?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Introduction
This study aimed to investigate the association between cardiorespiratory fitness (CRF) and perioperative morbidity and long-term mortality in operable patients with early-stage non-small cell lung cancer (NSCLC).
Patients and Methods
This prospective study included consecutive patients with early-stage NSCLC who underwent presurgical cardiopulmonary exercise testing between November 2014 and December 2019 (registration number: ChiCTR2100048120). Logistic and Cox proportional hazards regression were applied to evaluate the correlation between CRF and perioperative complications and long-term mortality, respectively. Propensity score overlap weighting was used to adjust for the covariates. We performed sensitivity analyses to determine the stability of our results.
Results
A total of 895 patients were followed for a median of 40 months [interquartile range 25]. The median age of the patients was 59 years [range 26–83], and 62.5% were male. During the study period, 156 perioperative complications and 146 deaths were observed. Low CRF was associated with a higher risk of death (62.9 versus 33.6 per 1000 person-years; weighted incidence rate difference, 29.34 [95% CI, 0.32 to 58.36] per 1000 person-years) and perioperative morbidity (241.6 versus 141.9 per 1000 surgeries; weighted incidence rate difference, 99.72 [95% CI, 34.75 to 164.70] per 1000 surgeries). A CRF of ≤ 20 ml/kg/min was significantly associated with a high risk of long-term mortality (weighted hazard ratio, 1.98 [95% CI, 1.31 to 2.98], p < 0.001) and perioperative morbidity (weighted odds ratio, 1.93 [1.28 to 2.90], p = 0.002) compared to higher CRF.
Conclusion
The study found that low CRF is significantly associated with increased perioperative morbidity and long-term mortality in operable patients with early-stage NSCLC.
KEY MESSAGES
Low cardiorespiratory fitness is significantly associated with increased perioperative morbidity and long-term mortality in operable patients with early-stage non-small cell lung cancer.
Future research is recommended to investigate the potential prognostic role of integrating cardiorespiratory fitness into the currently used prognosis algorithm for patients with non-small cell lung cancer.
Introduction
Lung cancer is responsible for the highest cancer-related mortality worldwide, with five-year survival rates ranging from 26% to 64% [Citation1]. Surgical lung resection remains the standard treatment for early-stage non-small cell lung cancer (NSCLC), comprising of approximately 80–85% of lung cancer cases [Citation2]. This procedure, however, carries a relatively high risk of perioperative morbidity. The incidence rate is estimated 20% to 40% [Citation3]. Therefore, investigating non-invasive methods for identifying surgical candidates who are at a high risk of perioperative morbidity and worse long-term prognosis has great clinical significance.
Cardiorespiratory fitness (CRF) serves as an indicator of comprehensive physiological cardiorespiratory function [Citation4], and it finds broad applications across various clinical contexts [Citation5,Citation6]. The CRF directly measured by cardiopulmonary exercise testing (CPET), peak oxygen consumption ( O2peak), is recommended as a clinical vital sign by the American Heart Association [Citation7]. Despite the reported association between CRF and perioperative morbidity in several surgical procedures [Citation8], existing research linking CRF and perioperative morbidity in patients with NSCLC remains somewhat inconclusive, largely due to limited sample sizes in these studies [Citation9–13]. As a result, the use and interpretation of CRF for presurgical assessment in lung resection candidates remain debatable.
Although CRF has emerged as a robust predictor of all-cause cardiovascular disease and cancer mortality in apparently healthy adults over the last three decades [Citation14], only three studies with relatively small sample sizes have explored the relationship between CRF and mortality in patients with lung cancer, yielding inconclusive results [Citation15–17]. Two studies suggest that lower CRF could correspond to an increased risk of all-cause mortality [Citation15,Citation17], while one study found no significant association [Citation16]. Given these discrepancies, large-scale studies are necessary to further investigate the correlation between CRF and long-term all-cause mortality in patients with lung cancer. The results of such studies could provide clinicians with research evidence to help them determine whether CRF should be used to improve the management of patients with lung cancer.
This study aimed to investigate the association between directly measured CRF and perioperative morbidity and long-term mortality in operable patients with early-stage NSCLC using a relatively large sample size.
