Abstract
Objectives
To investigate the clinical features and long-term outcomes of Chinese anti-neutrophil cytoplasmic antibodies (ANCAs)-associated vasculitis (AAV) patients with different ANCA serotypes.
Methods
Two hundred and twenty-four AAV patients from January 2010 to June 2021 were divided into myeloperoxidase (MPO)-ANCA and proteinase 3 (PR3)-ANCA groups. Clinical and long-term outcomes were compared.
Results
In this study, the average follow-up was 46.4 months (range 0.3–188.4 months). One hundred and seventy-seven (79.0%) patients were MPO–ANCA-positive and 47 were PR3–ANCA-positive; the mean age of MPO–ANCA positive patients at diagnosis was elder than that of PR3–ANCA positive patients (67.0 vs. 60.0 years, p = .004). Among PR3–ANCA-positive patients, ear, nose and throat symptoms were more common (p = .014). Between two ANCA serotypes, there were no differences in complement 3 (C3), Birmingham vasculitis activity score (BVAS), five-factor score (FFS) or other organ involvements. For all AAV patients, the overall survival rates at one, three and five years were 80.0%, 67.0% and 56.4%, respectively. The cumulative relapse-free rates of one, three and five years were 89.5%, 76.4% and 68.4%, respectively. The survival of AAV patients was unaffected by the ANCA serotype (p = .23). The ANCA serotype also had no effect on either disease relapse (p = .20) or remission rates (p = .10). In our study, PR3–ANCA patients showed a better long-term survival, as the 5-year survival rate and the 5-year relapse-free survival rate of PR3–ANCA patients were 60.7% and 76.9%, while that of MPO–ANCA patients were 55.2% and 65.8%, respectively. Rather than ANCA serotype, younger patients with milder kidney involvement and lower disease assessment scores (BVAS and FFS) might be more relevant to better prognosis.
Conclusions
The likelihood of induced remission, patient survival or disease recurrence is all unaffected by ANCA serotypes. A better prognosis is seen in younger patients with milder kidney involvement and lower BVAS/FFS scores.
Introduction
Anti-neutrophil cytoplasmic antibodies (ANCAs)-associated vasculitis (AAV) is a necrotizing small-vessel vasculitis, classified into microscopic polyangiitis (MPA), granulomatosis with polyangiitis (GPA) and eosinophilic GPA (EGPA) depending on clinical and pathology features [Citation1]. Myeloperoxidase (MPO)-ANCA and proteinase 3 (PR3)-ANCA are intricately related to several genetic and epidemiological factors [Citation1]. The most striking genetic correlation of AAV is with ANCA serotypes rather than with clinical manifestations [Citation2]. Emerging evidence suggests the potential for categorizing individuals into distinct phenotype classes utilizing ANCA serotypes rather than clinical diagnosis [Citation1,Citation2].
Different ANCA serotypes guide immunosuppression and affect clinical symptoms in AAV patients [Citation3]. However, there remains no consensus on whether ANCA serotype affects disease prognosis. Compared to PR3–ANCA + patients, MPO–ANCA + patients exhibit a higher likelihood of demise from cardiovascular disease [Citation4]. Nonetheless, another study revealed that no divergence in survival rates existed between the PR3–ANCA and MPO–ANCA vasculitis [Citation5]. A Chinese retrospective single-centre analysis indicated a lower relapse rate in the MPO group. However, the long-time prognosis was uncertain due to the brief duration of follow-up (median time 16.0 months) [Citation6]. Regrettably, the other research papers on Chinese AAV cases were based on short-term follow-up [Citation7–11]. How the ANCA serotypes affect the long-term outcomes of Chinese AAV patients were not clear. Therefore, we retrospectively examined the differences between MPO-AAV and PR3-AAV in terms of clinical characteristics and long-term outcomes in Chinese patients.
Methods
Patient population and baseline evaluation
Eligible patients with AAV diagnosed at Tianjin Medical University General Hospital from January 2010 to June 2021 were included. All patients fulfilled the following criteria: (1) adherence to the AAV classification criteria delineated by the American College of Rheumatology in 1990 or the International Chapel Hill Consensus Conference in 2012 [Citation12,Citation13]; (2) ≥18 years; patients with any of the following conditions were excluded: with other co-morbidities connective tissue diseases; ANCA antibody negative or double positive. The study was approved by the Ethics Committees of Tianjin Medical University General Hospital and fulfilled the Declaration of Helsinki.
Baseline information of diagnosed, treatment patterns and outcomes were collected. Disease activity was assessed by Birmingham vasculitis activity score (BVAS) and five-factor score (FFS) [Citation14,Citation15] and renal function was assessed by eGFR stratification. ANCA was detected by enzyme-linked immunosorbent assay (ELISA), classified as either PR3–ANCA or MPO–ANCA.
