterotypic regulators of gene expression in endothelial cells and other receptor cells of the circulatory system. The size and shape of the EVs formed by different activation or release methods and the composition they contain are different. For example, EVs released from platelets activated by thrombin contain large amounts of microRNA-223, whereas resting state platelets release EVs with less microRNA-223.microRNA-223 may be associated with cell proliferation. Edward et al. [Citation63] showed that after platelet activation by thrombin and collagen, the amount of CXCL4, CXCL7 and HMGB1 in EVs increased. In addition to chemical stimulation, platelet-derived EVs can be produced by physical methods, such as repeated freezing and thawing [Citation64]. In addition, the particle size of platelet-derived EVs has been analyzed under different activation conditions. Regardless of activation conditions, platelet-derived EVs were mainly distributed in the range of 100–250 nm, and more than 90% of the vesicles were smaller than 500 nm [Citation58]. Although there are differences in the degree of activation of platelets by different activation pathways, there is currently no absolute advantage or disadvantage to the activation conditions of PRP-Evs.
The differences in the biological characteristics of PRP-EVs under different activation conditions, subsequently affect their biological functions and clinical therapeutic effects. A standardized preparation process is important for the research and application of PRP-EVs. However, there is no unified standard for the preparation of PRP-EVs, especially the activation conditions of PRP have not yet reached a unified consensus. For future clinical applications of PRP-Evs, targeted studies based on application pathways are needed.
5. Storage of PRP-EVs
Storage conditions of PRP-EVs are also another important issue for their use, and most of the relevant articles so far do not mention their specific storage conditions. Here we elaborate on the storage conditions of EVs. Keeping the biological activity of EVs stable during storage is a crucial step, and different preservation conditions may have a large impact on the function of EVs, so it is especially important to find a specific storage condition. In the study of Gao et al. [Citation65], platelets were shaken on a flatbed agitator at 22 ± 2 °C for 5 days, and the results showed that the microvesicle content was three times higher at 5 days of storage than at 1 day of storage. EVsRNA in plasma remains stable for 2 weeks at 4 °C, can be stored for at least 2 months at −80 °C, and can be stored for ≥5 years at −20 °C. There is only a small change in individual mi RNA levels, but repeated freeze-thawing decreases the RNA content [Citation66]. What one can now determine is that −80 °C preservation conditions keep EVs intact and that −80 °C preserves the proteins in EVs better than −20 °C [Citation67]. EVs are protected by phospholipid bilayers and contain biologically active molecules such as RNA, DNA, proteins and lipids, so their preservation conditions have been a major concern for researchers. In conclusion, the optimal preservation conditions for EVs are still being explored, and there is no absolutely uniform preservation method. However, the overall preservation result trend is more or less the same, with 4 °C suitable for short-term storage and −80 °C/-20 °C more suitable for long-term storage.
6. Application of PRP-EVs
The biological function of EVs has been of great interest in recent years (). The currently known biological functions of EVs include regulation of gene transcription and translation in recipient cells, regulation of cell metabolism and proliferation, promotion of angiogenesis and wound healing, involvement in central and peripheral immune regulation, involvement in apoptosis and differentiation, and a role in cell migration and metastatic disease. Cell-free therapies are currently emerging as clinical treatments, making EVs a hot spot for research on cell-free therapies [Citation68]. Here we focus on an overview of its application in orthopedics and trauma repair.
6.1. PRP-EVs and orthopedics
The results of the big data-based analysis suggest that the main research and application areas for PRP-EVs are in orthopedics, especially in cartilage regeneration and osteoarthritis [Citation69](). There is evidence that PRP-EVs are enriched with molecular mediators responsible for the healing action of PRP. In 2014, Torreggiani et al. [Citation70] isolated EVs from PRP and demonstrated their potential beneficial effects on proliferation, migration and osteogenic differentiation of bone mesenchymal stem cells (BMSC). This is the first report to describe the role of platelet-derived EVs in tissue regeneration. In the study by Torreggiani et al. the growth factors bFGF, VEGF, PDGF-BB, and TGF-β1 in PRP-EVs and PRP were detected and compared by ELISA, and it was found that the same volume of PRP-EVs showed clearly higher concentrations of growth factors compared to PRP than the PRP group. The concentrations of bFGF, PDGF-BB and TGF-β1 were 3. 3 times, 2.7 times and 35.5 times higher than those of PRP, respectively. This suggests that the majority of the most important acting component of PRP, growth factor, is stored in PRP-EVs, which are important new effectors of PRP activity and may provide a favorable nano-delivery system for improved bone repair. However, the study did not elaborate on the in vivo role of PRP-EVs in tissue regeneration and their potential molecular mechanisms.