Methods
Study design and participants
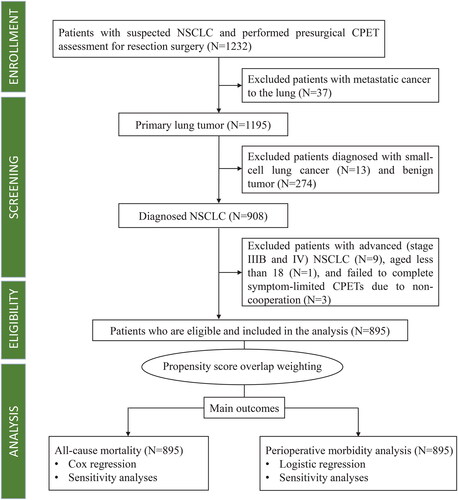
This prospective, observational study is part of the Xiangya Hospital Exercise Testing (X-ET) project [Citation18,Citation19]. Between November 1, 2014, and December 31, 2019, all suspected patients with NSCLC who underwent CPET one week before surgery were consecutively enrolled. The Multidisciplinary Tumour Board confirmed that the indications and contraindications for lung tumour resection surgery were consistent with current clinical practice guidelines [Citation20,Citation21]. We excluded patients with metastatic, advanced, and small-cell lung cancers, as well as benign tumours. Additionally, patients who were younger than 18 years or failed to complete a symptom-limited CPET were also excluded. A flowchart of patient inclusion and exclusion criteria is presented in . The Ethics Committee of the Xiangya Hospital Central South University approved this study (approval number 202010145). Because this was an observational study with no impact on patient management, the requirement for informed consent was waived. The study was registered in the Chinese Clinical Trial Registry (registry number: ChiCTR2100048120) and reported following the guideline of the Strengthening the Reporting of Observational Studies in Epidemiology.
Definition of exposed group
The O2peak, which is the gold standard measure of CRF, was determined by symptom-limited CPET using a cycle ergometer with a ramp protocol (CARDIOVIT system [Schiller Switzerland]). All CPETs were performed following the standard exercise testing procedures [Citation18,Citation19], which were adapted from the Exercise Standards for Testing and Training published by the American Heart Association [Citation22]. The optimal cut-off point of CRF for the outcomes was determined by the Receiver Operating Characteristics (ROC) curve and the Youden index [Citation23]. Patients with CRF values equal to or less than the cut-off points were assigned to the exposed group.
Perioperative management
All surgeries were performed by board-certified thoracic surgeons. The patients were managed by the same team of anaesthesiologists and surgeons. Following the surgery, patients underwent extubation in the surgical suite and managed in the post-anaesthesia recovery room for 24h. The patients were then transferred to the Department of Thoracic Surgery. Postoperative management was standardized, emphasizing early feeding, careful fluid balance, active mobilization, lung expansion exercises, and multimodal analgesia.
Outcomes
Primary outcome
The primary outcome of this study was all-cause mortality after lung surgery, and before the study was censored on December 31, 2021. We confirmed mortality during the follow-up period using three independent methods: (1) the residents’ registration office, (2) the electronic medical record system, and (3) contacting participants’ families.
Secondary outcomes
The secondary outcomes of the study were complications that occurred during hospitalization and within 30 days of discharge. These complications included respiratory, cardiovascular, technical, and a composite of all complications. Respiratory complications include atelectasis, respiratory failure, pneumonia, pulmonary embolism, and acute respiratory distress syndrome. Cardiovascular complications include cardiac arrhythmias requiring drug therapy, acute coronary syndrome, cardiac failure, and stroke. Technical complications include chylothorax, prolonged lung air leakage, blood loss and massive haemothorax requiring blood transfusion, and wound or chest infections.
Covariates
At enrollment, we collected data on biological sex, age, smoking history, body mass index (BMI), medical history, and CPET parameters. Data on tumour histology, clinical stage, and type of lung resection were obtained after surgery using the electronic medical record system. We cross-checked all data for accuracy and anonymized patient information to maintain confidentiality.
Sample size
Typically, to ensure model accuracy, a minimum of ten equivalent deaths or perioperative complications is recommended for each adjusted covariate [Citation24]. However, we used the propensity score overlap weighting technique to adjust covariates, which involves weighting and combining all covariates into one regression covariate. Thus, the 146 deaths and 156 complications observed were adequate for developing regression models.
Statistical analysis
We conducted the Shapiro–Wilk test to evaluate the normality of continuous variables. Mean ± standard deviation was presented for normally distributed continuous variables while median (IQR) was presented for non-normally distributed variables. Categorical variables are reported as counts (percentages). Standardized mean difference (SMD) was used to measure the balance among individual covariates before and after propensity score weighting. Conventionally, an SMD less than 0.1 is deemed appropriate for balance [Citation25].
To balance the covariates and minimize the impact of extreme propensities, we used the overlap weighting method to establish a propensity score model with covariates [Citation26]. This method assigns weights based on the probability of an individual belonging to an alternate group [Citation26,Citation27].
The Kaplan-Meier method was used for time-to-event analyses and compared with the two-sided log-rank test. Cox proportional hazard models were fitted with weights derived from overlap weighting to assess the relationship between CRF and all-cause mortality. Additionally, crude and weighted incidence rates were computed as the mortality rate/1000 person-years [Citation27].
We used a logistic regression model with propensity score overlap weighting to investigate the relationship between CRF and perioperative morbidity. We estimated the crude and weighted odds ratios as well as the perioperative morbidity rate (per 1000 surgeries).
