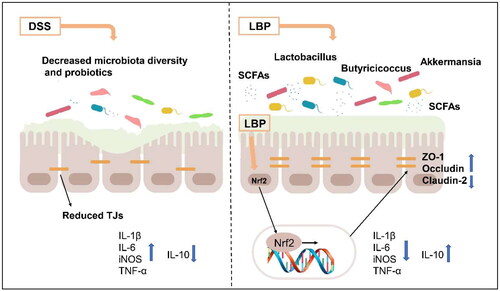
Abstract
Purpose
This study examined the protective effects and mechanism of Lycium barbarum polysaccharides (LBP) in the context of intestinal barrier function and intestinal microbiota in mice with dextran sulfate sodium (DSS)-induced chronic ulcerative colitis (UC).
Methods
C57BL/6J male mice were assigned to a standard normal diet without DSS (control group), a normal diet with DSS (DSS group, 2% DSS given discontinuously for 3 weeks) or a normal diet supplemented with LBP (1% dry feed weight, LBP group, 2% DSS given discontinuously for 3 weeks) for a total of 8 weeks, at which point colonic tissues and caecal contents were collected.
Results
LBP exerted a significant effect against colitis by increasing body weight, colon length, DAI and histopathological scores. LBP inhibited proinflammatory cytokines (IL-1β, IL-6, iNOS and TNF-α) expression, improved anti-inflammatory cytokine (IL-10) expression, promoted the expression of tight junction proteins (Occludin and ZO-1) via nuclear factor erythroid 2-related factor 2 (Nrf2) activation and decreased Claudin-2 expression to maintain the intestinal mucosal barrier. In addition, the abundances of some probiotics (Ruminococcaceae, Lactobacillus, Butyricicoccus, and Akkermansia) were decreased with DSS treatment but increased obviously with LBP treatment. And LBP reduced the abundance of conditional pathogens associated with UC (Mucispirillum and Sutterella). Furthermore, LBP improved the production of short-chain fatty acids (SCFAs), including acetic acid, propionic acid, butyric acid and isobutyric acid.
Conclusion
LBP can alleviate DSS-induced UC by regulating inflammatory cytokines and tight junction proteins. Moreover, LBP promotes probiotics, suppresses conditional pathogens and increases SCFAs production, showing a strong prebiotic effect.
1. Introduction
Ulcerative colitis (UC) is a chronic, relapsing, and non-infectious condition characterized by gastrointestinal tract inflammation; it has evolved into a global disease with an increasing prevalence and represents a substantial economic and disease burden on society [Citation1–4]. Conventional drugs for UC include corticosteroids, 5-aminosalicylic acid and immunosuppressants, but these drugs have undesirable side effects and can only reduce symptoms in acute episodes instead of fundamentally altering relapse episodes [Citation5]. Therefore, exploring novel drugs for UC treatment is imperative.
Extensive research has shown that searching for novel agents from natural products for the treatment of UC is a promising research direction [Citation6]. Goji berries are medicinal foods that have been used in Chinese medicine since ancient times [Citation7]. Lycium barbarum polysaccharide (LBP) is the major active ingredient isolated from Goji berries and exerts a strong anti-inflammatory effect, constituting a potential prebiotic for the treatment of colitis [Citation8]. For instance, Cao et al. reported that Lycium barbarum arabinogalactan, a subtype of LBP, alleviates DSS-induced chronic colitis via modulating metabolome [Citation9]. Moreover, LBP combined with capsaicin can alleviate DSS-induced colitis by inhibiting oxidative stress and inflammatory signalling [Citation10]. At present, the alleviation effect of LBP on IBD has been preliminarily confirmed, but the exact anti-inflammatory mechanism has not been fully revealed [Citation11]. The existing literature suggests that LBP is fermented to short-chain fatty acids (SCFAs) by intestinal microflora in vitro and increases the relative abundances of beneficial bacteria such as Bifidobacterium and Bacteroides [Citation12]. UC is characterized by an overall decrease in microbial diversity, with a loss of beneficial symbionts, which may result in increased mucosal adherence and translocation of symbiotic microorganisms, thus triggering chronic inflammation [Citation13]. UC is also associated with a loss of microbiota-derived SCFAs metabolites, bile acid dysmetabolism and increased tryptophan metabolism [Citation14]. Therefore, we surmise that LBP could ameliorate UC by balancing the intestinal microflora and enhancing SCFAs production.
Additionally, an in vitro study indicated that LBP ameliorated intestinal barrier dysfunction and inflammation through the MLCK-MLC signalling pathway in Caco-2 cells [Citation15]. The literature suggests that epithelial barrier defect is one of the pathogenic mechanisms of UC [Citation16]. Gut barrier integrity is maintained by tight junction (TJ) proteins such as claudins, zona occludins, and occludin, which are critical for the intestinal mucosa mechanical barrier and prevent the spread of pathogens and harmful antigens across the epithelium [Citation17]. In addition, there is a strong relationship between the intestinal microflora and intestinal barrier function. Intestinal microflora dysbiosis decreases intestinal mucosal barrier function, and intestinal pathogenic bacteria damage structural barriers by changing intestinal TJ proteins [Citation18]. Thus, in addition to gut microbiota, our research will focus on the role of LBP in regulating intestinal mucosal barrier function for the treatment of UC.
In the present study, the curative effect and mechanism of LBP were evaluated in a DSS-induced chronic UC mouse model by alterations in the intestinal microflora and the expression of tight junction proteins. In addition, we initially validated the role of LBP in the regulation of barrier function via the Nrf2 signalling pathway. We attempted to provide novel insight into the application of LBP in the prevention and treatment of UC as an effective adjuvant drug.
2. Materials and methods
2.1. Animals and the chronic colitis model
C57BL/6J male mice (weight 20-22 g) were obtained and housed in the Experimental Animal Laboratory Unit at Beijing Friendship Hospital. After a one-week acclimatization period, the mice were randomly assigned to 3 groups: the CON group (normal water and regular chow diet, n = 10), DSS group (DSS water and regular chow diet, n = 7) and LBP group (DSS water and fed 1% LBP supplemented in rodent chow; n = 8; LBP was purchased from Shanxi Ciyuan Biotechnology, 98% ultraviolet, No. CY191208). The DSS and LBP groups received 2.0% (w/v) DSS (M.W. 36,000–50,000 Da, MP Biomedicals, Cat. No. 9011-18-1) for one week, followed by regular water for fourteen days to recover. Model establishment last for three cycles (i.e. Day 0 to Day 54) [Citation19]. This project passed the experimental animal ethics review of the Institutional Animal Care and Use Committee of Beijing Friendship Hospital Affiliated to Capital Medical University (Permit Number 19-2022).
2.2. Assessment of colitis severity
Body weight loss, stool consistency, and gross bleeding were included to calculate the disease activity index (DAI) [Citation20]. Colonic damage was evaluated on the basis of both length assessment and histological scoring of the colon. For colon length, mice were sacrificed at day 54, and the colon was excised and measured from the ileocecal junction to the anal verge. For histological evaluation, sections from the distal colon were stained with H&E. Three fields of view were randomly selected for each slice to be examined microscopically using the scoring system reported by Dieleman LA () [Citation21] The histological score was defined as the sum of the four parameter scores and three fields were averaged.
