Abstract
Background
To investigate the application value of tumor abnormal protein in patients with type 2 diabetes mellitus complicated with lung adenocarcinoma in situ.
Materials and Methods
A total of 140 patients having type 2 diabetes mellitus complicated with lung adenocarcinoma in situ (Group A), 160 patients with type 2 diabetes mellitus (Group B), and 120 healthy controls (Group C) were enrolled in the Department of Thoracic Surgery of the First Affiliated Hospital of Soochow University from November 2021 to December 2022.
Results
The total cholesterol level was higher in Group A than in Group B (p < 0.05) and Group C (p < 0.01), and it was higher in Group B than in Group C (p < 0.01). The comparison results of cholesterol level were similar to those of tumor abnormal protein, low-density lipoprotein cholesterol, and glycosylated hemoglobin among the three groups. The triglyceride level was higher in Group A than in Group B and Group C (both p < 0.01). Group A had a higher level of high-density lipoprotein cholesterol than Group C (p < 0.01). The fasting plasma glucose level was higher in Group A than in Group B and Group C (both, p < 0.01). These findings indicated that tumor abnormal protein, glycosylated hemoglobin, high-density lipoprotein cholesterol, and fasting plasma glucose were independent factors for patients having type 2 diabetes mellitus complicated with lung adenocarcinoma in situ.
Conclusion
Therefore, detecting tumor abnormal protein levels may help diagnose lung adenocarcinoma in situ in patients with type 2 diabetes mellitus.
KEY MESSAGES
The study found that tumor abnormal protein, glycosylated hemoglobin, high-density lipoprotein cholesterol, and fasting plasma glucose were independent factors for patients having type 2 diabetes mellitus complicated with lung adenocarcinoma in situ. Detecting tumor abnormal protein levels may help diagnose lung adenocarcinoma in situ in patients with type 2 diabetes mellitus.
1. Introduction
Type 2 diabetes mellitus (T2DM) is a prevalent metabolic disorder. According to the 2021 data from the International Diabetes Federation, there were 140 million cases of T2DM in China, and the total number is projected to rise to 164 million in the next 10 years [Citation1]. According to the 2020 GLOBOCAN database, lung cancer is the most common tumor and the leading cause of cancer-related deaths in China, with 720,000 deaths resulting from it in 2020 [Citation2]. Notably, patients with diabetes are more likely to develop malignancies [Citation3]. Studies have shown that patients with diabetes have a 20%–25% higher risk of developing tumors than those without diabetes [Citation4]. In addition, this increase in T2DM cases may increase morbidity and mortality associated with lung cancer [Citation5]. However, there is a lack of sensitive biomarkers for the early diagnosis and screening of lung cancer, particularly for early-stage tumors like adenocarcinoma in situ (AIS).
Numerous carcinogenic factors can alter the glycan structure on the surface of normal cell membranes, leading to an increased number of N-glycan branches and the synthesis of many expression products, such as tumor abnormal protein (TAP) [Citation6]. Therefore, TAP is an aberrant glycoprotein generated during the growth of malignant tumors [Citation6]. It is present in various cancer types during abnormal cell proliferation [Citation6–8]. TAP is a suitable tumor marker as it can be detected in the peripheral blood at a certain concentration with a sensitivity over 80% [Citation9]. Moreover, previous research has confirmed a close relationship between TAP and several tumors, such as gastric cancer, bladder cancer, and esophageal squamous cell carcinoma [Citation9–11]. TAP was reportedly upregulated in these cancers and regarded as an essential biomarker for predicting tumor prognosis. However, there is no research on the association of TAP in patients having T2DM complicated with AIS. This study aims to investigate the clinical significance of TAP in patients having T2DM complicated with AIS.