Bootstrapping was used to assess the overall performance of the regression models [Citation28,Citation29]. We generated 1000 bootstrap samples to estimate the C-index, calibration, and Brier scores. Brier scores were evaluated on a scale of 0 (perfect accuracy) to 1 (perfect inaccuracy) to assess overall performance [Citation30]. The calibration slope was used to assess consistency between observed and predicted hazards, with values close to 1 indicating good overall agreement [Citation30]. Discrimination abilities were assessed with the C-index, where values of 0.5 and 1 indicated no discrimination and the best discrimination, respectively [Citation30].
We conducted interaction tests for all measured confounders and used the E-value to evaluate the unmeasured confounders. The E-value is a statistical measure that determines the minimum association strength required for an unmeasured confounder to explain the observed relationship between exposure and outcome [Citation31]. We also conducted pre-specified subgroup analyses based on birth sex, age, BMI, and smoking history. Before subgroup model development, overlap weighting propensity scores were recreated. Due to the potential for type I errors resulting from multiple comparisons, subgroup analyses were considered exploratory.
All analyses were performed using the R (version 4.2.0) software. The pROC, PSweight, tableone, cobalt, survival, survminer, fmsb, rms, boot, and E-value packages were used. Statistical significance was set at p < 0.05 (two-sided). The code and appropriate references for the statistical analyses are publicly available on the GitHub repository at https://github.com/YSDun/CRF/blob/3166c9378b911dc8c99ffe66f9b9c856c941b1b9/Code.Rmd.
Results
Participant characteristics
A total of 1232 patients suspected of having NSCLC underwent presurgical CPETs between November 1, 2014 and December 31, 2019. After the screening process, 895 patients were eligible and included in the study, while 337 were excluded because of metastatic lung cancer (n = 37), small cell lung cancer (n = 13), benign tumours (n = 274), stage IIIB and IV cancer (n = 9), failure to complete symptom-limited CPETs (n = 3), and age ≤ 18 years (n = 1) (). The median age of the 895 participants was 59 years (IQR, 13 years), and the majority of the participants were males (62.5%). The characteristics of the CPET data are shown in Supplement eTable 1. The ROC analysis and Youden index identified CRF ≤20 ml/kg/min as an optimal cut-off value for prognosis in this study, as illustrated by the ROC curves in Supplement eFigure 2. Among the 895 participants, 234 (26.1%) had a CRF ≤20 ml/kg/min, while 661 (73.9%) had a CRF >20 ml/kg/min. The mean CRF was 22.9 ml/kg/min (SD, 4.5).
Females, older people, those with higher BMI, and those with a history of hypertension, diabetes mellitus, and coronary artery disease were more likely to have low CRF. Further details are presented in . The Love plot displaying the covariates balance before and after weighting is provided in Supplement eFigure 1.
Table 1. Demographic and clinical characteristics grouped by high and low CRF, before and after applying propensity score overlap weighting.
The association between CRF and all-cause mortality
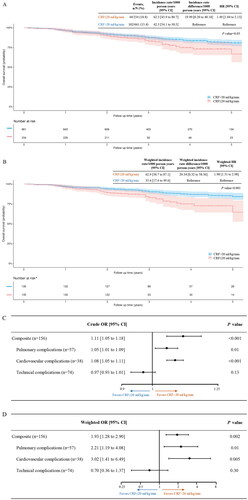
The median follow-up period was 40 months (IQR, 25 months). A total of 146 patients died during the study. After applying propensity score overlap weighting, the death rates per 1000 person-years were 62.9 in the low and 33.6 in the high CRF groups, respectively. The weighted incidence rate difference (IRD) between the two groups was 29.34 [95% CI, 0.32 to 58.36] per 1000 person-years. Participants with a CRF of ≤20 ml/kg/min had a 1.98 times higher risk of death than those with a CRF of >20 ml/kg/min (weighted HR, 1.98 [95% CI, 1.31 to 2.98]) ().
Figure 2. Kaplan–Meier survival curves (A–B) and Forest plots (C–D), before and after applying propensity score overlap weighting (N = 895). CRF, cardiorespiratory fitness; HR, hazard ratio; or, odds ratio. The shadow, along with the curves, represents the 95% confidence interval. *after propensity score overlap weighting, a single individual no longer represents a single data entity.