Table 1. Histological grading of colitis.
2.3 Myeloperoxidase (MPO) assay
MPO serves as a marker for colon tissue neutrophil infiltration. MPO activity in the distal colon was determined using an MPO Colorimetric Assay Kit (Elabscience Biotechnology Co., Cat. E-BC-K074-S) with a spectrophotometer at 460 nm, according to the manufacturer’s instructions. MPO activities are expressed as U/g protein.
2.4. Cell culture and treatment
Caco-2, a human colonic epithelial cell line, was obtained from American Type Culture Collection and cultured in DMEM (Gibco, Cat. No. C11995500BT) with 10% fetal bovine serum and 1% penicillin/streptomycin (Gibco, Cat. No. 15140122) at 37 °C in the presence of 5% CO2. Caco-2 cells were seeded in 6-well cell culture plates (6 × 105 cells per well) and incubated with LBP (dissolved in PBS; 100, 200, 400 µg/mL) for 24h to examine alterations in tight junction proteins. To investigate related mechanisms, ML385 (a Nrf2 inhibitor; dissolved in DMSO; 20 µM and 50 µM; Selleck Chemicals, Cat. No. 846557-71-9) were added to Caco-2 cells before treatment with LBP (dissolved in PBS; 400 µg/ml) for 24 h.
2.5. Quantitative real-time (qPCR) for cytokine levels
The mucosa of the distal colon was carefully separated from the muscle layer, and total RNA was extracted by grinding frozen mucosa with TRIzol reagent. For in vitro experiments, the Caco-2 cells were treated with Lipopolysaccharide (LPS, Sigma-Aldrich, Cat. No. L2880, 1 µg/ml) or LBP (100, 200, and 400 μg/mL) +LPS for 24 h. The total cellular RNA was isolated by utilizing TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Then, cDNA was synthesized using 5XAll-in-one RT MasterMix (Thermo Scientific, Sankt Leon-Rot, Germany). The primers for the target products (TNF-α, iNOS, IL-6, IL-1β, and IL-10) of mice colon and Caco-2 cells were designed as indicated in .
Table 2. PCR primers of cytokines.
2.6 Western blot analysis
Total protein of mucosa in the distal colon and cells was extracted using RIPA lysis buffer and then separated by 10% SDS/PAGE. The separated proteins were transferred onto PVDF membranes, which were then washed for 10 min with TBS and blocked with 5% non-fat dry milk in TBST for 2 h at 25 °C. The blot was incubated with a polyclonal primary antibody against ZO-1, Occludin, Claudin-2, and Nrf2 (Abcam) overnight at 4 °C. After washing in TBST (10 min, three times), the blot was incubated with a secondary antibody against rabbit IgG (Santa Cruz) for 1 h at 25 °C. The blot was finally washed with TBST (10 min, three times), and the protein bands were visualized with a chemiluminescence system. The resulting image was analysed using Image J software.
2.7. Immunofluorescence staining for fibrous actin (F-actin)
To determine the effect of LBP on F-actin, Caco-2 cells inoculated onto tablets in 24-well plates were collected after 24 h with LBP. Then, the culture medium was discarded, and the cells were washed with PBS for 5 min. The PBS was discarded, and the Caco-2 cells were fixed in 4% paraformaldehyde at room temperature for 10 min. Next, the Caco-2 cells were treated with 0.1% Triton X-100 for 5 min after washing with PBS (5 min, three times). Alexa Fluor 594-phalloidin (dissolved in PBS to a final concentration of 0.11 mol/L) was added (100 μL/well) for 30 min and then washed with PBS (5 min, three times). Finally, the samples were observed under a laser scanning confocal microscope after DAPI sealing, which was used to stain nuclei.
2.8. Permeability assay
Caco-2 cells incubated with or without LBP were cultured on Millipore Millicell plates, which were used for permeability experiments. After forming a confluent monolayer, the cells were assayed using fluorescein isothiocyanate-dextran FD4 (dissolved in PBS, 100 μg/mL, M.W. 3,000–5,000, Sigma-Aldrich, Cat. No. 46944) for 30 min in the incubator, and then the culture medium with FD4 leakage in the bottom plates was removed for fluorescence measurements (excitation 485 nm, emission 530 nm). In vivo, mice were fasted for 16 h followed by gavage with FD4 (dissolved in PBS, 0.5 μg/kg). After 3h, blood was sampled from mouse eyes for fluorescence measurements to evaluate intestinal permeability.
2.9 16S rRNA Gene and bioinformatics analysis
2.9.1 DNA extraction
The caecal contents of mice from caecum were collected quickly. Total genomic DNA samples were extracted using the OMEGA Soil DNA Kit (M5635-02) (OmegaBio-Tek, Norcross, GA, USA) following the manufacturer’s instructions and stored at −20 °C prior to further analysis. The quantity and quality of extracted DNA were measured using a NanoDrop NC2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) and agarose gel electrophoresis, respectively.
2.9.2 16S rRNA Gene amplicon sequencing
PCR amplification of the bacterial 16S rRNA gene V3-V4 region was performed using the forward primer 338 F (5′-ACTCCTACGGGAGGCAGCA-3′) and the reverse primer 806 R (5′-GGACTACHVGGGTWTCTAAT-3′). Sample-specific 7-bp barcodes were incorporated into the primers for multiplex sequencing. The PCR components contained 5 μl of buffer (5×), 0.25 μl of Fast pfu DNA Polymerase (5 U/μl), 2 μl (2.5 mM) of dNTPs, 1 μl (10 µM) of each forward and reverse primer, 1 μl of DNA template, and 14.75 μl of ddH2O. Thermal cycling consisted of initial denaturation at 98 °C for 5 min, followed by 24 cycles consisting of denaturation at 98 °C for 30 s, annealing at 52 °C for 30 s, and extension at 72 °C for 45 s, with a final extension of 5 min at 72 °C. PCR amplicons were purified with Vazyme VAHTSTM DNA Clean Beads (Vazyme, Nanjing, China) and quantified using the Quant-iT PicoGreen dsDNA Assay Kit (Invitrogen, Carlsbad, CA, USA). After the individual quantification step, amplicons were pooled in equal amounts, and paired-end 2 × 250 bp sequencing was performed using the Illumina NovaSeq platform with a NovaSeq 6000 SP Reagent Kit (500 cycles) at Shanghai Personal Biotechnology Co., Ltd. (Shanghai, China).
2.9.3 Sequence analysis
Microbiome bioinformatics was performed with QIIME2 according to the official tutorials, with slight modification (https://docs.qiime2.org/2019.4/tutorials/).