The conventional treatment was based on high dose glucocorticoids (1 mg/kg/daily) in combination with traditional disease-modifying anti-rheumatic drugs (tDMARDs). Intravenous CYC or daily oral MMF or azathioprine or leflunomide was given for maintenance management.
Disease severity definitions and follow-up data
Remission was defined as a BVAS score of 0 (complete remission) or a decrease in BVAS score of >50% without new symptoms (partial remission) after 3 months following treatment with an induction remission regimen. Patients with primary AAV who have an elevated BVAS score or a decrease in BVAS score of less than 50% following 3 months of therapy with an induction remission regimen were defined as refractory AAV. Relapse was the term used to determine by two physicians specializing in rheumatology assessed according to the BVAS of new organ activity or deterioration due to AAV.
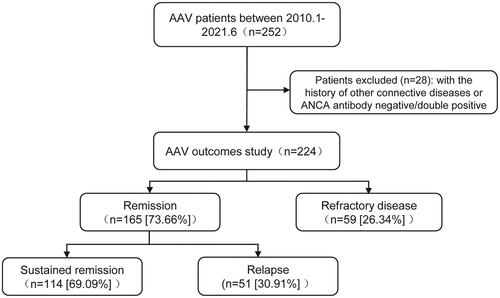
The primary endpoint was all-cause mortality, and the second endpoint was disease recurrence. The cause of death was independently categorized by two rheumatologists by medical records. A serious infection was referred to as an infection requiring intravenous antibiotic therapy or hospitalization. Each outcome event was censored at the time of occurrence during the follow-up. Data were collected at each visit. The flowchart of the study is shown in . The follow-up data were available until January 2022.
Statistical analysis
Continuous variables were presented as mean standard ± deviation or median (interquartile spacing, IQR) and were compared using the Student t-test or Mann–Whitney’s test depending on their distribution. Categorical variables were expressed as frequencies (percentages) and compared using the Chi-square test or Fisher’s exact probability method. Predicting poor prognostic variables were investigated by using multivariate Cox regression. According to the quantity of events, variables with statistically significant findings from the respective univariate analyses or those thought to be linked with clinical events were included in the multifactorial Cox regression analysis. The Kaplan–Meier method was used to plot the survival curves, and the log-rank test was used to compare variations in the survival curves. Data were statistically analysed using SPSS 22.0 (SPSS Inc., Chicago, IL) and GraphPad Prism 8.0 software (La Jolla, CA). A two-side p value <.05 was statistically significant.
Results
Demography and disease characteristics
A total of 28 patients were unenrolled due to concomitant other connective tissue diseases (n = 22) and ANCA antibody negative/double positive (n = 6); consequently, 224 patients were included; all the cases were followed up for average 46.4 months (0.3–188.4 months); baseline data are detailed in . The median age at diagnosis was 65.0 (59.0–72.8) and 90.2% are over 50 years old. One hundred and twenty-one (54%) patients were female, whilst 211 patients (94.2%) exhibited more than one organ involvement upon diagnosis. Lung (90.2%) and renal (77.7%) involvement were more common. Among 224 patients, 79.0% were positive for MPO–ANCA and 21.0% for PR3–ANCA. MPO–ANCA-positive patients were diagnosed at a relatively older age (mean age, 67.0 years vs. 60.0 years, p = .004); notably, ear, nose and throat involvement were more prevalent in PR3–ANCA patients (p = .014). Other clinical manifestations, including pulmonary and renal involvement were not significant difference between the two groups. There was also no difference in C-reactive protein, erythrocyte sedimentation rate, serum creatinine, estimated glomerular filtration rate (eGFR), nor the BVAS or FFS scores between the two groups (). ANCA titers were elevated in the mortality group (p = .022) among MPO–ANCA patients, yet inconsequential to disease recurrence (Table S1). Interestingly, in PR3–ANCA patients, ANCA antibody titers were neither associated with survival status nor relapse (Table S1).
Table 1. Baseline clinical characteristics of ANCA-associated vasculitis patients according to ANCA type.
Treatment
Regarding therapeutic strategies, 82.9% patients were treated with cyclophosphamide (CYC), whereas 11.8% chose mycophenolate mofetil (MMF) treatment. In addition, four patients received plasma exchange therapy for aggressive disease.