Figure 1. Extraction, identification and application of PRP-derived extracellular vesicles (PRP-EVs).
In 2017, TAO et al. [Citation71] found that PRP-EVs promote maintenance and regeneration of bone tissue and promote cell proliferation. In vitro experiments showed that under endoplasmic reticulum stress, PRP-EVs promote Bcl-2 expression through the Akt/Bad/Bcl-2 signaling pathway. Also, PRP-EVs increased the expression of osteogenic-related proteins such as runt-related transcription factor 2 (Runx 2), type I collagen and β-catenin, and the percentage of apoptotic cells was less in bone marrow mesenchymal stem cells and osteogenic precursor cells treated with PRP-EVs. PRP-EVs were found to prevent apoptosis in a rat model of glucocorticoid-induced femoral head necrosis.
Liu et al. [Citation72] demonstrated that PRP-EVs promote the proliferation of rabbit chondrocytes. The addition of interleukin 1β ((IL-1β)) to chondrocytes in an in vitro experiment simulating an inflammatory environment revealed that PRP-EVs possess similar chondroprotective effects. This study also found that PRP-EVs promoted chondrocyte proliferation and migration and inhibited chondrocyte apoptosis via the wnt/β-catenin signaling pathway, with a more pronounced effect at high concentrations (50 μg/mL) of PRP-EVs. Also, PRP-EVs can reduce the expression level of tumor necrosis factor-α (TNF-α), a pro-inflammatory mediator of osteoarthritis. In addition, PRP-EVs reversed the osteoarthritis-induced decrease in type II collagen expression and the upregulation of Wnt5a protein. Type II collagen is the major fibrous component of the hyaline cartilage matrix, and Wnt5a protein induces an increase in matrix metalloproteinase (MMP) and inhibits the expression of type II collagen [Citation73].
Otahal et al. [Citation74] compared the regulation of chondrocytes by pure PRP-EVs versus hyperacute phase serum-derived extracellular vesicles. The results suggest that both can promote extracellular matrix anabolism. However, PRP-EVs can significantly reduce the expression levels of inflammatory factors such as IL-1. This indicates that PRP-EVs have an inhibitory effect on inflammatory factors.
PRP-EVs have also been associated with muscle regeneration. One investigator found that in a rat model, EVs from PRP induced an increase in tissue regeneration markers, such as centrally located nuclear fibers. Its ability to promote recovery from muscle strain injuries may be due to factors that can modulate inflammation, fibrosis and muscle production [Citation75].
However, PRP-EVs have the disadvantage of low retention rate and short treatment effect. Thermosensitive gel is an ideal injection vehicle for delivering drugs [Citation76]. In a recent study in the Journal of Nanobiotechnology, Zhang et al. [Citation42] demonstrated through in vivo experiments that the incorporation of PRP-EVs into thermosensitive hydrogels increased the local retention of EVs in tissues. In a mouse model of lateral ankle instability after severance of the anterior talofibular ligament (ATFL)/heel-fibular ligament (CFL), the incorporation of PRP-EVs in thermosensitive hydrogels increased the retention of EVs in the ankle joint compared with PRP-EVs alone, resulting in improved outcomes in subtrochanteric osteoarthritis (STOA). The mechanism may be the induction of endogenous bone marrow mesenchymal stem cells (mBMSCs) to the site of injury through homing to induce cartilage differentiation and attenuate inflammation-induced cartilage damage.
There is no doubt that PRP-EVs offer a new treatment pathway for bone injuries. Although there are no controlled clinical studies on the application of PRP-EVs for bone repair, scholars at the Mayo Clinic in the United States have successfully prepared a lyophilized powder of PRP-EVs that retains a good vesicular structure after re-solubilization and has a restorative effect on wound healing and tendons [Citation77]. Therefore, PRP-EVs are expected to be a mass-produced platelet derivative product that can be standardized for production and allogeneic use and has a broad clinical prospect.
In a study by Graca et al. [Citation78] using a three-dimensional bioengineered in vitro tendon model, platelet-derived Evs were found to promote tendon-derived differentiation of stem cells on bioengineered living fibers. The study by Lu et al. [Citation79] confirmed the feasibility of platelet-derived Ev-parallel stripe bionic scaffolds loaded with recombinant Yap1 as a de novo tissue-engineered material for in situ repair of tendon defects. Therefore, platelet-derived EV is a promising biochemical tool for tissue engineering and regenerative medicine applications and deserves further exploration. Its application in tissue engineering can be investigated in the future.