2.10. Determination of SCFAs
The caecal contents of mice were collected quickly on a clean bench, and 30 mg was placed into a 1.5 mL EP tube. After grinding and homogenate with 300 μL ultrapure water, the samples were centrifuged at 18000 g for 20 min at 4 °C. Next, 200 μL of supernatant was extracted, and 50 μL of 50% H2SO4 and 200 μL of ether solution were added; the samples were shaken for 1 min, sonicated for 1 min, centrifuged at 12,000 rpm for 20 min at 4 °C and allowed to stand for 10 min. Finally, the supernatant (ether extract) was filtered by anhydrous sodium sulfate and tested on the instrument. The GC-MS (Pegasus HT, Leco Corp., USA) parameters used to detect SCFAs (acetic acid, propionic acid, n-butyric acid, isobutyric acid, n-valeric acid, isovaleric acid and hexanoic acid) were as follows: GC: Column: DB-FFAP (30 m ×0.25 mm × 0.25 µm), Oven Programmed Temp: 100 (1 min), 100-160 (5 °C/min),160-240 (40 °C/min), 240 (10 min), Inlet Temp: 250 °C, Injection Vol: 1.0 μL, Carrier Gas: Helium (99.9999%), Transfer Line Temp: 240 °C, and Flow Rate: 1.0 mL/min; MS: Ionization Mode: electron impact, Electron Energy: 70 ev, Detector Voltage: 1700 V, Source Temp: 220 V, Mass Range: 33-400 Da.
2.11. Statistical analysis
All data are expressed as the mean ± SEM. The differences between groups were analysed by two-way analysis of variance (ANOVA) or one-way ANOVA (post hoc analysis: Tukey’s multiple comparison test) and the Kruskal-Wallis test. Statistical analysis was performed with GraphPad Prism V.8.0.2 (San Diego, California, USA). A value of p < 0.05 was considered to indicate statistical significance.
3. Results
3.1. LBP administration ameliorated the symptoms of DSS-induced colitis in mice
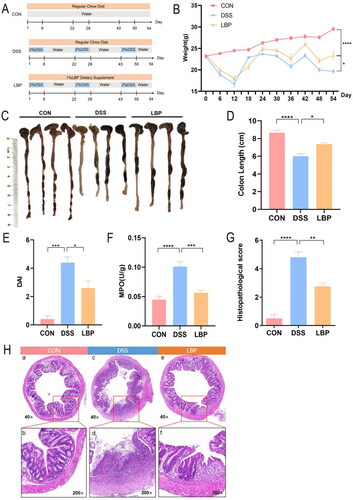
To investigate whether LBP supplementation exerts a protective effect on UC, a DSS mouse model was induced in C57BL/6J mice at the age of 6-8 weeks in three cycles (Day 0 to Day 54) by using 2% DSS in drinking water for one week, followed by 14 days of remission. LBP (1% of dry feed weight) was added to the rodent chow fed to the mice during the whole chronic colitis induction period. The control group received a regular chewing diet and normal drinking water (). The DSS group exhibited serious inflammation symptoms compared to control group and LBP supplementation could alleviate DSS-induced colitis, as indicated by body weight gain (p < 0.05), colon length (p < 0.05), DAI score (p < 0.05), MPO (p < 0.05) and histopathological scores (p < 0.05) (). Furthermore, HE staining indicated less neutrophil infiltration and better mucosal integrity in mice supplemented with LBP than in the DSS group (). Together, these results indicated that LBP treatment significantly ameliorated DSS-induced colitis.
Figure 1. The symptoms and inflammation of DSS-induced colitis can be ameliorated by LBP supplementation. (A) Schematic diagram illustrating the animal study design. (B) Changes in body weight were recorded every six days during the disease process. (C, D) Representative pictures of colon gross appearance and colon length. (E) DAI scoring of DSS-induced colitis. (F) Distal colonic MPO levels were measured by a MPO colorimetric assay kit. (G, H) Distal colon tissues were collected for histopathologic examination after haematoxylin and eosin (HE) staining at 40× and 200×; a and b: the colonic structure of the control group was normal, the glands were neatly arranged, the crypts were normal, and there were no inflammatory cell infiltration; c and d: the DSS groups indicated disturbed architecture of colon and extensive glandular defects, crypt destruction and inflammatory cell infiltration; e and f: the LBP groups showed reduced numbers of infiltrating cells, a lesser degree of glandular and crypt damage and repaired partial mucosal injury. Statistical analysis was performed using two-way ANOVA (weight) and one-way ANOVA (post hoc analysis: Tukey’s multiple comparison test). Data indicate the mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
3.2. Effect of LBP on the levels of pro-inflammatory and anti-inflammatory cytokines in colon tissues and Caco-2 cells
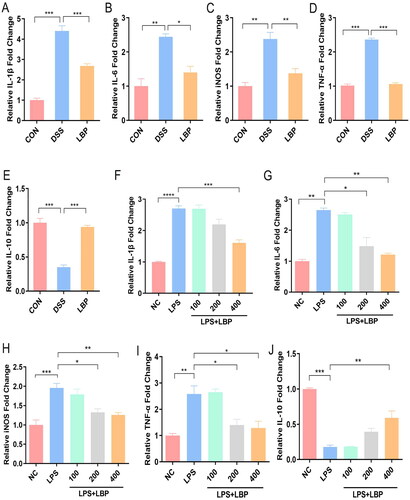
The overproduction of inflammatory cytokines, such as IL-1β, IL-6, TNF-α and iNOS, is known to play a key role in the pathogenesis of colitis [Citation22]. In this study, expressions of pro-inflammatory cytokines (IL-1β, IL-6, iNOS and TNF-α) in the distal colonic tissues were significantly increased in the DSS group compared with the CON group (p < 0.05), whereas LBP reversed these changes (p < 0.05) (). Anti-inflammatory cytokine IL-10 expression in the DSS group was significantly decreased (p < 0.05), but increased in the LBP group (p < 0.05) (). To further reveal the effect of LBP on the production of inflammatory factors, the expression levels of IL-1β, IL-6, iNOS, TNF-α and IL-10 were analyzed in Caco-2 cells treated with LPS or LBP (100, 200, and 400 μg/mL) +LPS for 24 h. LPS increased the expressions of IL-1β, IL-6, iNOS and TNF-α, but decreased the expressions of IL-10(p < 0.05). However, expressions of IL-1β, IL-6, iNOS and TNF-α in Caco-2 cells were significantly downregulated in a dose-dependent manner by LBP (LPS vs. LPS + LBP-400 μg/mL, p < 0.05) (). IL-10 expression was significantly upregulated in a dose-dependent manner (p < 0.05) (). Above results indicated that LBP can alleviate DSS-induced colitis via modulating anti-inflammatory and pro-inflammatory cytokines.
Figure 2. LBP inhibited the expression of pro-inflammatory factors and improved the expression of anti-inflammatory factors. (A-E) Levels of pro-inflammatory factors (IL-1β, IL-6, iNOS and TNF-α) and anti-inflammatory factors (IL-10) were measured by RTq-PCR in distal colon tissues from the DSS and LBP groups. (F-J) Levels of pro-inflammatory factors (IL-1β, IL-6, iNOS and TNF-α) and anti-inflammatory factors (IL-10) were measured by RTq-PCR in Caco-2 cells that were treated with LPS (1 μg/mL) or LBP (100, 200, and 400 μg/mL) + LPS for 24 h. NC: negative control. Statistical analysis used unpaired t-test and one-way ANOVA (post hoc analysis: Tukey’s multiple comparison test). Data indicate the mean ± SEM. ns: p > 0.05, * p < 0.05, **p < 0.01, ***p < 0.001.