Long-term outcomes and their predictors
Ninety-six patients died during follow-up. The overall 1-year, 3-year and 5-year survival rates were 80.0%, 67.0% and 56.4%, respectively. Notably, the main causes of death were disease activity (45.8%) and infection (28.1%). Patients with lower eGFR and higher BVAS exhibited a poorer prognosis (p < .001 and p = .004, respectively) (). The PR3–ANCA group experienced survival rates at 1, 3 and 5 years of 89.4%, 70.0% and 60.7%, respectively; the MPO–ANCA group had rates of 77.3%, 66.2% and 55.2%, respectively. However, ANCA serotype seemed unrelated to mortality (p = .23). Male, age ≥65 years, FFS ≥2, eGFR <15 mL/(min*1.73 m2) and infection were independently predictors related with increased risk of death in multivariable Cox analysis ().
Figure 2. Long-term survival of AAV patients (Kaplan–Meier’s analysis). The survival curves for AAV patients with different ANCA types (A), gender (B), BVAS (C), eGFR (D) and ESKD (E).
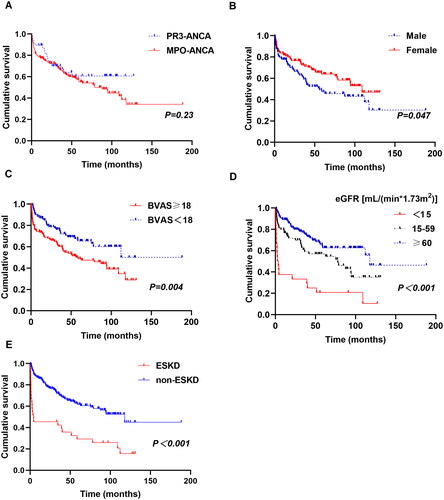
Table 2. Univariate and multivariate analyses for prognostic factors of death.
It is noteworthy that almost half of death events (45 of 96) occurred within the first year; while 70 patients endured survival beyond 5 years with the median survival time of 94.5 months; of the 70 longer survival patients, 11 individuals survived for over a decade. Comparatively, those who died within 1 year were older (median age 72.0 vs. 63.0 years, p < .001) with a greater prevalence of kidney and lung involvement; these patients also faced an augmented risk of worsening renal failure, higher BVAS and FFS scores ().
Table 3. Characteristics of ANCA-associated vasculitis patient survival less than 1 year and more than 5 years.
Relapse
One hundred and sixty-five (73.7%) AAV patients achieved remission after initial treatment, while 51 patients (30.9%) relapsed with a median relapse time of 39.0 months (). Cumulative relapse-free rates after 1, 3 and 5 years were 89.5%, 76.4% and 68.4%, respectively. The remission rates were 83.0% and 71.2% in PR3–ANCA and MPO–ANCA group, respectively; there was no difference between the two groups (p = .10). The 1-year cumulative recurrence-free rate of MPO–ANCA (+) and PR3–ANCA (+) was 89.4% vs. 89.7%; the 3-year cumulative recurrence-free rate was 76.3% vs. 76.9%; the 5-year rate was 65.8% and 76.9%, respectively. However, the disease relapse rate appeared to be unaffected by ANCA serotype (p = .20). After eliminating four individuals relapsed due to self-medication discontinuation, multivariable Cox regression analysis showed male (HR 1.787, 95%CI 1.020–3.130, p = .042) and infection (HR 2.872, 95%CI 1.628–5.066, p < .001) were independently associated with disease relapse ().
Figure 3. Relapse-free survival (A, C, D) and the number of relapses (B) in AAV patients in whom remission was achieved (Kaplan–Meier’s analysis).
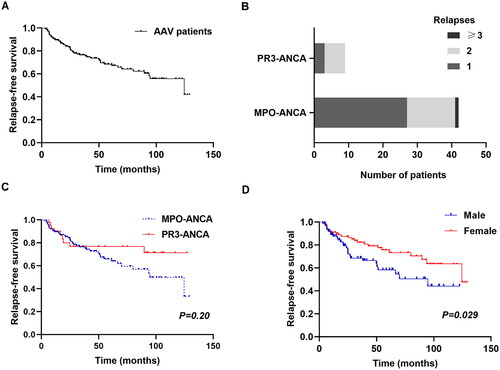
Table 4. Univariate and multivariate analyses for prognostic factors of AAV relapse in whom remission was achieved.
End-stage kidney disease (ESKD)
Thirty-three AAV patients (14.7%) progressed to ESKD during follow up with the median time of 35.3 (10.8–63.6) months. The two serotypes had similar rates of developing ESKD (Table S3). Patients with ESKD had lower survival rates (p < .001, ).