6.2. PRP-EVs and wound repair
Chronic wounds are difficult to heal because of the presence of infected or necrotic tissues, poor local microcirculation, low quantity and activity of growth factors due to the destruction of growth factors by protein hydrolases, and uncoupling of repair cell membrane receptors from growth factors. Most of the growth factors of PRP are stored in PRP-EVs. In pathological situations such as trauma, the number of platelet-derived EVs in the body’s blood rises significantly. Since EVs can initiate processes that mediate inflammation and vascular regeneration, this suggests that platelet-derived EVs may be involved in the repair process of post-traumatic tissues of the organism [Citation80,Citation81].
Diabetes mellitus(DM) is a common metabolic disease. Diabetic wounds are a common complication in diabetic patients. EVs have become a hot topic of research related to diabetic wound healing because of their safety, ease of use, and batch preparation. It may play an important role in the development and treatment of diabetes and its complications [Citation82]. Guo et al. [Citation83] established full skin trauma on the back of diabetic rats. They were randomly divided into control group, sodium alginate hydrogel group, PRP gel group and PRP-EVs gel group and given the appropriate treatment. The most significant effect was found in the PRP-EVs group. PRP-EVs promoted the proliferation and migration of human microvascular endothelial cell line HMEC-1 cells and fibroblasts more significantly than PRP.PRP-EVs have a stronger fibrillogenic and angiogenic capacity, while the repair effect lasts longer. The main mechanism may be that EVs enhance PRP-induced angiogenesis by activating the extracellular regulatory protein kinase (Erk), Akt signaling pathway.
Once produced, PRP-EVs can be injected in liquid form or mixed with chitosan solution and applied as a hydrogel sponge dressing. The use of PRP-EVs-loaded chitosan/silk gel sponges for the treatment of diabetic mouse skin wounds was also confirmed in a study by Xu et al. [Citation84]. PRP-EVs alone or in combination with homogeneous polysaccharide (ZWP) significantly accelerated trabecular collagen synthesis and deposition, re-epithelialization, and skin angiogenesis in diabetic rats. This helps to reduce the size of the wound, thus speeding up the healing of diabetic wounds. Otherwise, no side effects were observed in all treatment groups during the healing process. In terms of angiogenesis, PRP-EVs/ZWP combined therapy kept stronger efficiency than PRP-EVs or ZWP single administration. These findings provide a mechanistic basis for the use of PRP-EVs as a potential therapeutic strategy to accelerate diabetic skin repair.
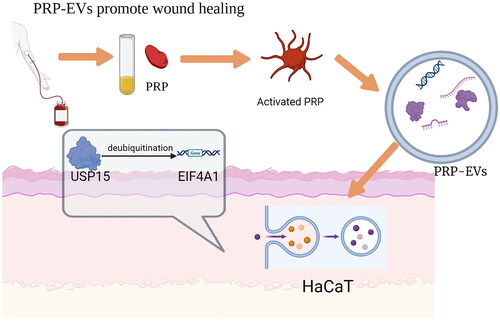
A study by Schlegelmilch et al. [Citation85] concluded that the Hippo pathway and its downstream effectors, namely the Yes-associated protein (YAP) and PDZ-binding motif (TAZ), promote tissue and cell regeneration during animal development and regeneration. By establishing an in vivo skin wound model in mice, Xu et al. [Citation57] found that mice treated with PRP-EVs healed faster and had smaller scars compared to mice treated with PRP alone. PRP-EVs treatment was found to significantly increase the proliferation and migration of immortalized human keratin-forming cells (HaCaT cells) by experiment. USP15 is an important driver of PRP-EVs-mediated wound healing. At the mechanistic level, USP15 enhances the functional properties of HaCaT cells by promoting EIF4A1 deubiquitination. The investigators assessed the levels of the protein in PRP and PRP-EVs samples by Western blotting and found that it was present at higher levels in PRP-EVs. qPCR analysis also showed that USP15 expression was increased at the mRNA level in PRP-EVs ().
Figure 2. USP15 is one of the major mRNAs in PRP-EVs that can be taken up by HaCaT and subsequently deubiquitinates EIF4A1, which then accelerates re-epithelialisation and promotes wound healing.