3.3. LBP regulated gut microbiota and ameliorated microflora dysbiosis of DSS-induced colitis in mice
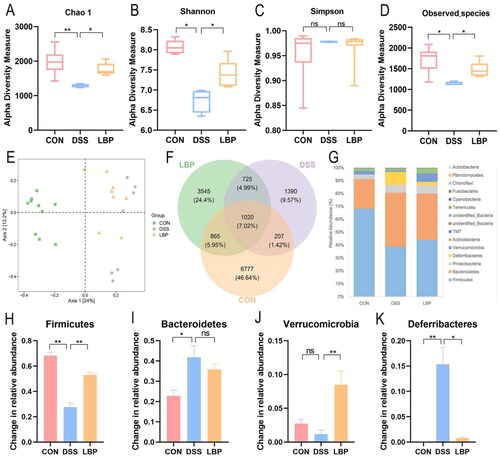
To investigate whether the gut microbiota is altered by LBP administration, we performed the gut microbial alpha diversity indices, including the Chao1, Shannon, Simpson, and observed species (OTUs). The results showed that the Chao1, Shannon, and OTUs were significantly decreased in DSS-treated mice compared to the CON group (p < 0.05), and these changes were reversed after LBP treatment (p < 0.05) (). Based on the weighted UniFrac distance, PCoA showed distinct clustering of microbiota composition for the CON, DSS and LBP groups, which indicated that DSS induced a significant change in the gut microbiota composition, but LBP shifted this change toward the CON group (). In addition, the OTU differences between the three groups are depicted in the Venn diagram, which showed that LBP obviously reshaped the gut microbiota (). Modification of the microbial community was quantified at the phylum level by 16S rRNA gene sequencing (). The three groups mainly consisted of Bacteroidetes and Firmicutes, which composed approximately 90% of the total bacterial community according to total relative abundance. Compared to the CON group, the abundance of Firmicutes was decreased (p < 0.05), while the abundances of Deferribacterias and Bacteroidetes were dramatically increased in DSS group (p < 0.05), and LBP treatment can significantly reversed the abundance changes of Firmicutes, Verrucomicrobia and Deferribacterias (p < 0.05) (). The abundance of Bacteroidetes showed no difference between DSS group and LBP group (p > 0.05) (). In conclusion, LBP ameliorated intestinal microflora dysbiosis in DSS-treated mice by significantly altering the gut microbiota diversity and composition.
Figure 3. LBP mitigated intestinal microflora dysbiosis in DSS-treated mice. (A-D) Alpha diversity boxplot (Chao1, Shannon, and Simpson indices and observed species). (E) Principal coordinate analysis (PCoA) using Bray-Curtis metric distances of beta diversity. (F) Venn graph of the OTUs from gut microbiota of CON (orange), DSS (purple), and LBP (green) groups. (G) Average percentage of community abundance at the phylum level in the CON, DSS and LBP groups. (H-K) Relative abundance of Firmicutes, Deferribacterias, Verrucomicrobia and Bacteroidetes in the CON, DSS and LBP groups. Statistical analysis used the Kruskal-Wallis test. Data indicate the mean ± SEM. ns: p > 0.05,*p < 0.05, **p < 0.01.
3.4. LBP showed a strong prebiotic effect and increased the abundance of SCFAs-producing bacteria
Alteration of the microbial community was measured at the genus level by determining the dominant genus (). Compared to the CON group, the relative abundance of Lactobacillus, Butyricicoccus and Akkermansia in the DSS group was decreased (p < 0.05), while the relative abundance of Sutterella and Mucispirillum significantly increased (p < 0.05), and these changes were reversed by LBP treatment (p < 0.05) (). Recent report indicated that intestinal inflammation may be alleviated by Akkermansia [Citation23]. Butyricicoccus, a butyrate-producing bacterium, was seen as the next generation of probiotics and a promising candidate for IBD treatment [Citation24]. Nevertheless, Mucispirillum and Sutterella may drive the development of colitis [Citation25,Citation26]. The LEfSe analysis revealed that LBP significantly impacted 21 kinds of bacteria and the top group increased by LBP feeding was Ruminococcaceae (LDA score = 4.3098, p < 0.05) (). Previous studies had confirmed that Ruminococcaceae families were classified in the Clostridium cluster XIVa, a major SCFAs-producing group [Citation27]. The above findings implied that LBP may play a prebiotic role by modulating abundances of intestinal beneficial and pathogenic bacteria, and increasing the production of SCFAs.
Figure 4. LBP treatment regulated the gut microbiota at the genus level. (A) The dominant genera were compared between the different groups. (B-F) Changes in the relative abundances of Lactobacillus, Butyricicoccus, Akkermansia, Mucispirillum and Sutterella. (G) LEfSe analysis of the gut microbiota differed among the three groups. The statistical test was performed using the LDA effect size method. Statistical analysis used the Kruskal-Wallis test. Data indicate the mean ± SEM. ns: p > 0.05, *p < 0.05, **p < 0.01.
3.5. LBP treatment increased the levels of microbial SCFAs metabolites
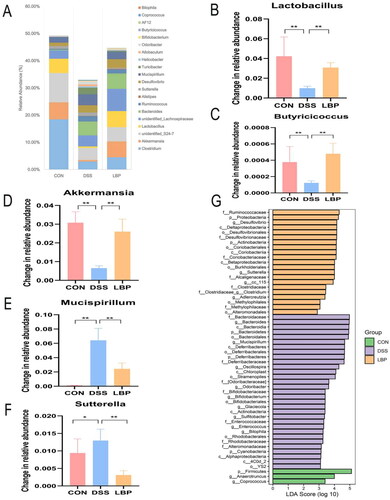
To further investigate whether LBP had an impact on microbial metabolic output, the SCFAs concentrations in cecal contents were assessed by GC-MS. The concentrations of certain microbial metabolites, such as SCFAs, have been shown to be reduced in IBD patients [Citation28]. Consistent with these changes, the DSS group showed a significant reduction in acetic acid (p < 0.05), propionic acid (p < 0.05), butyric acid (p < 0.05), and isobutyric acid (p < 0.05) levels compared to the control group (). Previous 16S rRNA sequencing analysis showed that the gut microbiota in the LBP group displayed a predominance of Ruminococcaceae, Lactobacillus and Butyricicoccus, which were associated with SCFAs metabolism. In accordance with the changes in microbial community structure and composition, the LBP group had higher amounts of acetic acid (p < 0.05), propionic acid (p < 0.05), butyric acid (p < 0.05), and isobutyric acid (p < 0.05) in the faeces (). However, there were no significant differences in valeric acid, isovaleric acid or hexanoic acid levels (p > 0.05) (). These results suggested that LBP can enhance the production of beneficial microbial SCFAs metabolites.