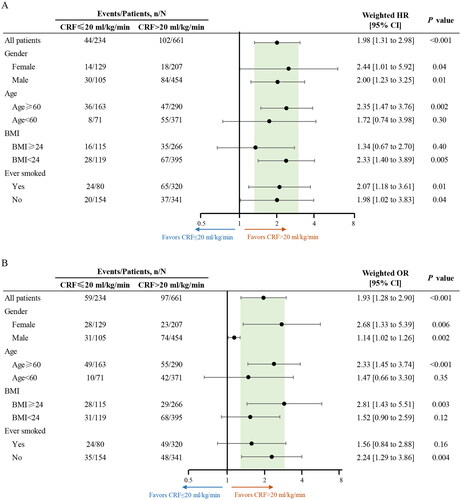
Several sensitivity analyses were conducted to evaluate the potential sources of bias that might impact the observed association between CRF and all-cause mortality. First, interaction tests were conducted to examine the role of the measured covariates. When testing for interactions, only age exhibited a significant interaction with the observed association between CRF and all-cause mortality (Supplement eTable 2). Pre-specified subgroup analyses were also conducted, revealing that the relationship between CRF and all-cause mortality remained statistically significant across the male, female, age ≥ 60 years, BMI < 24 kg/m2, ever smoked, and never smoked subgroups ().
Figure 3. Subgroup analyses on the association between measured CRF and all-cause mortality (A) and perioperative morbidity (B). BMI, body mass index; CRF, cardiorespiratory fitness; HR, hazard ratio; or, odds ratio.
Moreover, this study employed the E-value test to evaluate the potential for bias resulting from unmeasured confounders. The calculated E-value was 2.58, which exceeds the HRs of most recognized risk factors, such as male sex (HR = 1.29), for lung cancer prognosis as reported in previous literature [Citation32], indicating that the observed association between CRF and all-cause mortality is less likely to be explained by an unmeasured confounder.
In addition, we conducted internal validation using bootstrapping resampling to assess the robustness of the observed association. The analyses showed that the Brier score was 0.11 [95% CI, 0.10 to 0.12], the calibration slope was 0.85 [0.81 to 0.89], and the C-index was 0.62 [0.51 to 0.73]. These results indicate good overall performance of the regression model on the relationship between CRF and all-cause mortality [Citation30].
The association between CRF and perioperative morbidity
Among the 895 participants, 156 experienced a total of 169 perioperative complications, including 57 pulmonary complications, 38 cardiovascular complications, and 74 technical-related complications. There were 241.6 and 141.9 complications per 1000 surgeries among patients with low and high CRF, respectively (weighted IRD per 1000 surgeries, 99.72 [95% CI, 34.75 to 164.70]). Participants with a CRF of ≤20 ml/kg/min had a 1.93 times higher risk of perioperative complications than those with a CRF of >20 ml/kg/min, with a weighted OR of 1.93 [95% CI, 1.28 to 2.90] (). presents the incidence rates and corresponding ORs for pulmonary, cardiovascular, and technical-related complications.
Table 2. Association of measured CRF with all-cause mortality and perioperative morbidity in operable patients with early-stage NSCLC, after applying propensity score overlap weighting.
Coronary artery diseases and clinical stage exhibited significant interactions with the observed association between CRF and perioperative morbidity (Supplement eTable 2). In the subgroup analyses, the association between CRF and perioperative morbidity remained statistically significant across the male, female, age ≥ 60 years, BMI ≥ 24 kg/m2, and never smoked groups (). The calculated E-value was 2.12, which exceeded the HRs of the most recognized risk factors for perioperative morbidity in patients with NSCLC, indicating a lower likelihood of the observed association being explained by an unmeasured confounding variable. The results of internal validation analysis showed that the Brier score was 0.14 [95% CI, 0.13 to 0.16], the calibration slope was 0.78 [0.75 to 0.81], and the C-index value was 0.60 [0.53 to 0.67]. These findings indicate that the regression model for the association between CRF and perioperative morbidity demonstrated good overall performance [Citation30].
Discussion
This study investigated the association between CRF and perioperative morbidities and long-term mortality in a relatively large sample size of patients with early-stage NSCLC using propensity score overlap weighting. The results indicate a significant association between low CRF and both heightened perioperative morbidity and elevated long-term mortality rates in patients with early-stage NSCLC (eFigure 4).
While CRF is well-established as a robust predictor of cancer incidence [Citation33], cancer-related mortality [Citation34], and all-cause mortality in apparently healthy adults [Citation32], studies assessing the relationship between CRF and all-cause mortality in patients with lung cancer are scarce, with inconsistent results. For example, Jones et al. demonstrated that CRF significantly predicted survival in a study with 398 patients with NSCLC [Citation16], whereas Lindenmann et al. found no notable association between cancer-related death and CRF [Citation17]. Notably, Cundrle et al. [Citation35] demonstrated that CRF may not be the optimal predictor for CPET parameters related to cardiovascular complications in lung resection. This study, which features the largest sample size and longest follow-up period to date, supports the hypothesis that CRF is significantly associated with all-cause mortality in patients eligible for surgery with early-stage NSCLC. Further investigations are imperative to explore the prognostic implications of combining CRF with the other established CPET predictors [Citation36] to improve outcomes in NSCLC patients.