Adverse events
Fifty-two cardiovascular events (CVE) were noted among 45 individuals (20.1%) with the median time of 29.7 (6.2–62.1) months, comprising 13 acute myocardial infarction, 37 strokes and two sudden cardiac death. Besides, 27 venous thromboembolic events (VTEs) occurred with the median time of 35.3 (10.9–68.9) months. No difference was detected in CVE and VTE events between the MPO–ANCA and PR3–ANCA groups (p = .159 and p = .644, respectively).
We observed 71 infections that needed intravenous antibiotics; the infections happened mostly (50/71, 70.4%) within the first six months, with a median time to infection of 1.63 (0.4–10.6) months. Out of the 71 recorded infections, 41 were caused by bacteria, 12 by fungi, five by viruses, and 13 by multiple pathogens. The most frequent infection sites were the lungs, sepsis and urinary tract. Regarding the various ANCA serotypes, no significant difference was observed in infection frequency or timing (Table S2; p = .169 and p = .313, respectively).
Discussion
The present comprehensive investigation assessed the clinical and outcomes of two ANCA serotypes in Chinese patients undergoing prolonged monitoring. MPO–ANCA was predominant and it accounted for 79.0% in our study. Overall, it was found that discernible clinical distinctions exist between individuals with these two serotypes. MPO–ANCA positive patients were older than PR3–ANCA positive patients, which is consistent with POLVAS registry data [Citation16]. Mentionable is the higher morbidity involving ears, nose and throat in PR3–ANCA individuals. In line with previous data [Citation17], PR3–ANCA patients were predominantly male (61.5% vs. 43%, p < .001). However, no significant disparities were observed in terms of nervous system, skin, lung and renal involvement between the two subtypes. It stands to reason that MPO–ANCA might contribute to a more severe renal prognosis due to serious glomerular damage [Citation18], although ANCA specificity alone does not indicate the risk of renal outcome [Citation19].
Genetic and other evaluations illustrate that the clinical disparities between PR3-AAV and MPO-AAV are more extensive than those between GPA and MPA, indicating that antigen specificity holds significance from a pathogenetic perspective [Citation2, Citation20]. Nevertheless, due to substantial regional variances in AAV and ANCA types, MPO–ANCA predominates in China, Japan and other Asian nations, whereas PR3–ANCA dominates Europe and the United States, leading to differences in clinicopathologic features, treatment response and prognosis. While the correlations between the HLA-DQA and DQB1 loci are more robust with MPO–ANCA patients, the result of two genome-wide association studies (GWAS) studies in European AAV populations has revealed that HLADPB1 variants seem to be more significantly related with a subgroup of PR3–ANCA-positive patients than with a subgroup of patients with GPA [Citation2, Citation21,Citation22]. Moreover, DRB1*0901, DRB1*1302, DQA1*0302 and DQB1*0303 are typical HLA alleles intimately linked to MPO–ANCA positive vasculitis in East Asian populations [Citation23–26]. Comparing our data with those of MPO–ANCA positive patients in Europe and the United States, non-specific systemic symptoms such as irregular fever, fatigue, joint and muscle pains and weight loss were observed, and lung and kidney involvement were very common. Pulmonary interstitial fibrosis is more common in MPO–ANCA positive patients, and ear, nose, throat and eye involvement account for a lower proportion of MPO–ANCA, but is more likely to occur in PR3–ANCA patients [Citation27–29].
The cumulative 1-year, 3-year and 5-year survival rates for AAV patients in our study were 80%, 67% and 56%, respectively. Mortality outcomes were somewhat unfavourable in comparison to established studies from Europe. According to the European Vasculitis Collaboration study, the 1-year, 2-year and 5-year survival rates are 88%, 85% and 78% for patients with AAV, respectively [Citation30]. ANCA serotype appears to have minimal impact on the survival of these patients. With respect to demographic facts, male, older patients, worse kidney function and higher disease assessment scores (BVAS and FFS) in our study may contribute to this divergence. According to Haris et al.’s research, patients with PR3–ANCA are more likely to exhibit a higher BVAS score and consequently, a less favourable outcome [Citation31]. However, such a correlation was not discovered in our study. Our research implies that age ≥65 years is an independent predictor correlated with an amplified risk of mortality. As previously reported, advanced age at AAV onset is associated with a primary risk factor for short-term death [Citation27]. Interestingly, other research found the two-year and five-year elderly AAV patients (median age 74 years, IQR 65–92) survival rates to be 83 and 75%, respectively [Citation28]. However, whether advancing age does indeed equate to poor outcomes requires further examination. Considering that our cohort suffered from more severe organ damage (lung and kidney) as well as elevated C-reactive protein, this may explain the survival disparity. Nonetheless, elderly patients, particularly those affected by multi-organ involvement, should receive additional attention.