Figure 5. LBP treatment increased the production of microbial SCFA metabolites. Concentration differences measured by GC-MS among the CON, DSS and LBP groups in fecal levels of (A) acetic acid, (B) propionic acid, (C) butyrate acid, (D) isobutyric acid, (E) valeric acid, (F) isovaleric acid and (G) hexanoic acid (boxplot). Statistical analysis used the Kruskal-Wallis test. ns: p > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
3.6. LBP treatment reduced intestinal permeability in vivo and in vitro
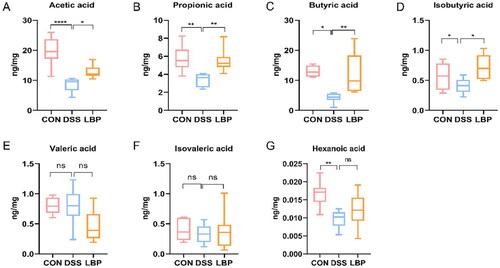
It is generally considered that intestinal mucosal barrier damage plays a major role in UC occurrence and development [Citation29]. Thus, to characterize the effect of LBP on gut barrier function, the intestinal permeability of the DSS and LBP groups was measured via the detection of FD4 in serum 3 h post FD4 gavage. The LBP group showed a smaller amount of FD4 permeated through the intestinal epithelium than the DSS group (p < 0.05) (). Moreover, consistent with this finding, FD4 flux to the Caco-2 monolayer (100% confluency) was measured after LBP treatment for 24 h. LBP decreased the amount of FD4 that permeated through the Caco-2 cell monolayer (p < 0.05) (). Previous research confirmed that the integrity of the gut barrier can also be assessed by measuring the F-actin filaments of the intestinal epithelium [Citation30]. Therefore, to investigate the effect of LBP on F-actin, the F-actin filaments of Caco-2 cells were labeled with phalloidin after treatment with 400 μg/mL LBP for 24 h. Compared with the NC cells, the Caco-2 cells treated with LBP displayed a more exaggerated and prolonged F-actin phenotype (). These findings indicated that LBP can reduce intestinal permeability in vivo and in vitro.
Figure 6. LBP treatment decreased intestinal permeability in vivo and Caco-2 cell monolayer permeability in vitro. (A) The gut barrier permeability of the DSS and LBP groups was measured by detection of FD4 in serum 3 h post FD4 gavage. (B) Effect of LBP on Caco-2 cell monolayer permeability to FD4. (C) The F-actin filaments of Caco-2 cells were labeled with Alexa Fluor 594-phalloidin after treatment with 400 μg/mL LBP for 24 h. Scale bar = 50 μm. NC: negative control. Statistical analysis was performed using one-way ANOVA (post hoc analysis: Tukey’s multiple comparison test). Data indicate the mean ± SEM. **p < 0.01, ****p < 0.0001.
3.7. LBP enhanced intestinal mucosal barrier function by regulating the expression of tight junctions
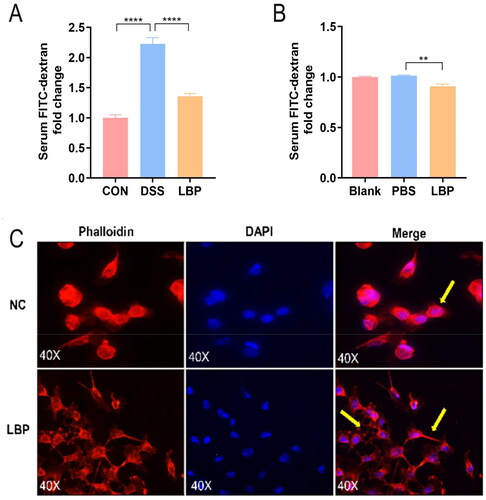
TJ proteins are involved in maintaining intestinal barrier function, and a decrease in its expression level can lead to barrier dysfunction and increase intestinal epithelial paracellular permeability [Citation31]. Indeed, Western blotting analysis showed that the expression levels of ZO-1 and Occludin in distal colonic mucosa were significantly increased in the LBP treatment group compared with the DSS group. In addition, the expression of Claudin-2 was significantly decreased in this group (p < 0.05) (). Further in vitro experiments demonstrated that LBP treatment increased the expression of ZO-1 and Occludin and decreased the expression of Claudin-2 in a concentration-dependent manner (p < 0.05) (). The above results indicated that LBP can enhance intestinal mucosal barrier function by regulating the expression of tight junctions.
Figure 7. LBP upregulated TJ protein expression in Caco-2 cells via activation of Nrf2. (A, B) Relative expression of TJ proteins (ZO-1, Occludin, and Claudin-2) was measured by Western blotting in the distal colon mucosa of the DSS and LBP groups. (C, D) Relative expression of TJ protein (ZO-1, Occludin, Claudin-2) were measured by Western blotting in Caco-2 cells after treatment with LBP 0, 100, 200, or 400 μg/mL for 24 h. (E, F) Caco-2 cells were pretreated with 20 μmol/L and 50 μmol/L ML385 (Nrf2 inhibitor) respectively for 1 h and then treated with 400 μg/mL LBP for 24 h. Relative expression of TJ proteins (ZO-1 and Occludin) and Nrf2 in Caco-2 cells was measured by Western blot. The gray value was calculated using ImageJ software. Statistical analysis was performed using one-way ANOVA (post hoc analysis: Tukey’s multiple comparison test). Data indicate the mean ± SEM. *p < 0.05, **p < 0.01.
Some reports have indicated that mice lacking Nrf2 are more susceptible to colitis than wild-type mice [Citation32]. Interestingly, LBP exerts protective effects against oxidative stress by up-regulating Nrf2/HO-1 signaling [Citation33]. In addition, Nrf2 was involved in enhancing gut barrier integrity via the upregulation of Occludin and Claudin-4 [Citation34]. To evaluate the role of Nrf2 in the regulation of TJ proteins by LBP, the Nrf2 inhibitor ML385 was added to Caco-2 cells before LBP treatment. The results showed that the expression of Nrf2 was significantly downregulated by ML385 (LBP vs. LBP + ML385-50 μM: p < 0.05) and that LBP-induced ZO-1 and Occludin expression was significantly repressed by ML385 (LBP vs. LBP + ML385-50 μM: p < 0.05) (). Overall, these results indicated that LBP may improve the expression of ZO-1 and Occludin via activating Nrf2 signalling pathway.
4. Discussion
UC has become a health problem of global concern, and its prevalence is on the rise [Citation35]. Conventional drugs for UC have undesirable side effects and cannot alter relapse episodes [Citation36]. Therefore, exploring novel natural drugs for IBD treatment is imperative. Previous researches have demonstrated that LBP can have a protective effect on DSS-induced chronic colitis [Citation37,Citation38]. However, the functional mechanisms of LBP in colitis remission remain largely unclear. This study showed that LBP can alleviate DSS-induced colitis by regulating the levels of inflammatory cytokines and promoting the expression of tight junction proteins. In addition, LBP could modulate the intestinal microbiota in UC mice by exerting a prebiotic effect. This finding adds to the evidence that LBP could be developed as an adjuvant drug or potential prebiotic for the prevention and treatment of UC.