In our study, 146 out of 895 NSCLC patients passed away during a median follow-up period of 40 months (IQR, 25 months). It is noteworthy that the mortality rate might be slightly elevated compared to the latest statistics. This variation could be attributed to diverse cancer stages, age and general health of patients, the effectiveness of the treatment plan, or advancements in medical treatment techniques over the years.
The role of CRF in pre-surgical assessment of patients with NSCLC remains a contentious issue. While certain studies indicate that CRF as a valuable marker for predicting perioperative morbidity, others present conflicting results. From a systematic literature search, six out of fourteen studies revealed a significant association between low CRF and a heightened risk of perioperative complications in lung resection [Citation13,Citation37–41], yet the remaining eight did not show any such correlation [Citation9–12,Citation42–45]. Moreover, there exists a lack of consensus on the optimal cut-off value for CRF in NSCLC patients. While 20 ml/kg/min is commonly utilized as the cut-off in heart failure patients [Citation46], a study by Jones et al. [Citation16] proposed different peak VO2 cut-offs, specifically ‘<13.9 ml/kg/min, 14.0–17.3 ml/kg/min, and >17.4 ml/kg/min’ in NSCLC patients. In our study involving Chinese NSCLC patients (median age, 59 years) undergoing lung resection, we observed that a CRF of ≤20 ml/kg/min was associated with a higher rate of perioperative morbidity. Two factors may contribute to this disparity. Firstly, Jones et al. determined the cut-off through tertile split of all patient distributions, while we established the cut-off via ROC analysis and the Youden index. Additionally, the difference may be influenced by ethnicities, as our prior research has highlighted variations in the normal CRF range across different ethnic groups [Citation18,Citation47]. A future multicentre study that includes participants from multiple countries is warranted to determine an optimal CRF cut-off point for identifying patients with NSCLC at an increased risk of perioperative morbidity.
Although CRF has been linked to patient prognosis across various health conditions, the underlying mechanisms remain elusive. A recent study suggested that the mitochondrial oxygen affinity of skeletal muscles may be closely associated with CRF [Citation48]. Supporting this notion, our pre-clinical research demonstrated an association between mitochondrial function and the ability of mice to resist stress-induced myocardial [Citation49] and skeletal muscle damage [Citation50,Citation51]. Thus, we conducted a pilot experiment to evaluate the correlation between all-cause mortality and the expression of a mitochondrial volume biomarker, 2-oxoglutarate dehydrogenase E1 component (OGDH), using immunohistochemical staining with an OGDH antibody. The results showed that OGDH expression in both tumour and tumour-adjacent tissues in the death group was significantly lower than that in the survival group (Supplementary eFigure 3). These findings suggest that mitochondrial function may be one of the mechanisms underlying the relationship between CRF and disease prognosis. Future studies are needed to verify this hypothesis and explore other mechanisms underlying the association between low CRF and adverse events.
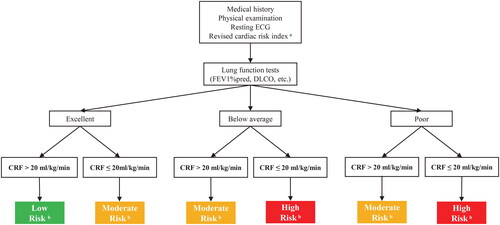
Clinical outcomes are widely acknowledged to be influenced by various factors in real-world settings. Previously established risk models, including The American College of Surgeons National Surgical Quality Improvement Program Surgical Risk Calculator [Citation52] and The Society of Thoracic Surgeons Adult Cardiac Surgery Risk Model [Citation53], has been validated their effectiveness in predicting risk. Aligning with current clinical guidelines for CRF usage in lung cancer patients, we propose an algorithm that incorporates CRF measurement as an additional parameter with a cut-off point of 20 ml/kg/min. This approach aims to assess perioperative morbidity risk and long-term prognosis in operable patients with early-stage NSCLC () [Citation54,Citation55]. Further investigations are essential to explore the prognostic significance of combining CRF measurement with other established and emerging predictors and risk models [Citation52,Citation53] to enhance outcomes in NSCLC patients.
Figure 4. A Suggested algorithm for using CRF in the assessment of perioperative morbidity risk and long-term mortality in operable patients with early-stage NSCLC. CRF, cardiorespiratory fitness; DLCO, diffusing capacity of the lungs for carbon monoxide; ECG, electrocardiograph; FEV1, forced expiratory volume in one second.
aRevised cardiac risk index: (1) high-risk surgery (including lobectomy or pneumonectomy), (2) ischaemic heart disease (prior myocardial infarction, angina pectoris), (3) heart failure, (4) insulin-dependent diabetes, (5) previous stroke of transient ischemic attack, and (6) creatinine ≥2 mg·dL−1.
bLow risk in green indicates excellent prognosis and a low risk of perioperative complications. Moderate risk in yellow and high risk in red suggest a progressively worse prognosis and higher a risk of perioperative complications. Patients who are classified as moderate- and high-risk warrant strong consideration of more aggressive medical management and surgical options.