2. Materials and methods
2.1. General data
A total of 140 patients having T2DM complicated by AIS presented to the Department of Thoracic Surgery of the First Affiliated Hospital of Soochow University from November 2021 to December 2022. Group A comprised the 140 patients having T2DM complicated by AIS (70 men and 70 women; age, 62–72 years). Group B comprised 160 patients with T2DM admitted to the Department of Endocrinology over the same time (80 men and 80 women; age range, 56–64 years). Group C comprised 120 healthy outpatients (60 men and 60 women; age range, 52–68 years) as normal controls recruited over the same period; all of them were confirmed as not having diabetes using the oral glucose tolerance test. Lung cancer patients were confirmed as having AIS by postoperative pathology and the lung adenocarcinoma staging criteria jointly proposed by the International Association for the Study of Lung Cancer, American Thoracic Society, and European Respiratory Society in 2011 [Citation12]. Patients were diagnosed as having T2DM according to the World Health Organization (1999) diagnostic criteria for diabetes [Citation13]. The exclusion criteria were as follows: Patients diagnosed with T2DM and AIS at the same time or after a diagnosis of AIS, patients with pulmonary metastasis caused by other diseases, and patients diagnosed as having type 1 diabetes, special types of diabetes, gestational diabetes, cardiovascular and cerebrovascular diseases, hypertension, autoimmune diseases, acute and chronic infections, severe liver and kidney diseases, or other endocrine diseases. The study was approved by the institutional review board of the First Affiliated Hospital of Soochow University (2021-268), all methods were carried out in accordance with relevant guidelines and regulations. The written informed consent to participate was obtained from each subject.
2.2. Methods
Fifteen milliliters of morning venous blood was collected from each subject after 12 h of fasting, of which 10 mL was submitted to the biochemical panel and 5 mL was sent for the TAP test. Hitachi 7600 automatic biochemical analyzer was used for the biochemistry test. The glycosylated hemoglobin (HbA1C) was measured by high-performance liquid chromatography using a TOSOH G8 glycosylated hemoglobin detector in Japan. The TAP test was performed using a coagulation test with the corresponding detection kit and an image analyzer provided by Zhejiang Ruisheng Medical Technology Co., Ltd.
2.3. Indicators
2.3.1. Biochemical parameters
The following were the biochemical parameters studied: total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), fasting plasma glucose (FPG), and HbA1C.
2.3.2. TAP
As for TAP, a condensate area of <121 μm2 indicated no significant condensate and negative TAP expression. A condensate area of 121–225 μm2 suggested small condensates and positive TAP expression. A condensate area of >225 μm2 suggested large condensates and positive TAP expression.
2.4. Statistical analysis
We used the Prism8 software (San Diego, CA, USA) for statistical analysis. Measurement data were expressed as mean ± standard deviation (x ± SD). The one-way analysis of variance was used for pairwise comparisons among the three groups, and the t-test was used for comparisons between the two groups. Pearson’s correlation analysis was performed to examine the relationship between TAP and other clinical parameters. The logistic regression model was applied to analyze the risk factors of T2DM complicated with AIS. A P value of <0.05 was considered statistically significant.
3. Results
3.1. Comparison of general data among the three groups
Gender and age distribution did not statistically significantly differ among the three groups (all, p > 0.05). One-way analysis of variance revealed the following findings. The TC level was higher in Group A than in Group B (p < 0.05) and Group C (p < 0.01), and it was higher in Group B than in Group C (p < 0.01). The TG level was higher in group A than in Group B and Group C (both p < 0.01). The HDL-C level was higher in Group A than in Group C (p < 0.01). The LDL-C level was higher in Group A than in Group C (p < 0.01), and it was higher in Group B than in Group C (p < 0.05). The FBG level was higher in Group A than in Group B and Group C (both p < 0.01). The HbA1C level was higher in Group A than in Group B and Group C (both, p < 0.01), and it was higher in Group B than in Group C (p < 0.01). The TAP level was higher in Group A than in Group B and Group C (both, p < 0.01), and it was higher in Group B than in Group C (p < 0.01). The positive rate of TAP was 100% in Group A, which was significantly higher than that in Group B (37.14%, p < 0.01; and ).
Table 1. General data of three groups (x ± SD).
Table 2. TAP levels in the three groups (x ± SD).
3.2. Correlation analysis on TAP and other clinical parameters
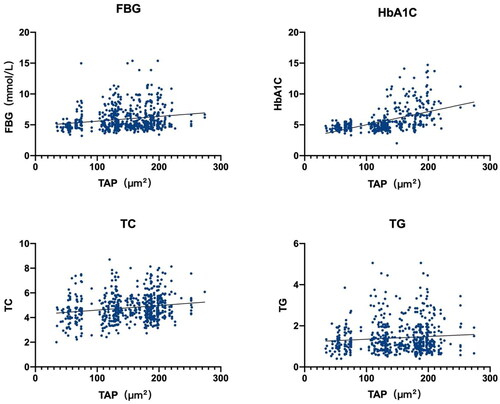
Pearson’s correlation analysis was performed using TAP as the dependent variable and sex, age, FPG, TC, TG, LDL-C, and HDL-C as independent variables. The findings revealed a positive correlation of TAP with FPG (r = 0.132, F = 90.58, p < 0.01), HbA1C (r = 0.062, F = 27.61, p < 0.01), TC (r = 0.048, F = 29.83, p < 0.01), and TG (r = 0.012, F = 6.98, p < 0.01; ).