The intestinal mucosal barrier dysfunction has been considered as one crucial factor driving IBD pathogenesis [Citation39]. Tight junction proteins, mainly including ZO-1, Occludin and Claudin-2, are key molecules that regulate the integrity of the intestinal epithelial barrier [Citation40]. Among them, ZO-1 and Occludin are crucial barrier protector and alterations in their expression and distribution result in direct impairment of intestinal epithelial mechanical barrier [Citation41]. Zhou et al. also found that LBP mitigated the intestinal barrier damage by up-regulating the expression of ZO-1 [Citation42]. Li et al. demonstrated that LBP treatment restored the protein expression of Occludin in TNF-α-evoked Caco-2 cell barrier dysfunction [Citation15]. Conversely, several studies have documented that excess Claudin-2, a tight junction protein part of the paracellular barrier, typically increased intestinal permeability through the pore pathway [Citation43,Citation44]. Moreover, Claudin-2 was overexpressed in the inflamed gut of patients with IBD, while ZO-1 and Occludin were down-regulated [Citation45,Citation46]. Similarly, our findings showed that under LBP treatment, the expression levels of ZO-1 and Occludin were up-regulated, while Claudin-2 expression was down-regulated. It might suggest a potential reparative and mitigating role of LBP in chronic UC.
Nrf2, a central regulator of cellular antioxidant stress and anti-inflammatory reactions, plays a potent role in the pathogenesis of UC [Citation47]. In addition, activation of the Nrf2 pathway could lead to the up-regulation of epithelial TJ proteins [Citation34,Citation48]. It was documented that LBP can relieve inflammatory kidney injury or acute lung injury, protect against neurotoxicity, and attenuate acute pancreatitis via Nrf2 pathway [Citation49–51]. The above evidence suggests that LBP may alleviate UC by regulating TJ proteins via the Nrf2 pathway. Indeed, our findings demonstrated that LBP could improve the expression of ZO-1 and Occludin via Nrf2 activation in Caco-2 cells. In addition, our results showed that LBP exerted a significant anti-inflammatory effect by enhancing anti-inflammatory cytokine (IL-10) production while down-regulating the proinflammatory cytokines (IL-1β, IL-6, iNOS and TNF-α). Collectively, LBP can alleviate DSS-induced chronic colitis by restoring epithelial barrier function and reducing intestinal inflammation.
Gut microbiota is commonly considered to be another key factor in UC [Citation52]. In this study, we also found that the anti-inflammatory effect of LBP is mainly mediated by the gut microbiota. At the family level, the abundance of butyrate-producing Ruminococcaceae was increased by LBP feeding, which was consistent with a report from Kang et al. who found that dietary Goji markedly increased Lachnospiraceae and Ruminococcaceae abundance in IL-10-deficient mice [Citation27]. At the genus level, LBP administration increased the emergence of some potential probiotic genera (e.g. Akkermansia, Lactobacillus, and Butyricicoccus) in mice with UC, which are butyrate-producing bacteria. Among them, Akkermansia (phylum Verrucomicrobia) has been widely accepted as a new generation of probiotics because of its potential anti-inflammatory properties [Citation53]. Conversely, a considerable decrease in Akkermansia abundance was generally observed in UC [Citation54]. Butyricicoccus is a butyrate-producing genus in clostridial cluster IV that shows reduced abundance in the stool of patients with UC, and many studies have confirmed that Butyricicoccus pullicaecorum could reduce intestinal inflammation [Citation55,Citation56]. In addition, Lactobacillus, a traditional probiotic, has been mixed with various symbiotic bacteria to treat UC patients [Citation57]. Besides, it was reported that LBP treatment could enhance the abundance of Lactobacillus in normal mice or in media [Citation58,Citation59]. In fact, Lactobacillus was reported to protect the intestinal mucosa of rats from enteropathy by preventing reduced expression of ZO-1 and Occludin [Citation60]. It has been reported that butyrade can effectively reverse intestinal barrier damage by increasing the expression of ZO-1 and Occludin and reducing the expression of Claudin-2, indicating that the butyrate-producing bacteria may exert barrier repair effects through butyric acid [Citation61,Citation62]. In other words, LBP may regulate the expression of TJ proteins by increasing the level of butyrate-producing bacteria and their-derived butyrate. In contrast, we found that LBP treatment significantly reduced the abundance of Mucispirillum and Sutterella, which are conditional pathogens associated with UC. Thus, LBP could be used as a potential prebiotic for UC treatment by boosting beneficial bacterial levels and reducing conditional pathogen levels.
Although the prebiotic effects of LBP are well researched, few reports have focused on the use of LBP as a ‘synbiotic’, which refers to a mixture of probiotics and LBP to make its prebiotic effect more effective and lasting. According to our findings, we can infer that combining LBP with Akkermansia, Lactobacillus or Butyricicoccus may generate a highly potent synbiotic for UC therapy. Therefore, further animal experiments and clinical studies should be undertaken to investigate the development of LBP synbiotics. In addition, to investigate the barrier regulation role of LBP, we only detect the expression of Nrf2 under LBP treatment in vitro. Thus, there is a lack of animal experiments to validate this preliminary result. It is necessary to conduct further experiments to explore the exact mechanisms by which LBP and its metabolites improve the intestinal barrier in future research.
4. Conclusion
In conclusion, we found that LBP alleviated DSS-induced chronic colitis mainly by promoting the expression of tight junction proteins to enhance intestinal barrier function. In addition, LBP showed a strong prebiotic effect: enhancing the abundance of probiotics (e.g. Ruminococcaceae, Lactobacillus, Butyricicoccus and Akkermansia), reducing the abundance of conditional pathogens (e.g. Mucispirillum and Sutterella) and increasing short-chain fatty acid (e.g. acetic acid, propionic acid, butyrate acid and isobutyric acid) production (). Thus, LBP could be developed as an adjuvant drug for the prevention and treatment of UC.
Authors contributions
Z-YL performed the experiments and drafted the paper; L-HL, H-JL,Y-QL, F-QZ, T-YS and Z-YL performed the experiments and analyzed the data; J-YZ, FG, J-NX and Q-YH contributed to the acquisition, analysis, and interpretation of data. D-SZ and H-HZ contributed to conception and design of the study. All authors contributed to manuscript revision, read, and approved the submitted version.
Ethics statement
Animal procedures in this work followed the Arrive guidelines. This project passed the experimental animal ethics review of the Institutional Animal Care and Use Committee of Beijing Friendship Hospital Affiliated to Capital Medical University (Permit Number 19-2022).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
We confirm that the data supporting the findings of this study are available within the article. The original data of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Dejban P, Rahimi N, Takzare N, et al. Biochemical and histopathological evidence for the beneficial effects of modafinil on the rat model of inflammatory bowel disease: involvement of nitric oxide pathway. Pharmacol Rep. 2020;72(1):1–17. doi: 10.1007/s43440-019-00054-5.
- Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390(10114):2769–2778. doi: 10.1016/S0140-6736(17)32448-0.
- Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015;12(12):720–727. doi: 10.1038/nrgastro.2015.150.
- Windsor JW, Kaplan GG. Evolving epidemiology of IBD. Curr Gastroenterol Rep. 2019;21(8):40. doi: 10.1007/s11894-019-0705-6.
- Shen B, Kochhar G, Navaneethan U, et al. Role of interventional inflammatory bowel disease in the era of biologic therapy: a position statement from the global interventional IBD group. Gastrointest Endosc. 2019;89(2):215–237. doi: 10.1016/j.gie.2018.09.045.