Limitations
This study has several limitations that should be considered. First, despite using the propensity score overlap weighting technique and conducting multiple sensitivity analyses, the possibility of residual and unmeasured confounding factors could not be fully excluded. Second, although we provided information regarding clinical staging, which can affect the eligibility and accessibility of neoadjuvant radiotherapy and chemoradiotherapy, we did not specifically address these treatments. Third, caution should be exercised when interpreting the subgroup results because the number of events in some subgroups was limited.
Conclusions
Reduced CRF is significantly associated with perioperative morbidity and long-term all-cause mortality in operable patients with early-stage NSCLC. Future studies are recommended to investigate the potential prognostic role of integrating CRF into the currently used prognosis algorithm for patients with NSCLC eligible for surgery.
Author contributions
YSD and SXL were involved in the conception and design of the study. YSD, NC, SPW, SQF, JWR, NJZ, THZ, DZL, MC, YR and YD were involved in the analysis. YSD, NC, SPW and SXL were involved in the interpretation of the findings. YSD, NC, SPW and JWR were involved in drafting the manuscript. YSD, WYL, RJT, RWS and TPO were involved in revising it critically for intellectual content. All authors reviewed and approved the final manuscript and agree to be held accountable for all aspects of the work.
Funding statement
This work was funded by the National Natural Science Foundation of China [82272613 to Yaoshan Dun, 82172549 to Suixin Liu, and 82101407 to Yang Du], Natural Science Foundation of Hunan Province [2021JJ70073 to Suixin Liu and 2022JJ40777 to Yang Du].
Supplemental Material
Download MS Word (4.1 MB)Acknowledgments
We express our sincere appreciation to all patients who took part in this study, as their valuable contributions were essential to its success. Additionally, We extend our heartfelt gratitude to Ms. Ning Xu from the Department of Data Analytics and Application, Ping An Technology, Shanghai, China, for generously sharing her time and invaluable experiences. Her contributions were instrumental in ensuring the seamless execution of the statistical analysis for this study.
Disclosure statement of interest
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, SXL, upon reasonable request.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):1–12. doi: 10.3322/caac.21708.
- Duma N, Santana-Davila R, Molina JR. Non-small cell lung cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc. 2019;94(8):1623–1640. doi: 10.1016/j.mayocp.2019.01.013.
- Brioude G, Gust L, Thomas PA, et al. Postoperative complications after major lung resection. Rev Mal Respir. 2019;36(6):720–737. doi: 10.1016/j.rmr.2018.09.004.
- Albouaini K, Egred M, Alahmar A, et al. Cardiopulmonary exercise testing and its application. Postgrad Med J. 2007;83(985):675–682. doi: 10.1136/hrt.2007.121558.
- ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167:211–277.
- Radtke T, Crook S, Kaltsakas G, et al. ERS statement on standardisation of cardiopulmonary exercise testing in chronic lung diseases. Eur Respir Rev. 2019;28(154):180101. doi: 10.1183/16000617.0101-2018.
- Ross R, Blair SN, Arena R, et al. Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American heart association. Circulation. 2016;134(24):e653–e99. doi: 10.1161/CIR.0000000000000461.
- Guazzi M, Arena R, Halle M, et al. 2016 Focused update: clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Eur Heart J. 2018;39(14):1144–1161. doi: 10.1093/eurheartj/ehw180.
- Begum S, Papagiannopoulos K, Falcoz P, et al. Outcome after video-assisted thoracoscopic surgery and open pulmonary lobectomy in patients with low VO2 max: a case-matched analysis from the ESTS database. Eur J Cardiothorac Surg. 2016;49(4):1054–1058; discussion 1058. doi: 10.1093/ejcts/ezv378.
- Miyazaki T, Callister MEJ, Franks K, et al. Minute ventilation-to-carbon dioxide slope is associated with postoperative survival after anatomical lung resection. Lung Cancer. 2018;125:218–222. doi: 10.1016/j.lungcan.2018.10.003.
- Rodrigues F, Grafino M, Faria I, et al. Surgical risk evaluation of lung cancer in COPD patients - a cohort observational study. Rev Port Pneumol (2006). 2016;22(5):266–272. doi: 10.1016/j.rppnen.2016.02.010.
- Mao YS, He J, Yan SP, et al. Cardiopulmonary exercise testing in the evaluation of high risk patients with lung cancer. Chin Med J. 2010;123:3089–3094.
- Bobbio A, Chetta A, Internullo E, et al. Exercise capacity assessment in patients undergoing lung resection. Eur J Cardiothorac Surg. 2009;35(3):419–422. doi: 10.1016/j.ejcts.2008.11.004.