We found no significant disparity in baseline kidney function between PR3–ANCA and MPO–ANCA groups. The earliest indication of AAV development, which ultimately results in ESKD, is typically acute renal failure [Citation29, Citation32,Citation33]. Nevertheless, this does not discredit the efficacy of immunosuppressive therapy, which can induce remission and maintain kidney function in certain AAV patients, irrespective of the severity of renal dysfunction [Citation34]. In addition to this, a renal risk scoring system was meticulously developed based on thorough clinicopathologic features, which divided patients into three well-defined risk groups (low, medium and high) for predicting ESKD in ANCA-associated glomerulonephritis [Citation35]. Similarly, a separate investigation discovered that the renal risk score manifested a superior predictive value for ESKD compared to the histopathological classification [Citation36].
Previous studies consistently affirms that the predominant causes of mortality are infection and vasculitis activity. Kidney failure and pulmonary fibrosis emerge as key complications of vasculitis activity. Flossmann et al. identified cardiovascular causes are the third primary death causes in AAV patients, especially within the first year [Citation30]. Steinberg et al. analysed the causes of death of more than 10,000 AAV patients in the United States over the past decade and found that more than 50% of patients died from vasculitis itself [Citation37]. Our findings accentuate that disease activity is a potentially fatal determinant. Possible culprits include unequal distribution of healthcare resources and delayed diagnosis. Additionally, the unapproved indication for AAV and the high cost of rituximab in China limit its utilization. Consistent with the results of previous studies [Citation38–40], mortality is pronounced during the initial year of diagnosis, particularly for patients with FFS ≥ 2 points, advanced age, severe infection and renal impairment. Contrary to a life expectancy of under 1 year, those surviving beyond 5 years are younger, with milder kidney damage and lower disease assessment scores (BVAS/FFS). Simultaneously, successful induction of remission in new diagnosed patients with AAV is instrumental to favourable prognosis. This may aid future intervention randomized clinical trials by identifying a cohort with severe diseases that could benefit from more comprehensive treatment strategies.
Previous reported AAV relapse occurs in 8–63.9% individuals with a 2-year relapse rate of 18–40% [Citation41,Citation42]. Our findings indicate that the recurrence rate was 30.91% consistent with other studies; male and infection are the independent risk factor for disease recurrence, while ANCA serotype dose not influence disease relapse. The presence of PR3–ANCA in European studies has been affiliated with a higher risk of illness recurrence [Citation34, Citation43,Citation44]. Nonetheless, this finding has not been verified in Asian AAV cohorts [Citation7, Citation45], which is also true for our study. It may be attributed to regional variations and ethnic variances. PR3–ANCA-positive patients are the majority in studies of vasculitis conducted in Europe [Citation34, Citation43], while only 20.43% AAV patients in our cohort are PR3–ANCA positive. Lung involvement are risk variables for disease recurrence in the Glomerular Disease Collaborative Network cohort [Citation43]. However, lung involvement is not linked to relapse in our study, and the same in the trial conducted by the French Vasculitis Collaborative Group [Citation34]. Additionally, elevated blood creatinine at diagnosis was related to a decreased relapse in the analysis of a relapse cohort according to data from the European Vasculitis Collaborative Group [Citation44]. Although complex to study, AAV recurrence merits examination for considering different background population and an extensively observation of specific organ impairment.
Although infections may transpire at any juncture of the ailment, they are predominantly observed within the initial year subsequent to diagnosis [Citation10, Citation46]. The most frequent sites of infection were the lungs and urinary tract [Citation10, Citation47]. A study of 325 patients discovered that 40% of AAV patients developed severe infections with significant mortality, and higher BVAS scores predicted infection [Citation47]. These observations emulate our findings significantly. Concurrently, severe infection denoted a substantial risk of mortality and disease recurrence. A better prognosis for individuals with AAV hinges on striking equilibrium between eliciting disease remission and side effects stemming from the treatment.
This study has several limitations. First, it is a single-centre retrospective study, potentially introducing selection bias. Second, we are unable to explicitly investigate the impact of a specific therapy or medication on the clinical results of AAV patients. In addition, we also apologize that we cannot do renal histological staging due to insufficient renal pathology data. Although no Coronavirus Disease 2019 (COVID-19)-related deaths were observed, the COVID-19 pandemic remains affected by its collateral damage to patients in different ways. However, our study may offer some ‘real-world’ clinical data on the prognostic outcomes of disease under current treatment protocols.
Conclusions
In this work, we aim to highlight the distinctive clinical characteristics of MPO–ANCA (+) and PR3–ANCA (+) in AAV patients. Nevertheless, ANCA serotype appears to have no bearing on patient survival and disease relapse. Younger patients with milder kidney involvement and lower disease assessment scores (BVAS/FFS) generally exhibit a more favourable prognosis.