3.3. Multivariate unconditional logistic regression analysis in patients having T2DM complicated with AIS
With the presence or absence of lung cancer as the dependent variable and TAP, FBG, HbA1C, TG, TC, LDL-C, and HDL-C as independent variables, the logistic regression analysis for binary data was carried out in Group A. The outcome indicated that TAP [odds ratio (OR) = 1.067, 95% confidence interval (CI) = 1.050–1.083, p < 0.01], HbA1C (OR = 1.520, 95% CI = 1.238–1.865, p < 0.01), HDL-C (OR = 1.520, 95% CI = 1.238–1.865, p = 0.024), and FPG (OR = 0.845, 95% CI = 0.714–0.999, p = 0.048) were independent variables for patients having T2DM complicated with AIS ().
Table 3. Logistic regression analysis of influencing factors of T2DM complicated with AIS.
4. Discussion
Lung AIS is difficult to diagnose. Current diagnostic methods include lung puncture and bronchoscopic biopsy, which are invasive and may cause pulmonary infection [Citation14]. Although tumor biomarkers like carcinoembryonic antigen, CYFRA 21-1, and NSE can help identify advanced-stage lung cancer, they cannot distinguish AIS from benign lung diseases [Citation15]. As mentioned above, TAP is formed during the clonal proliferation of tumor cells [Citation16]. It is a product of mutations in proto-oncogenes and tumor suppressor genes, which could be applied as an indicator for many tumors [Citation6,Citation7,Citation11].
Our investigation demonstrated that TAP was positive in patients having T2DM complicated with AIS and that the condensate area of TAP was significantly higher in these patients than in patients with T2DM and healthy controls. The structure of glycans covered on the cell membrane could change and circulate across the body to various sites during lung cancer development [Citation17], which may explain the presence and high expression of TAP in AIS patients. Interestingly, some patients with T2DM displayed higher TAP levels and larger condensate areas than healthy controls, which could possibly be due to the long-term and persistent hyperglycemia of patients with diabetes [Citation18]. The biochemical tests also revealed that patients having T2DM and those having T2DM complicated with AIS had elevated FPG and HA1C levels. Hyperglycemia could provide energy substrates for tumor growth. It would damage the normal structure of the capillary basement membrane, increase its thickness, decrease its permeability, and induce mitochondrial dysfunction and aerobic respiratory disorders [Citation19]. The consequent excessive production of anaerobic end-products and slow metabolic rate would result in abnormal cell division and carcinogenesis.
The findings indicated that HbA1C and HDL-C were independent risk factors for patients having T2DM complicated with AIS. The lipid parameters included in this research, including TC, TG, HDL-C, and LDL-C, were significantly elevated in patients with T2DM and those having T2DM complicated with AIS than in healthy controls. Several meta-analyses have confirmed that the high levels of lipid indicators were closely associated with an increased risk of T2DM, which may serve as complementary indicators with other biomarkers to predict the development of lung AIS [Citation20–22].
In the present study, the positive rate of TAP was 37.14% in patients with T2DM. Ma et al. reported a positive rate of TAP of 86.67% in patients with endometrial cancer [Citation23]. Zhang et al. reported a positive rate of TAP of 68.4% in gastric adenocarcinoma patients and noted that patients with advanced stages had higher expression of TAP [Citation24]. Xu et al. performed TAP detection in 87 cases with bladder cancer and discovered that 78.6% of the patients were positive for TAP [Citation10]. The positive rate of TAP was significantly lower in our study than in other reports. This discrepancy could be explained by AIS being an early-stage lung cancer with restricted tumor cell proliferation and lower levels of aberrant glycoproteins. Additionally, prior research has shown that malignant cells or healthy cells with a propensity to become cancerous had concomitant alterations in their glycan structures [Citation25], which may lead to aberrant glycoprotein and elevated TAP levels. Nevertheless, long-term follow-up is required to determine whether TAP-positive patients with T2DM can subsequently develop lung cancer.