- Imboden MT, Harber MP, Whaley MH, et al. Cardiorespiratory fitness and mortality in healthy men and women. J Am Coll Cardiol. 2018;72(19):2283–2292. doi: 10.1016/j.jacc.2018.08.2166.
- Brunelli A, Pompili C, Salati M, et al. Preoperative maximum oxygen consumption is associated with prognosis after pulmonary resection in stage I non-small cell lung cancer. Ann Thorac Surg. 2014;98(1):238–242. doi: 10.1016/j.athoracsur.2014.04.029.
- Jones LW, Watson D, Herndon Ii JE, et al. Peak oxygen consumption and long-term all-cause mortality in nonsmall cell lung cancer. Cancer. 2010;116(20):4825–4832. doi: 10.1002/cncr.25396.
- Lindenmann J, Fink-Neuboeck N, Fediuk M, et al. Preoperative peak oxygen consumption: a predictor of survival in resected lung cancer. Cancers (Basel). 2020;12(4):836. doi: 10.3390/cancers12040836.
- Dun Y, Olson TP, Li C, et al. Characteristics and reference values for cardiopulmonary exercise testing in the adult chinese population - the xiangya hospital exercise testing project (the X-ET project). Int J Cardiol. 2021;332:15–21. doi: 10.1016/j.ijcard.2021.03.013.
- Dun Y, Olson TP, Ripley-Gonzalez JW, et al. Safety of exercise testing in the clinical chinese population. Front Cardiovasc Med. 2021;8:638682. doi: 10.3389/fcvm.2021.638682.
- Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e93S–e120S. doi: 10.1378/chest.12-2351.
- Brunelli A, Kim AW, Berger KI, et al. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: diagnosis and management of lung cancer, 3rd ed: american college of chest physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e166S–ee90S. doi: 10.1378/chest.12-2395.
- Fletcher GF, Ades PA, Kligfield P,., et al. Exercise standards for testing and training: a scientific statement from the American heart association. Circulation. 2013;128(8):873–934. doi: 10.1161/CIR.0b013e31829b5b44.
- Fluss R, Faraggi D, Reiser B. Estimation of the Youden index and its associated cutoff point. Biom J. 2005;47(4):458–472. doi: 10.1002/bimj.200410135.
- Peduzzi P, Concato J, Feinstein AR, et al. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol. 1995;48(12):1503–1510. doi: 10.1016/0895-4356(95)00048-8.
- Normand ST, Landrum MB, Guadagnoli E, et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54(4):387–398. doi: 10.1016/s0895-4356(00)00321-8.
- Thomas LE, Li F, Pencina MJ. Overlap weighting: a propensity score method that mimics attributes of a randomized clinical trial. JAMA. 2020;323(23):2417–2418. doi: 10.1001/jama.2020.7819.
- Orkaby AR, Driver JA, Ho YL, et al. Association of statin use with all-cause and cardiovascular mortality in us veterans 75 years and older. JAMA. 2020;324(1):68–78. doi: 10.1001/jama.2020.7848.
- Steyerberg EW, Harrell FE, Jr., Borsboom GJ, et al. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54(8):774–781. doi: 10.1016/s0895-4356(01)00341-9.
- Sauerbrei W, Schumacher M. A bootstrap resampling procedure for model building: application to the cox regression model. Stat Med. 1992;11(16):2093–2109. doi: 10.1002/sim.4780111607.
- Steyerberg EW, Vickers AJ, Cook NR, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology. 2010;21(1):128–138. doi: 10.1097/EDE.0b013e3181c30fb2.
- Haneuse S, VanderWeele TJ, Arterburn D. Using the E-value to assess the potential effect of unmeasured confounding in observational studies. JAMA. 2019;321(6):602–603. doi: 10.1001/jama.2018.21554.
- Ochman B, Kiczmer P, Ziora P, et al. Incidence of concomitant neoplastic diseases, tumor characteristics, and the survival of patients with lung adenocarcinoma or squamous cell lung carcinoma in tobacco smokers and non-smokers-10-year retrospective single-Centre cohort study. Cancers (Basel). 2023;15(6):1896. doi: 10.3390/cancers15061896.
- Vainshelboim B, Lima RM, Kokkinos P, et al. Cardiorespiratory fitness, lung cancer incidence, and cancer mortality in male smokers. Am J Prev Med. 2019;57(5):659–666. doi: 10.1016/j.amepre.2019.04.020.
- Boidin M, Lip GY, Thijssen D. Role of cardiorespiratory fitness in cancer development: time for an update. Eur J Prev Cardiol. 2021;28(17):e14–e16. doi: 10.1177/2047487320935228.
- Mazur A, Brat K, Homolka P, et al. Ventilatory efficiency is superior to peak oxygen uptake for prediction of lung resection cardiovascular complications. PLoS One. 2022;17(8):e0272984. doi: 10.1371/journal.pone.0272984.