Author contributions
FQ: study conception and design, statistical analyses and writing of manuscript. JH: study conception and design, writing of manuscript. WW: study conception and design. All authors read and revised manuscript. All authors approved the final manuscript.
Ethical approval
Ethics approval was granted by the Tianjin Medical University General Hospital Ethics Committees (IRB2021-YX-001-01), and the study was conducted in accordance with the Declaration of Helsinki.
Consent form
Informed consent was not required because of the retrospective study.
Supplemental Material
Download MS Word (16.5 KB)Acknowledgements
Not applicable.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Requests for data should be directed to the senior author but will be conditioned on the legal premises under which they were collected.
Additional information
Funding
References
- Walker BS, Peterson LK, Koening C, et al. Performance of MPO–ANCA and PR3–ANCA immunoassays for the stratification of specific ANCA-associated vasculitis: a systematic review and meta-analysis. Autoimmun Rev. 2022;21(6):1. doi: 10.1016/j.autrev.2022.103100.
- Lyons PA, Rayner TF, Trivedi S, et al. Genetically distinct subsets within ANCA-associated vasculitis. N Engl J Med. 2012;367(3):214–11. doi: 10.1056/NEJMoa1108735.
- Unizony S, Villarreal M, Miloslavsky EM, et al. Clinical outcomes of treatment of anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis based on ANCA type. Ann Rheum Dis. 2016;75(6):1166–1169. doi: 10.1136/annrheumdis-2015-208073.
- Wallace ZS, Fu X, Harkness T, et al. All-cause and cause-specific mortality in ANCA-associated vasculitis: overall and according to ANCA type. Rheumatology. 2020;59(9):2308–2315. doi: 10.1093/rheumatology/kez589.
- Murosaki T, Sato T, Akiyama Y, et al. Difference in relapse-rate and clinical phenotype by autoantibody-subtype in Japanese patients with anti-neutrophil cytoplasmic antibody-associated vasculitis. Mod Rheumatol. 2017;27(1):95–101. doi: 10.1080/14397595.2016.1192760.
- Hong Y, Shi P, Liu X, et al. Distinction between MPO–ANCA and PR3–ANCA-associated glomerulonephritis in Chinese patients: a retrospective single-center study. Clin Rheumatol. 2019;38(6):1665–1673. doi: 10.1007/s10067-019-04458-9.
- Li Z-Y, Chang D-Y, Zhao M-H, et al. Predictors of treatment resistance and relapse in antineutrophil cytoplasmic antibody-associated vasculitis: a study of 439 cases in a single Chinese center: relapse and treatment resistance in AAV. Arthritis Rheumatol. 2014;66(7):1920–1926. doi: 10.1002/art.38621.
- Chen Y, Bao H, Liu Z, et al. Risk factors for renal survival in Chinese patients with myeloperoxidase–ANCA-associated GN. Clin J Am Soc Nephrol. 2017;12(3):417–425. doi: 10.2215/CJN.06200616.
- Huang L, Shen C, Zhong Y, et al. The association of neutrophil-to-lymphocyte ratio with all-cause mortality in Chinese patients with MPO–ANCA associated vasculitis. Clin Exp Med. 2020;20(3):401–408. doi: 10.1007/s10238-020-00629-0.
- Yang L, Xie H, Liu Z, et al. Risk factors for infectious complications of ANCA-associated vasculitis: a cohort study. BMC Nephrol. 2018;19(1):138. doi: 10.1186/s12882-018-0933-2.
- Liu S, Han L, Liu Y, et al. Clinical significance of MPO–ANCA in eosinophilic granulomatosis with polyangiitis: experience from a longitudinal Chinese cohort. Front Immunol. 2022;13:885198. doi: 10.3389/fimmu.2022.885198.
- Jennette JC, Falk RJ, Bacon PA, et al. 2012 Revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Arthritis Rheum. 2013;65(1):1–11. doi: 10.1002/art.37715.
- Chung SA, Langford CA, Maz M, et al. 2021 American College of Rheumatology/Vasculitis Foundation Guideline for the management of antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol. 2021;73(8):1366–1383. doi: 10.1002/art.41773.
- Mukhtyar C, Lee R, Brown D, et al. Modification and validation of the Birmingham Vasculitis Activity Score (version 3). Ann Rheum Dis. 2009;68(12):1827–1832. doi: 10.1136/ard.2008.101279.
- Guillevin L, Pagnoux C, Seror R, et al. The five-factor score revisited: assessment of prognoses of systemic necrotizing vasculitides based on the French Vasculitis Study Group (FVSG) Cohort. Medicine. 2011;90(1):19–27. doi: 10.1097/MD.0b013e318205a4c6.