Studies have found that the glycan structure of glycoproteins was significantly different between tumor cells and normal cells [Citation26]. The normal glycoproteins could undergo glycosylation under the stimulation of oncogenes, which could produce TAP and stimulate the clonal proliferation of tumor cells. The structural alterations of sugar chains on glycoproteins were closely related to tumor cell invasion and metastasis, which laid a theoretical basis for early cancer screening. TAP could be detected when the number of cancer cells exceeded 10,000. Moreover, the process by which the number of cancer cells increased from 10,000 to 100,000,000 took 1–3 years [Citation23]. This suggested that TAP could be detectable in the peripheral blood 1–3 years before mass formation, which may facilitate the early identification of the tumor.
The primary limitation of this research was that only age and a few biochemical parameters were included, and we did not consider other risk factors, such as smoking and drinking. Besides, 420 cases were incorporated into this retrospective study, which may not fully reflect the relationship of TAP with patients having T2DM complicated with AIS. Prospective studies with larger sample sizes are required to identify the role of TAP in patients having T2DM complicated with AIS.
5. Conclusion
Taken together, the study showed that TAP was an independent risk factor for patients having T2DM complicated with AIS. Moreover, TAP could easily help diagnose lung cancer following aberrant CT results in patients with T2DM because the detection was simple and only required a drop of blood. These findings suggested that TAP could serve as an early screening index in patients having T2DM complicated with AIS.
Authors’ contributions
YZ concepted, designed the study and revised the manuscript. KC and XZ analyzed the data and contributed to the discussion of the article. JH created the artwork. MW interpretation of the data. All authors have read and approved the final version of the manuscript.
Ethics approval and consent to participate
The study was approved by the institutional review board of the First Affiliated Hospital of Soochow University (2021-268), all methods were carried out in accordance with relevant guidelines and regulations. The written informed consent to participate was obtained from each subject.
| List of abbreviations | ||
| T2DM | = | Type 2 diabetes mellitus |
| AIS | = | adenocarcinoma in situ |
| TAP | = | tumor abnormal protein |
| HbA1C | = | glycosylated hemoglobin |
| TC | = | total cholesterol |
| TG | = | triglyceride |
| HDL-C | = | high-density lipoprotein cholesterol |
| LDL-C | = | low-density lipoprotein cholesterol |
| FPG | = | fasting plasma glucose |
| OR | = | odds ratio |
| CI | = | confidence interval. |
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The datasets are not publicly available due to restrictions used under the license for the current study. There are available on reasonable request from the corresponding author.
Additional information
Funding
References
- Ogurtsova K, Guariguata L, Barengo NC, et al. IDF diabetes atlas: global estimates of undiagnosed diabetes in adults for 2021. Diabetes Res Clin Pract. 2022;183:1. doi:10.1016/j.diabres.2021.109118.
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–6. doi:10.3322/caac.21660.
- Briana DD, Malamitsi-Puchner A. Galectin-3: an early marker of gestational diabetes, subclinical atherosclerosis, and tumor progression. Angiology. 2020;71(5):474–474. doi:10.1177/0003319719831870.
- Nam H, Hong SS, Jung KH, et al. A serum marker for early pancreatic cancer with a possible link to diabetes. J Natl Cancer Inst. 2022;114(2):228–234. doi:10.1093/jnci/djab191.
- Hong T, Qin N, Zhao X, et al. Investigation of causal effect of type 2 diabetes mellitus on lung cancer: a mendelian randomization study. Front Genet. 2021;12:673687. doi:10.3389/fgene.2021.673687.
- Liu Z, Cai J, Yu Y, et al. Tumor abnormal protein as a novel biomarker in papillary thyroid carcinoma. Clin Lab. 2017;63(3):479–485. doi:10.7754/Clin.Lab.2016.160903.
- Chen WX, Yang LG, Cheng L, et al. Tumor abnormal protein in the diagnosis of breast cancer in patients with a palpable mass. Ann Clin Lab Sci. 2019;49(3):297–301.
- Chen R, Jiang C, Zhu Q, et al. Combining the tumor abnormal protein test with tests for carcinoembryonic antigens, cancer antigen 15-3, and/or cancer antigen 125 significantly increased their diagnostic sensitivity for breast cancer. Medicine (Baltimore). 2020;99(29):e21231. doi:10.1097/MD.0000000000021231.