- Algieri F, Rodriguez-Nogales A, Rodriguez-Cabezas ME, et al. Botanical drugs as an emerging strategy in inflammatory bowel disease: a review. Mediators Inflamm. 2015;2015:179616–179614. doi: 10.1155/2015/179616.
- Ma ZF, Zhang H, Teh SS, et al. Goji berries as a potential natural antioxidant medicine: an insight into their molecular mechanisms of action. Oxid Med Cell Longev. 2019;2019:2437397–2437399. doi: 10.1155/2019/2437397.
- Cao C, Wang Z, Gong G, et al. Effects of lycium barbarum polysaccharides on immunity and metabolic syndrome associated with the modulation of gut microbiota: a review. Foods. 2022;11(20):3177. doi: 10.3390/foods11203177.
- Cao C, Wang L, Ai C, et al. Impact of lycium barbarum arabinogalactan on the fecal metabolome in a DSS-induced chronic colitis mouse model. Food Funct. 2022;13(16):8703–8716. doi: 10.1039/d2fo01283a.
- Chen YS, Lian YZ, Chen WC, et al. Lycium barbarum polysaccharides and capsaicin inhibit oxidative stress, inflammatory responses, and pain signaling in rats with dextran sulfate Sodium-Induced colitis. Int J Mol Sci. 2022;23(5):2423. doi: 10.3390/ijms23052423.
- Wang J, Gao H, Xie Y, et al. Lycium barbarum polysaccharide alleviates dextran sodium sulfate-induced inflammatory bowel disease by regulating M1/M2 macrophage polarization via the STAT1 and STAT6 pathways. Front Pharmacol. 2023;14:1044576. doi: 10.3389/fphar.2023.1044576.
- Xiao Z, Deng Q, Zhou W, et al. Immune activities of polysaccharides isolated from lycium barbarum L. What do we know so far? [Pharmacol Ther. 2022;229:107921. doi: 10.1016/j.pharmthera.2021.107921.
- Muller M, Hansmannel F, Arnone D, et al. Genomic and molecular alterations in human inflammatory bowel disease-associated colorectal cancer. United European Gastroenterol J. 2020;8(6):675–684. doi: 10.1177/2050640620919254.
- Lavelle A, Sokol H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2020;17(4):223–237. doi: 10.1038/s41575-019-0258-z.
- Li W, Gao M, Han T. Lycium barbarum polysaccharides ameliorate intestinal barrier dysfunction and inflammation through the MLCK-MLC signaling pathway in caco-2 cells. Food Funct. 2020;11(4):3741–3748. doi: 10.1039/d0fo00030b.
- Du L, Ha C. Epidemiology and pathogenesis of ulcerative colitis. Gastroenterol Clin North Am. 2020;49(4):643–654. doi: 10.1016/j.gtc.2020.07.005.
- Landy J, Ronde E, English N, et al. Tight junctions in inflammatory bowel diseases and inflammatory bowel disease associated colorectal cancer. World J Gastroenterol. 2016;22(11):3117–3126. doi: 10.3748/wjg.v22.i11.3117.
- Ren X, Zhu Y, Gamallat Y, et al. E. coli O124 K72 alters the intestinal barrier and the tight junctions proteins of Guinea pig intestine. Biomed Pharmacother. 2017;94:468–473. doi: 10.1016/j.biopha.2017.07.123.
- Wirtz S, Popp V, Kindermann M, et al. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat Protoc. 2017;12(7):1295–1309. doi: 10.1038/nprot.2017.044.
- Cho CW, Ahn S, Lim TG, et al. Cynanchum wilfordii polysaccharides suppress dextran sulfate Sodium-Induced acute colitis in mice and the production of inflammatory mediators from macrophages. Mediators Inflamm. 2017;2017:3859856–3859814. doi: 10.1155/2017/3859856.
- Dieleman LA, Palmen MJ, Akol H, et al. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114(3):385–391. doi: 10.1046/j.1365-2249.1998.00728.x.
- Pedersen J, Coskun M, Soendergaard C, et al. Inflammatory pathways of importance for management of inflammatory bowel disease. World J Gastroenterol. 2014;20(1):64–77. doi: 10.3748/wjg.v20.i1.64.
- Everard A, Belzer C, Geurts L, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110(22):9066–9071. doi: 10.1073/pnas.1219451110.
- Geirnaert A, Steyaert A, Eeckhaut V, et al. Butyricicoccus pullicaecorum, a butyrate producer with probiotic potential, is intrinsically tolerant to stomach and small intestine conditions. Anaerobe. 2014;30:70–74. doi: 10.1016/j.anaerobe.2014.08.010.
- Caruso R, Mathes T, Martens EC, et al. A specific gene-microbe interaction drives the development of Crohn’s disease-like colitis in mice. Sci Immunol. 2019;4(34):eaaw4341. doi: 10.1126/sciimmunol.aaw4341.
- Kaakoush NO. Sutterella species, IgA-degrading bacteria in ulcerative colitis. Trends Microbiol. 2020;28(7):519–522. doi: 10.1016/j.tim.2020.02.018.
- Kang Y, Yang G, Zhang S, et al. Goji berry modulates gut microbiota and alleviates colitis in IL-10-deficient mice. Mol Nutr Food Res. 2018;62(22):e1800535. doi: 10.1002/mnfr.201800535.
- Parada VD, De la Fuente MK, Landskron G, et al. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol. 2019;10:1486. doi: 10.3389/fimmu.2019.01486.
- Li C, Cai YY, Yan ZX. Brain-derived neurotrophic factor preserves intestinal mucosal barrier function and alters gut microbiota in mice. Kaohsiung J Med Sci. 2018;34(3):134–141. doi: 10.1016/j.kjms.2017.11.002.
- Borisova MA, Achasova KM, Morozova KN, et al. Mucin-2 knockout is a model of intercellular junction defects, mitochondrial damage and ATP depletion in the intestinal epithelium. Sci Rep. 2020;10(1):21135. doi: 10.1038/s41598-020-78141-4.
- Ding Y, Yan Y, Chen D, et al. Modulating effects of polysaccharides from the fruits of Lycium barbarum on the immune response and gut microbiota in cyclophosphamide-treated mice. Food Funct. 2019;10(6):3671–3683. doi: 10.1039/c9fo00638a.
- Khor TO, Huang MT, Kwon KH, et al. Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium-induced colitis. Cancer Res. 2006;66(24):11580–11584. doi: 10.1158/0008-5472.CAN-06-3562.
- Cao S, Du J, Hei Q. Lycium barbarum polysaccharide protects against neurotoxicity via the Nrf2-HO-1 pathway. Exp Ther Med. 2017;14(5):4919–4927. doi: 10.3892/etm.2017.5127.
- Singh R, Chandrashekharappa S, Bodduluri SR, et al. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat Commun. 2019;10(1):89. doi: 10.1038/s41467-018-07859-7.
- Kaplan GG, Windsor JW. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2021;18(1):56–66. doi: 10.1038/s41575-020-00360-x.