To sum up, PRP-derived EVs have a greater angiogenic capacity to accelerate wound healing and reduce scar formation in wound healing. Notably, the combination of PRP-EVs and ZWP has a stronger angiogenic capacity, providing a new way to promote wound repair.
7. Conclusions and future prospects
This article reviews the historical background of PRP-EVs, their biological properties, and their value for orthopedic and trauma repair applications. Regarding the research on PRP-EVs, the current tendency is to study in the field of bone regeneration, in the field related to cartilage or osteoarthritis, and in the repair of diabetic traumas. However, the article also acknowledges that there are still many areas that need further research, such as the effects of different activation modalities on EVs, the impact of centrifugation on PRP-EVs, and the optimal storage conditions for EVs.
EVs are not immunogenic and there are no species differences, and signals can be transmitted across species. Therefore, it is expected to be obtained in large quantities from other species, mass produced and used clinically as cell-free therapies [Citation64]. Research on the application of PRP-EVs is generally still in its infancy. There are still many barriers to break between basic research and clinical applications in the future. Its biosafety, reproducibility, and biological function are not fully understood. From the current evidence-based medical evidence, the literature on the application of PRP-EVs is mainly based on a few animal studies. To date there have been few clinical trials of EVs treatment. More importantly, few adverse effects have been reported regarding its treatment. To overcome the hurdles from laboratory to clinical use, studies including preclinical and clinical trials need to be refined to explore the therapeutic effects and mechanisms of EVs and to find viable clinical practice options. It is a treatment with a bright future once we achieve the ability to control its content, but there is a long way to go.
Due to the limited efficacy and unintended side effects of traditional drug delivery systems, nanoparticle-based drug delivery systems have become part of the next generation of drug delivery technologies. Recent studies have shown that PRP-Evs has the potential to be a novel delivery vehicle for inflammation targeting [Citation86]. The further development of utilizing PRP-Evs as drug delivery systems is full of opportunities and challenges. More attention can be put on studying the role of PRP-Evs in drug delivery in the future.
Authors contributions
Writing—original draft, Y.H.; writing—review and editing, X.W., L.Z., and X.F. All authors have read and agreed to the published version of the manuscript.
Disclosure statement
The authors declare no conflict of interest.
Data availability statement
All figures and tables are original and are not taken from other publications. Data sharing is not applicable to this article, as no new data were created or analysed in this study.
Table 1. Some preclinical applications of PRP-EVs in regenerative medicine.
Additional information
Funding
References
- Alves R, Grimalt R. A review of platelet-rich plasma: history, biology, mechanism of action, and classification. Skin Appendage Disord. 2018;4(1):1–11. doi: 10.1159/000477353.
- Qiao J, An N, Ouyang X. Quantification of growth factors in different platelet concentrates. Platelets. 2017;28(8):774–778. doi: 10.1080/09537104.2016.1267338.
- Amable PR, Carias RB, Teixeira MV, et al. Platelet-rich plasma preparation for regenerative medicine: optimization and quantification of cytokines and growth factors. Stem Cell Res Ther. 2013;4(3):67. doi: 10.1186/scrt218.
- Katz JN. Platelet-Rich plasma for osteoarthritis and achilles tendinitis. JAMA. 2021;326(20):2012–2014. doi: 10.1001/jama.2021.19540.
- Belk JW, Kraeutler MJ, Houck DA, et al. Platelet-Rich plasma versus hyaluronic acid for knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Am J Sports Med. 2021;49(1):249–260. doi: 10.1177/0363546520909397.
- Anitua E, Fernandez-de-Retana S, Alkhraisat MH. Platelet rich plasma in oral and maxillofacial surgery from the perspective of composition. Platelets. 2021;32(2):174–182. doi: 10.1080/09537104.2020.1856361.
- Kang JS, Zheng Z, Choi MJ, et al. The effect of CD34+ cell-containing autologous platelet-rich plasma injection on pattern hair loss: a preliminary study. J Eur Acad Dermatol Venereol. 2014;28(1):72–79. doi: 10.1111/jdv.12062.
- Alam M, Hughart R, Champlain A, et al. Effect of platelet-rich plasma injection for rejuvenation of photoaged facial skin: a randomized clinical trial. JAMA Dermatol. 2018;154(12):1447–1452. doi: 10.1001/jamadermatol.2018.3977.
- Everts P, Onishi K, Jayaram P, et al. Platelet-Rich plasma: new performance understandings and therapeutic considerations in 2020. Int J Mol Sci. 2020;21(20):7794. doi: 10.3390/ijms21207794.
- Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009;27(3):158–167. doi: 10.1016/j.tibtech.2008.11.009.
- Kawase T. A strategic and worldwide cooperative challenge required for the next generation of platelet concentrates. Int J Mol Sci. 2022;23(7):3437. doi: 10.3390/ijms23073437.
- Wu J, Piao Y, Liu Q, et al. Platelet-rich plasma-derived extracellular vesicles: a superior alternative in regenerative medicine? Cell Prolif. 2021;54:e13123.
- Żmigrodzka M, Guzera M, Miśkiewicz A, et al. The biology of extracellular vesicles with focus on platelet microparticles and their role in cancer development and progression. Tumour Biol. 2016;37(11):14391–14401. doi: 10.1007/s13277-016-5358-6.
- Zhang B, Tian X, Hao J, et al. Mesenchymal stem cell-derived extracellular vesicles in tissue regeneration. Cell Transplant. 2020;29:963689720908500. doi: 10.1177/0963689720908500.
- Veerman RE, Güçlüler Akpinar G, Eldh M, et al. Immune cell-derived extracellular Vesicles - Functions and therapeutic applications. Trends Mol Med. 2019;25(5):382–394. doi: 10.1016/j.molmed.2019.02.003.
- Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–383. doi: 10.1083/jcb.201211138.
- Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci USA. 2004;101(36):13368–13373. doi: 10.1073/pnas.0403453101.
- Dutta S, Hornung S, Kruayatidee A, et al. α-Synuclein in blood exosomes immunoprecipitated using neuronal and oligodendroglial markers distinguishes parkinson’s disease from multiple system atrophy. Acta Neuropathol. 2021;142(3):511–513. doi: 10.1007/s00401-021-02332-0.
- Yan C, Chen J, Wang C, et al. Milk exosomes-mediated miR-31-5p delivery accelerates diabetic wound healing through promoting angiogenesis. Drug Deliv. 2022;29(1):214–228. doi: 10.1080/10717544.2021.2023699.
- Li K, Lin Y, Luo Y, et al. A signature of saliva-derived exosomal small RNAs as predicting biomarker for esophageal carcinoma: a multicenter prospective study. Mol Cancer. 2022;21(1):21. doi: 10.1186/s12943-022-01499-8.
- Zhang Y, Kim MS, Jia B, et al. Hypothalamic stem cells control ageing speed partly through exosomal miRNAs. Nature. 2017;548(7665):52–57. doi: 10.1038/nature23282.
- Gebara N, Scheel J, Skovronova R, et al. Single extracellular vesicle analysis in human amniotic fluid shows evidence of phenotype alterations in preeclampsia. J Extracell Vesicles. 2022;11(5):e12217. doi: 10.1002/jev2.12217.
- Machtinger R, Laurent LC, Baccarelli AA. Extracellular vesicles: roles in gamete maturation, fertilization and embryo implantation. Hum Reprod Update. 2016;22(2):182–193. doi: 10.1093/humupd/dmv055.
- Armstrong JPK, Stevens MM. Strategic design of extracellular vesicle drug delivery systems. Adv Drug Deliv Rev. 2018;130:12–16. doi: 10.1016/j.addr.2018.06.017.
- Cocucci E, Meldolesi J. Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol. 2015;25(6):364–372. doi: 10.1016/j.tcb.2015.01.004.
- Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. doi: 10.1080/20013078.2018.1535750.
- Valadi H, Ekström K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596.
- Cai J, Han Y, Ren H, et al. Extracellular vesicle-mediated transfer of donor genomic DNA to recipient cells is a novel mechanism for genetic influence between cells. J Mol Cell Biol. 2013;5(4):227–238. doi: 10.1093/jmcb/mjt011.
- Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478) :eaau6977.doi: 10.1126/science.aau6977.
- Wu Y, Wu M, Zhang Y, et al. Lyophilization is suitable for storage and shipment of fresh tissue samples without altering RNA and protein levels stored at room temperature. Amino Acids. 2012;43(3):1383–1388. doi: 10.1007/s00726-011-1212-8.
- Mathieu M, Martin-Jaular L, Lavieu G, et al. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21(1):9–17. doi: 10.1038/s41556-018-0250-9.
- Luan X, Sansanaphongpricha K, Myers I, et al. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol Sin. 2017;38(6):754–763. doi: 10.1038/aps.2017.12.
- Xiong Y, Chen L, Yu T, et al. Inhibition of circulating exosomal microRNA-15a-3p accelerates diabetic wound repair. Aging (Albany NY). 2020;12(10):8968–8986. doi: 10.18632/aging.103143.
- Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol. 1967;13(3):269–288. doi: 10.1111/j.1365-2141.1967.tb08741.x.
- Crawford N. The presence of contractile proteins in platelet microparticles isolated from human and animal platelet-free plasma. Br J Haematol. 1971;21(1):53–69. doi: 10.1111/j.1365-2141.1971.tb03416.x.
- Berckmans RJ, Nieuwland R, Böing AN, et al. Cell-derived microparticles circulate in healthy humans and support low grade thrombin generation. Thromb Haemost. 2001;85(04):639–649. doi: 10.1055/s-0037-1615646.
- Zhang M, Hu W, Cai C, et al. Advanced application of stimuli-responsive drug delivery system for inflammatory arthritis treatment. Materials Today Bio. 2022;14:100223. doi: 10.1016/j.mtbio.2022.100223.
- Haemmerle M, Stone RL, Menter DG, et al. The platelet lifeline to cancer: challenges and opportunities. Cancer Cell. 2018;33(6):965–983. doi: 10.1016/j.ccell.2018.03.002.
- Gardin C, Ferroni L, Leo S, et al. Platelet-Derived exosomes in atherosclerosis. Int J Mol Sci. 2022;23(20):12546. doi: 10.3390/ijms232012546.
- Nyam-Erdene A, Nebie O, Delila L, et al. Characterization and chromatographic isolation of platelet extracellular vesicles from human platelet lysates for applications in neuroregenerative medicine. ACS Biomater Sci Eng. 2021;7(12):5823–5835. doi: 10.1021/acsbiomaterials.1c01226.
- Delila L, Nebie O, Le NTN, et al. Neuroprotective activity of a virus-safe nanofiltered human platelet lysate depleted of extracellular vesicles in parkinson’s disease and traumatic brain injury models. Bioeng Transl Med. 2023;8(1):e10360. doi: 10.1002/btm2.10360.
- Zhang Y, Wang X, Chen J, et al. Exosomes derived from platelet-rich plasma administration in site mediate cartilage protection in subtalar osteoarthritis. J Nanobiotechnology. 2022;20(1):56. doi: 10.1186/s12951-022-01245-8.
- Qian Z, Wang H, Bai Y, et al. Improving chronic diabetic wound healing through an injectable and Self-Healing hydrogel with platelet-rich plasma release. ACS Appl Mater Interfaces. 2020;12(50):55659–55674. doi: 10.1021/acsami.0c17142.
- Antich-Rosselló M, Forteza-Genestra MA, Monjo M, et al. Platelet-Derived extracellular vesicles for regenerative medicine. Int J Mol Sci. 2021;22(16):8580. doi: 10.3390/ijms22168580.
- Tang Q, Lim T, Shen LY, et al. Well-dispersed platelet lysate entrapped nanoparticles incorporate with injectable PDLLA-PEG-PDLLA triblock for preferable cartilage engineering application. Biomaterials. 2021;268:120605. doi: 10.1016/j.biomaterials.2020.120605.
- Harke H, Tanger D, Fürst-Denzer S[, et al. Effect of a preoperative separation of platelets on the postoperative blood loss subsequent to extracorporeal circulation in open heart surgery (author’s transl.). Anaesthesist. 1977;26(2):64–71.
- Chen P, Huang L, Ma Y, et al. Intra-articular platelet-rich plasma injection for knee osteoarthritis: a summary of meta-analyses. J Orthop Surg Res. 2019;14(1):385. doi: 10.1186/s13018-019-1363-y.
- Perez AG, Lana JF, Rodrigues AA, et al. Relevant aspects of centrifugation step in the preparation of platelet-rich plasma. ISRN Hematol. 2014;2014:176060–176068. doi: 10.1155/2014/176060.
- Dashore S, Chouhan K, Nanda S, et al. Preparation of platelet-rich plasma: national IADVL PRP taskforce recommendations. Indian Dermatol Online J. 2021;12(Suppl 1):S12–s23. doi: 10.4103/idoj.idoj_269_21.
- Li P, Kaslan M, Lee SH, et al. Progress in exosome isolation techniques. Theranostics. 2017;7(3):789–804. doi: 10.7150/thno.18133.
- Antwi-Baffour S, Adjei J, Aryeh C, et al. Understanding the biosynthesis of platelets-derived extracellular vesicles. Immun Inflamm Dis. 2015;3(3):133–140. doi: 10.1002/iid3.66.