- Zhang M, Wang X, Wang Z. Early study of tumor abnormal protein in gastric adenocarcinoma. Onco Targets Ther. 2021;14:1719–1726. doi:10.2147/OTT.S297413.
- Zhang L, Guo X, Min Y, et al. Tumor abnormal protein (TAP) examination contributes to primary diagnosis of bladder cancer. Int J Clin Exp Med. 2015;8(10):18528–18532.
- Cheng Y, Fang Q, Chen Y, et al. High expression of tumor abnormal protein preoperatively predicts poor prognosis of patients with esophageal squamous cell carcinoma. Front Surg. 2021;8:609719. doi:10.3389/fsurg.2021.609719.
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC lung cancer staging project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2(8):706–714. doi:10.1097/JTO.0b013e31812f3c1a.
- The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20(7):1183–1197. doi:10.2337/diacare.20.7.1183.
- Navani N, Booth HL, Kocjan G, et al. Combination of endobronchial ultrasound-guided transbronchial needle aspiration with standard bronchoscopic techniques for the diagnosis of stage I and stage II pulmonary sarcoidosis. Respirology. 2011;16(3):467–472. doi:10.1111/j.1440-1843.2011.01933.x.
- Fiala O, Pesek M, Finek J, et al. Prognostic significance of serum tumor markers in patients with advanced-stage NSCLC treated with pemetrexed-based chemotherapy. Anticancer Res. 2016;36(1):461–466.
- Wu FT, Wu W, Li XT, et al. Tumor abnormal protein level predicts disease response and progression of diffuse large B-cell lymphoma in the rituximab era. Clin Lab. 2019. doi:10.7754/Clin.Lab.2019.190631.
- Cheng Y, Chen Y, Zang G, et al. Increased expression of TAP is predictive of poor prognosis in patients with Non-Small cell lung cancer. Cancer Manag Res. 2020;12:1941–1946. doi:10.2147/CMAR.S239593.
- Yi ZH, Luther Y, Xiong GH, et al. Association between diabetes mellitus and lung cancer: meta-analysis. Eur J Clin Invest. 2020;50(10):e13332.
- Shlomai G, Neel B, LeRoith D, et al. Type 2 diabetes mellitus and cancer: the role of pharmacotherapy. J Clin Oncol. 2016;34(35):4261–4269. doi:10.1200/JCO.2016.67.4044.
- Zhu XW, Deng FY, Lei SF. Meta-analysis of atherogenic index of plasma and other lipid parameters in relation to risk of type 2 diabetes mellitus. Prim Care Diabetes. 2015;9(1):60–67. doi:10.1016/j.pcd.2014.03.007.
- Yang T, Liu Y, Li L, et al. Correlation between the triglyceride-to-high-density lipoprotein cholesterol ratio and other unconventional lipid parameters with the risk of prediabetes and type 2 diabetes in patients with coronary heart disease: a RCSCD-TCM study in China. Cardiovasc Diabetol. 2022;21(1):93. doi:10.1186/s12933-022-01531-7.
- Naseri K, Saadati S, Yari Z, et al. Beneficial effects of probiotic and synbiotic supplementation on some cardiovascular risk factors among individuals with prediabetes and type 2 diabetes mellitus: a grade-assessed systematic review, meta-analysis, and meta-regression of randomized clinical trials. Pharmacol Res. 2022;182:106288. doi:10.1016/j.phrs.2022.106288.
- Ma A, Fan D, Yan F. A study of the application of TAP combined with transvaginal ultrasound in the diagnosis of early-stage endometrial cancer. Oncol Lett. 2018;16(4):5186–5190. doi:10.3892/ol.2018.9250.
- Wang HJ, Liu H, Lin YH, et al. MiR-32-5p knockdown inhibits epithelial to mesenchymal transition and renal fibrosis by targeting SMAD7 in diabetic nephropathy. Hum Exp Toxicol. 2021;40(4):587–595. doi:10.1177/0960327120952157.
- Li LX, Zhang B, Gong RZ. Insights into the role of tumor abnormal protein in early diagnosis of cancer: a prospective cohort study. Medicine (Baltimore). 2020;99(11):e19382. doi:10.1097/MD.0000000000019382.
- Khateeb J, Fuchs E, Khamaisi M. Diabetes and lung disease: a neglected relationship. Rev Diabet Stud. 2019;15(1):1–15. doi:10.1900/RDS.2019.15.1.