- Wójcik K, Masiak A, Jeleniewicz R, et al. Association of antineutrophil cytoplasmic antibody (ANCA) specificity with the demographic and clinical characteristics of patients with ANCA-associated vasculitides. Pol Arch Intern Med. 2022;132(3):16187. doi: 10.20452/pamw.16187.
- Weiner M, Bjørneklett R, Hrušková Z, et al. Proteinase-3 and myeloperoxidase serotype in relation to demographic factors and geographic distribution in anti-neutrophil cytoplasmic antibody-associated glomerulonephritis. Nephrol Dial Transplant. 2018;34(2):301–308. doi: 10.1093/ndt/gfy106.
- Mohammad AJ, Segelmark M. A population-based study showing better renal prognosis for proteinase 3 antineutrophil cytoplasmic antibody (ANCA)-associated nephritis versus myeloperoxidase ANCA-associated nephritis. J Rheumatol. 2014;41(7):1366–1373. doi: 10.3899/jrheum.131038.
- Lionaki S, Blyth ER, Hogan SL, et al. Classification of antineutrophil cytoplasmic autoantibody vasculitides: the role of antineutrophil cytoplasmic autoantibody specificity for myeloperoxidase or proteinase 3 in disease recognition and prognosis. Arthritis Rheum. 2012;64(10):3452–3462. doi: 10.1002/art.34562.
- Pagnoux C, Springer J. Editorial: classifying antineutrophil cytoplasmic antibody (ANCA)-associated vasculitides according to ANCA type or phenotypic diagnosis: salt or pepper? Arthritis Rheumatol. 2016;68(12):2837–2840. doi: 10.1002/art.39860.
- Xie G, Roshandel D, Sherva R, et al. Association of granulomatosis with polyangiitis (Wegener’s) with HLA–DPB1*04 and SEMA6A gene variants: evidence from genome-wide analysis. Arthritis Rheum. 2013;65(9):2457–2468. doi: 10.1002/art.38036.
- Merkel PA, Xie G, Monach PA, et al. Identification of functional and expression polymorphisms associated with risk for antineutrophil cytoplasmic autoantibody-associated vasculitis. Arthritis Rheumatol. 2017;69(5):1054–1066. doi: 10.1002/art.40034.
- Tsuchiya N, Kobayashi S, Hashimoto H, et al. Association of HLA-DRB1*0901-DQB1*0303 haplotype with microscopic polyangiitis in Japanese. Genes Immun. 2006;7(1):81–84. doi: 10.1038/sj.gene.6364262.
- Tsuchiya N, Kobayashi S, Kawasaki A, et al. Genetic background of Japanese patients with antineutrophil cytoplasmic antibody-associated vasculitis: association of HLA-DRB1*0901 with microscopic polyangiitis. J Rheumatol. 2003;30(7):1534–1540.
- Kawasaki A, Hasebe N, Hidaka M, et al. Protective role of HLA-DRB1*13:02 against microscopic polyangiitis and MPO–ANCA-positive vasculitides in a Japanese population: a case-control study. PLOS One. 2016;11(5):e0154393. doi: 10.1371/journal.pone.0154393.
- Wang H-Y, Cui Z, Pei Z-Y, et al. Risk HLA class II alleles and amino acid residues in myeloperoxidase–ANCA-associated vasculitis. Kidney Int. 2019;96(4):1010–1019. doi: 10.1016/j.kint.2019.06.015.
- Monti S, Craven A, Klersy C, et al. Association between age at disease onset of anti-neutrophil cytoplasmic antibody-associated vasculitis and clinical presentation and short-term outcomes. Rheumatology. 2021;60(2):617–628. doi: 10.1093/rheumatology/keaa215.
- McGovern D, Williams SP, Parsons K, et al. Long-term outcomes in elderly patients with ANCA-associated vasculitis. Rheumatology. 2020;59(5):1076–1083. doi: 10.1093/rheumatology/kez388.
- de Lind van Wijngaarden RAF, Hauer HA, Wolterbeek R, et al. Clinical and histologic determinants of renal outcome in ANCA-associated vasculitis: a prospective analysis of 100 patients with severe renal involvement. J Am Soc Nephrol. 2006;17(8):2264–2274. doi: 10.1681/ASN.2005080870.
- Flossmann O, Berden A, de Groot K, et al. Long-term patient survival in ANCA-associated vasculitis. Ann Rheum Dis. 2011;70(3):488–494. doi: 10.1136/ard.2010.137778.