- Štukelj R, Schara K, Bedina-Zavec A, et al. Effect of shear stress in the flow through the sampling needle on concentration of nanovesicles isolated from blood. Eur J Pharm Sci. 2017;98:17–29. doi: 10.1016/j.ejps.2016.10.007.
- Fujioka Y, Ishikawa Y. Remnant lipoproteins as strong key particles to atherogenesis. J Atheroscler Thromb. 2009;16(3):145–154. doi: 10.5551/jat.e598.
- Cervenakova L, Saá P, Yakovleva O, et al. Are prions transported by plasma exosomes? Transfus Apher Sci. 2016;55(1):70–83. doi: 10.1016/j.transci.2016.07.013.
- Allan D, Tieu A, Lalu M, et al. Mesenchymal stromal cell-derived extracellular vesicles for regenerative therapy and immune modulation: progress and challenges toward clinical application. Stem Cells Transl Med. 2020;9(1):39–46. doi: 10.1002/sctm.19-0114.
- Royo F, Théry C, Falcón-Pérez JM, et al. Methods for separation and characterization of extracellular vesicles: results of a worldwide survey performed by the ISEV rigor and standardization subcommittee. Cells. 2020;9(9):1955. doi: 10.3390/cells9091955.
- Xu Y, Lin Z, He L, et al. Platelet-Rich plasma-derived exosomal USP15 promotes cutaneous wound healing via deubiquitinating EIF4A1. Oxid Med Cell Longev. 2021;2021:9674809–9674814. doi: 10.1155/2021/9674809.
- Aatonen MT, Ohman T, Nyman TA, et al. Isolation and characterization of platelet-derived extracellular vesicles. J Extracell Vesicles. 2014;3(1):24692. doi: 10.3402/jev.v3.24692.
- van der Meijden PEJ, Heemskerk JWM. Platelet biology and functions: new concepts and clinical perspectives. Nat Rev Cardiol. 2019;16(3):166–179. doi: 10.1038/s41569-018-0110-0.
- Saumell-Esnaola M, Delgado D, García Del Caño G, et al. Isolation of platelet-derived exosomes from human platelet-rich plasma: biochemical and morphological characterization. Int J Mol Sci. 2022;23(5):2861. doi: 10.3390/ijms23052861.
- Rui S, Yuan Y, Du C, et al. Comparison and investigation of exosomes derived from platelet-rich plasma activated by different agonists. Cell Transplant. 2021;30:9636897211017833. doi: 10.1177/09636897211017833.
- Laffont B, Corduan A, Plé H, et al. Activated platelets can deliver mRNA regulatory Ago2•microRNA complexes to endothelial cells via microparticles. Blood. 2013;122(2):253–261. doi: 10.1182/blood-2013-03-492801.
- Goetzl EJ, Goetzl L, Karliner JS, et al. Human plasma platelet-derived exosomes: effects of aspirin. Faseb J. 2016;30(5):2058–2063. doi: 10.1096/fj.201500150R.
- Spakova T, Janockova J, Rosocha J. Characterization and therapeutic use of extracellular vesicles derived from platelets. Int J Mol Sci. 2021;22(18):9701. doi: 10.3390/ijms22189701.
- Gao M, Zhang B, Zhang Y, et al. The effects of apheresis, storage time, and leukofiltration on microparticle formation in apheresis platelet products. Transfusion. 2018;58(10):2388–2394. doi: 10.1111/trf.14890.
- Ge Q, Zhou Y, Lu J, et al. miRNA in plasma exosome is stable under different storage conditions. Molecules. 2014;19(2):1568–1575. doi: 10.3390/molecules19021568.
- Zhou H, Yuen PS, Pisitkun T, et al. Collection, storage, preservation, and normalization of human urinary exosomes for biomarker discovery. Kidney Int. 2006;69(8):1471–1476. doi: 10.1038/sj.ki.5000273.
- Johnson J, Wu YW, Blyth C, et al. Prospective therapeutic applications of platelet extracellular vesicles. Trends Biotechnol. 2021;39(6):598–612. doi: 10.1016/j.tibtech.2020.10.004.
- Yin B, Ni J, Witherel CE, et al. Harnessing tissue-derived extracellular vesicles for osteoarthritis theranostics. Theranostics. 2022;12(1):207–231. doi: 10.7150/thno.62708.
- Torreggiani E, Perut F, Roncuzzi L, et al. Exosomes: novel effectors of human platelet lysate activity. Eur Cell Mater. 2014;28:137–151; discussion 151. doi: 10.22203/ecm.v028a11.