- Ng WK, Wong SH, Ng SC. Changing epidemiological trends of inflammatory bowel disease in Asia. Intest Res. 2016;14(2):111–119. doi: 10.5217/ir.2016.14.2.111.
- Philippe D, Brahmbhatt V, Foata F, et al. Anti-inflammatory effects of Lacto-Wolfberry in a mouse model of experimental colitis. World J Gastroenterol. 2012;18(38):5351–5359. doi: 10.3748/wjg.v18.i38.5351.
- Kang Y, Xue Y, Du M, et al. Preventive effects of goji berry on dextran-sulfate-sodium-induced colitis in mice. J Nutr Biochem. 2017;40:70–76. doi: 10.1016/j.jnutbio.2016.10.009.
- Rath T, Atreya R, Neurath MF. A spotlight on intestinal permeability and inflammatory bowel diseases. Expert Rev Gastroenterol Hepatol. 2023;17(9):893–902. doi: 10.1080/17474124.2023.2242772.
- Capaldo CT. Claudin barriers on the brink: how conflicting tissue and cellular priorities drive IBD pathogenesis. Int J Mol Sci. 2023;24(10):8562. doi: 10.3390/ijms24108562.
- Fizanne L, Villard A, Benabbou N, et al. Faeces-derived extracellular vesicles participate in the onset of barrier dysfunction leading to liver diseases. J Extracell Vesicles. 2023;12(2):e12303. doi: 10.1002/jev2.12303.
- Zhou W, Kan X, Chen G, et al. The polysaccharides from the fruits of Lycium barbarum L. modify the gut community profile and alleviate dextran sulfate sodium-induced colitis in mice. Int J Biol Macromol. 2022;222(Pt B):2244–2257. doi: 10.1016/j.ijbiomac.2022.10.015.
- Poplawska M, Dutta D, Jayaram M, et al. Genes modulating intestinal permeability and microbial community are dysregulated in sickle cell disease. Ann Hematol. 2022;101(5):1009–1013. doi: 10.1007/s00277-022-04794-y.
- Barrett KE. Claudin-2 pore causes leak that breaches the dam in intestinal inflammation. J Clin Invest. 2020;130(10):5100–5101. doi: 10.1172/JCI140528.
- Rankin CR, Lokhandwala ZA, Huang R, et al. Linear and circular CDKN2B-AS1 expression is associated with inflammatory bowel disease and participates in intestinal barrier formation. Life Sci. 2019;231:116571. doi: 10.1016/j.lfs.2019.116571.
- Woo JK, Choi S, Kang JH, et al. Fermented barley and soybean (BS) mixture enhances intestinal barrier function in dextran sulfate sodium (DSS)-induced colitis mouse model. BMC Complement Altern Med. 2016;16(1):498. doi: 10.1186/s12906-016-1479-0.
- Peng S, Shen L, Yu X, et al. The role of Nrf2 in the pathogenesis and treatment of ulcerative colitis. Front Immunol. 2023;14:1200111. doi: 10.3389/fimmu.2023.1200111.
- Liu Y, Bao Z, Xu X, et al. Extracellular Signal-Regulated kinase/nuclear factor-Erythroid2-like2/heme oxygenase-1 pathway-Mediated mitophagy alleviates traumatic brain injury-induced intestinal mucosa damage and epithelial barrier dysfunction. J Neurotrauma. 2017;34(13):2119–2131. doi: 10.1089/neu.2016.4764.
- Huang Y, Zhou F, Shen C, et al. LBP reduces the inflammatory injury of kidney in septic rat and regulates the Keap1-Nrf2∕ARE signaling pathway1. Acta Cir Bras. 2019;34(1):e631258493. doi: 10.1590/s0102-865020190010000003.
- Zheng G, Ren H, Li H, et al. Lycium barbarum polysaccharide reduces hyperoxic acute lung injury in mice through Nrf2 pathway. Biomed Pharmacother. 2019;111:733–739. doi: 10.1016/j.biopha.2018.12.073.
- Xiong GF, Li DW, Zheng MB, et al. The effects of Lycium barbarum polysaccharide (LBP) in a mouse model of Cerulein-Induced acute pancreatitis. Med Sci Monit. 2019;25:3880–3886. doi: 10.12659/MSM.913820.
- Khan I, Ullah N, Zha L, et al. Alteration of gut microbiota in inflammatory bowel disease (IBD): cause or consequence? IBD treatment targeting the gut microbiome. Pathogens. 2019;8(3):126. doi: 10.3390/pathogens8030126.
- Derrien M, Belzer C, de Vos WM. Akkermansia muciniphila and its role in regulating host functions. Microb Pathog. 2017;106:171–181. doi: 10.1016/j.micpath.2016.02.005.
- Zakerska-Banaszak O, Tomczak H, Gabryel M, et al. Dysbiosis of gut microbiota in polish patients with ulcerative colitis: a pilot study. Sci Rep. 2021;11(1):2166. doi: 10.1038/s41598-021-81628-3.
- Devriese S, Eeckhaut V, Geirnaert A, et al. Reduced mucosa-associated butyricicoccus activity in patients with ulcerative colitis correlates with aberrant claudin-1 expression. J Crohns Colitis. 2017;11(2):229–236. doi: 10.1093/ecco-jcc/jjw142.
- Steppe M, Van Nieuwerburgh F, Vercauteren G, et al. Safety assessment of the butyrate-producing Butyricicoccus pullicaecorum strain 25-3(T), a potential probiotic for patients with inflammatory bowel disease, based on oral toxicity tests and whole genome sequencing. Food Chem Toxicol. 2014;72:129–137. doi: 10.1016/j.fct.2014.06.024.
- Meijer BJ, Dieleman LA. Probiotics in the treatment of human inflammatory bowel diseases: update 2011. J Clin Gastroenterol. 2011;45 Suppl(Suppl):S139–S144. doi: 10.1097/MCG.0b013e31822103f7.
- Zhu W, Zhou S, Liu J, et al. Prebiotic, immuno-stimulating and gut microbiota-modulating effects of lycium barbarum polysaccharide. Biomed Pharmacother. 2020;121:109591. doi: 10.1016/j.biopha.2019.109591.
- Zhou F, Jiang X, Wang T, et al. Lyciumbarbarum polysaccharide (LBP): a novel prebiotics candidate for bifidobacterium and lactobacillus. Front Microbiol. 2018;9:1034. doi: 10.3389/fmicb.2018.01034.
- Orlando A, Linsalata M, Bianco G, et al. Lactobacillus rhamnosus GG protects the epithelial barrier of wistar rats from the pepsin-trypsin-digested gliadin (PTG)-induced enteropathy[J]. Nutrients. 2018;10(11):1698. doi: 10.3390/nu10111698.
- Wang R, Yang X, Liu J, et al. Gut microbiota regulates acute myeloid leukaemia via alteration of intestinal barrier function mediated by butyrate. Nat Commun. 2022;13(1):2522. doi: 10.1038/s41467-022-30240-8.
- Peng L, Li ZR, Green RS, et al. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in caco-2 cell monolayers. J Nutr. 2009;139(9):1619–1625. doi: 10.3945/jn.109.104638.