- Dun Y, Wu S, Cui N, et al. Prognostic role of minute ventilation/carbon dioxide production slope for perioperative morbidity and long-term survival in resectable patients with nonsmall-cell lung cancer: a prospective study using propensity score overlap weighting. Int J Surg. 2023;109(9):2650–2659. doi: 10.1097/JS9.0000000000000509.
- Bolliger CT, Jordan P, Solèr M, et al. Exercise capacity as a predictor of postoperative complications in lung resection candidates. Am J Respir Crit Care Med. 1995;151(5):1472–1480. doi: 10.1164/ajrccm.151.5.7735602.
- Brutsche MH, Spiliopoulos A, Bolliger CT, et al. Exercise capacity and extent of resection as predictors of surgical risk in lung cancer. Eur Respir J. 2000;15(5):828–832. doi: 10.1034/j.1399-3003.2000.15e03.x.
- Li Q, Cao M, Zhang G, et al. Evaluation of cardiopulmonary exercise test on predicting post-operative respiratory failure in patients with lung cancer. Chin J Lung Cancer. 2003;6:367–370.
- Licker M, Schnyder JM, Frey JG, et al. Impact of aerobic exercise capacity and procedure-related factors in lung cancer surgery. Eur Respir J. 2011;37(5):1189–1198. doi: 10.1183/09031936.00069910.
- Wang JS, Abboud RT, Evans KG, et al. Role of CO diffusing capacity during exercise in the preoperative evaluation for lung resection. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1435–1444. doi: 10.1164/ajrccm.162.4.2001117.
- Bayram A, Candan T, Gebitekin C. Preoperative maximal exercise oxygen consumption test predicts postoperative pulmonary morbidity following major lung resection. Respirology. 2007;12(4):505–510. doi: 10.1111/j.1440-1843.2007.01097.x.
- Bechard D, Wetstein L. Assessment of exercise oxygen consumption as preoperative criterion for lung resection. Ann Thorac Surg. 1987;44(4):344–349. doi: 10.1016/s0003-4975(10)63787-3.
- Markos J, Mullan BP, Hillman DR, et al. Preoperative assessment as a predictor of mortality and morbidity after lung resection. Am Rev Respir Dis. 1989;139(4):902–910. doi: 10.1164/ajrccm/139.4.902.
- Wang J, Olak J, Ultmann RE, et al. Assessment of pulmonary complications after lung resection. Ann Thorac Surg. 1999;67(5):1444–1447. doi: 10.1016/s0003-4975(99)00255-6.
- Mancini DM, Eisen H, Kussmaul W, et al. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991;83(3):778–786. doi: 10.1161/01.cir.83.3.778.
- Kaminsky LA, Arena R, Myers J, et al. Updated reference standards for cardiorespiratory fitness measured with cardiopulmonary exercise testing: data from the fitness registry and the importance of exercise national database (FRIEND). Mayo Clin Proc. 2022;97(2):285–293. doi: 10.1016/j.mayocp.2021.08.020.
- Larsen FJ, Schiffer TA, Zinner C, et al. Mitochondrial oxygen affinity increases after sprint interval training and is related to the improvement in peak oxygen uptake. Acta Physiol (Oxf). 2020;229:e13463.
- Xie M, Jiang L, Dun Y, et al. Trimetazidine combined with exercise improves exercise capacity and anti-fatal stress ability through enhancing mitochondrial quality control. Life Sci. 2019;224:157–168. doi: 10.1016/j.lfs.2019.03.027.
- Dun Y, Liu S, Zhang W, et al. Exercise combined with rhodiola sacra supplementation improves exercise capacity and ameliorates exhaustive exercise-induced muscle damage through enhancement of mitochondrial quality control. Oxid Med Cell Longev. 2017;2017:8024857–8024818. doi: 10.1155/2017/8024857.
- Jiang L, Shen X, Dun Y, et al. Exercise combined with trimetazidine improves anti-fatal stress capacity through enhancing autophagy and heat shock protein 70 of myocardium in mice. Int J Med Sci. 2021;18(7):1680–1686. doi: 10.7150/ijms.53899.
- Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg. 2013;217(5):833–842.e1-3. doi: 10.1016/j.jamcollsurg.2013.07.385.
- O’Brien SM, Feng L, He X, et al. The society of thoracic surgeons 2018 adult cardiac surgery risk models: part 2-statistical methods and results. Ann Thorac Surg. 2018;105(5):1419–1428. doi: 10.1016/j.athoracsur.2018.03.003.
- Brunelli A, Charloux A, Bolliger CT, et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy). Eur Respir J. 2009;34(1):17–41. doi: 10.1183/09031936.00184308.
- Ha D, Mazzone PJ, Ries AL, et al. The utility of exercise testing in patients with lung cancer. J Thorac Oncol. 2016;11(9):1397–1410. doi: 10.1016/j.jtho.2016.04.021.