- Tao SC, Yuan T, Rui BY, et al. Exosomes derived from human platelet-rich plasma prevent apoptosis induced by glucocorticoid-associated endoplasmic reticulum stress in rat osteonecrosis of the femoral head via the akt/bad/bcl-2 signal pathway. Theranostics. 2017;7(3):733–750. doi: 10.7150/thno.17450.
- Liu X, Wang L, Ma C, et al. Exosomes derived from platelet-rich plasma present a novel potential in alleviating knee osteoarthritis by promoting proliferation and inhibiting apoptosis of chondrocyte via wnt/β-catenin signaling pathway. J Orthop Surg Res. 2019;14(1):470. doi: 10.1186/s13018-019-1529-7.
- Wang S, Ding P, Xia X, et al. Bugan rongjin decoction alleviates inflammation and oxidative stress to treat the postmenopausal knee osteoarthritis through wnt signaling pathway. Biomed Eng Online. 2021;20(1):103. doi: 10.1186/s12938-021-00939-8.
- Otahal A, Kramer K, Kuten-Pella O, et al. Characterization and chondroprotective effects of extracellular vesicles from plasma- and Serum-Based autologous blood-derived products for osteoarthritis therapy. Front Bioeng Biotechnol. 2020;8:584050. doi: 10.3389/fbioe.2020.584050.
- Iyer SR, Scheiber AL, Yarowsky P, 3rd, et al. Exosomes isolated from platelet-rich plasma and mesenchymal stem cells promote recovery of function after muscle injury. Am J Sports Med. 2020;48(9):2277–2286. doi: 10.1177/0363546520926462.
- Liu D, Jiang T, Cai W, et al. An in situ gelling drug delivery system for improved recovery after spinal cord injury. Adv Healthc Mater. 2016;5(12):1513–1521. doi: 10.1002/adhm.201600055.
- Shi A, Li J, Qiu X, et al. TGF-β loaded exosome enhances ischemic wound healing in vitro and in vivo. Theranostics. 2021;11(13):6616–6631. doi: 10.7150/thno.57701.
- Graça AL, Domingues RMA, Gomez-Florit M, et al. Platelet-Derived extracellular vesicles promote tenogenic differentiation of stem cells on bioengineered living fibers. Int J Mol Sci. 2023;24(4):3516. doi: 10.3390/ijms24043516.
- Lu J, Yang X, He C, et al. Rejuvenation of tendon stem/progenitor cells for functional tendon regeneration through platelet-derived exosomes loaded with recombinant Yap1. Acta Biomater. 2023;161:80–99. doi: 10.1016/j.actbio.2023.02.018.
- Curry N, Raja A, Beavis J, et al. Levels of procoagulant microvesicles are elevated after traumatic injury and platelet microvesicles are negatively correlated with mortality. J Extracell Vesicles. 2014;3(1):25625. doi: 10.3402/jev.v3.25625.
- Zhang J, Guan J, Niu X, et al. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med. 2015;13(1):49. doi: 10.1186/s12967-015-0417-0.
- Cho H, Blatchley MR, Duh EJ, et al. Acellular and cellular approaches to improve diabetic wound healing. Adv Drug Deliv Rev. 2019;146:267–288. doi: 10.1016/j.addr.2018.07.019.
- Guo SC, Tao SC, Yin WJ, et al. Exosomes derived from platelet-rich plasma promote the re-epithelization of chronic cutaneous wounds via activation of Yap in a diabetic rat model. Theranostics. 2017;7(1):81–96. doi: 10.7150/thno.16803.
- Xu N, Wang L, Guan J, et al. Wound healing effects of a curcuma zedoaria polysaccharide with platelet-rich plasma exosomes assembled on chitosan/silk hydrogel sponge in a diabetic rat model. Int J Biol Macromol. 2018;117:102–107. doi: 10.1016/j.ijbiomac.2018.05.066.
- Schlegelmilch K, Mohseni M, Kirak O, et al. Yap1 acts downstream of α-catenin to control epidermal proliferation. Cell. 2011;144(5):782–795. doi: 10.1016/j.cell.2011.02.031.
- Burnouf T, Chou ML, Lundy DJ, et al. Expanding applications of allogeneic platelets, platelet lysates, and platelet extracellular vesicles in cell therapy, regenerative medicine, and targeted drug delivery. J Biomed Sci. 2023;30(1):79. doi: 10.1186/s12929-023-00972-w.