- Haris Á, Polner K, Arányi J, et al. Simple, readily available clinical indices predict early and late mortality among patients with ANCA-associated vasculitis. BMC Nephrol. 2017;18(1):76. doi: 10.1186/s12882-017-0491-z.
- Hilhorst M, Wilde B, van Paassen P, et al. Improved outcome in anti-neutrophil cytoplasmic antibody (ANCA)-associated glomerulonephritis: a 30-year follow-up study. Nephrol Dial Transplant. 2013;28(2):373–379. doi: 10.1093/ndt/gfs428.
- Tanna A, Guarino L, Tam FWK, et al. Long-term outcome of anti-neutrophil cytoplasm antibody-associated glomerulonephritis: evaluation of the international histological classification and other prognostic factors. Nephrol Dial Transplant. 2015;30(7):1185–1192. doi: 10.1093/ndt/gfu237.
- Hogan SL, Falk RJ, Chin H, et al. Predictors of relapse and treatment resistance in antineutrophil cytoplasmic antibody-associated small-vessel vasculitis. Ann Intern Med. 2005;143(9):621–631. doi: 10.7326/0003-4819-143-9-200511010-00005.
- Brix SR, Noriega M, Tennstedt P, et al. Development and validation of a renal risk score in ANCA-associated glomerulonephritis. Kidney Int. 2018;94(6):1177–1188. doi: 10.1016/j.kint.2018.07.020.
- Brilland B, Boud’hors C, Copin M-C, et al. Assessment of renal risk score and histopathological classification for prediction of end-stage kidney disease and factors associated with change in eGFR after ANCA-glomerulonephritis diagnosis. Front Immunol. 2022;13:834878. doi: 10.3389/fimmu.2022.834878.
- Steinberg AW, Wechsler ME, Fernández Pérez ER. Trends in antineutrophil cytoplasmic autoantibody-associated vasculitis-related mortality in the United States, 1999 to 2017. Ann Intern Med. 2020;172(2):160–163. doi: 10.7326/M19-1564.
- Westman KW, Bygren PG, Olsson H, et al. Relapse rate, renal survival, and cancer morbidity in patients with Wegener’s granulomatosis or microscopic polyangiitis with renal involvement. J Am Soc Nephrol. 1998;9(5):842–852. doi: 10.1681/ASN.V95842.
- Mahr A, Girard T, Agher R, et al. Analysis of factors predictive of survival based on 49 patients with systemic Wegener’s granulomatosis and prospective follow-up. Rheumatology. 2001;40(5):492–498. doi: 10.1093/rheumatology/40.5.492.
- Hruskova Z, Casian A, Konopasek P, et al. Long-term outcome of severe alveolar haemorrhage in ANCA-associated vasculitis: a retrospective cohort study. Scand J Rheumatol. 2013;42(3):211–214. doi: 10.3109/03009742.2012.754939.
- Mukhtyar C, Flossmann O, Hellmich B, et al. Outcomes from studies of antineutrophil cytoplasm antibody associated vasculitis: a systematic review by the European League Against Rheumatism Systemic Vasculitis Task Force. Ann Rheum Dis. 2008;67(7):1004–1010. doi: 10.1136/ard.2007.071936.
- Holle JU, Gross WL, Latza U, et al. Improved outcome in 445 patients with Wegener’s granulomatosis in a German Vasculitis Center over four decades. Arthritis Rheum. 2011;63(1):257–266. doi: 10.1002/art.27763.
- Pagnoux C, Hogan SL, Chin H, et al. Predictors of treatment resistance and relapse in antineutrophil cytoplasmic antibody-associated small-vessel vasculitis: comparison of two independent cohorts. Arthritis Rheum. 2008;58(9):2908–2918. doi: 10.1002/art.23800.
- Walsh M, Flossmann O, Berden A, et al. Risk factors for relapse of antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2012;64(2):542–548. doi: 10.1002/art.33361.
- Hara A, Wada T, Sada K, et al. Risk factors for relapse of antineutrophil cytoplasmic antibody-associated vasculitis in Japan: a nationwide, prospective cohort study. J Rheumatol. 2018;45(4):521–528. doi: 10.3899/jrheum.170508.
- Mohammad AJ, Segelmark M, Smith R, et al. Severe infection in antineutrophil cytoplasmic antibody-associated vasculitis. J Rheumatol. 2017;44(10):1468–1475. doi: 10.3899/jrheum.160909.
- Rathmann J, Jayne D, Segelmark M, et al. Incidence and predictors of severe infections in ANCA-associated vasculitis: a population-based cohort study. Rheumatology. 2021;60(6):2745–2754. doi: 10.1093/rheumatology/keaa699.