Abstract
Objective
To evaluate the clinical outcomes of using the extra-uterine placental transfusion (EPT) approach in very preterm infants (VPIs, gestational age <32 weeks) and compare this to delayed cord clamping (DCC) after birth.
Methods
In this matched pairs study, we compared the clinical outcomes of the EPT group to those of the DCC group. EPT were performed in fifty-three VPIs, of whom 27 were singletons and 25 were twins. The singleton VPIs were matched for gestational age (±5 days) and delivery mode, and the twin VPIs were matched between each other with the first twin subjected to DCC and the second twin to EPT. Data on the infants were collected and analysed as an overall group. A twin subgroup consisting of DCC and EPT groups was also analysed separately. The primary study outcome was either death or major morbidities.
Results
In total, 100 infants were included (n = 50 EPT group, n = 50 DCC group). The gestational ages of the DCC and EPT groups were (29.16 ± 1.76) and (29.12 ± 1.84) weeks, respectively. There were no differences in either deaths or major morbidities and other clinical outcomes, including the resuscitation variables, haemoglobin levels and red blood cell transfusion, between the two groups. In twin subgroups (gestational age 29.05 ± 1.89 weeks), EPT was associated with a higher rate of necrotizing enterocolitis (NEC) when compared with DCC (odds ratio = 7 (95% CI, 1.06 to 56.89), p = 0.031).
Conclusions
In twin subgroups, the incidence of NEC was higher in the EPT group when compared to the DCC group and therefore based on an abundance of caution the use of EPT in very preterm twins is not recommended.
KEY MESSAGE
Extra-uterine placental transfusion (EPT) is an alternative new form of placental transfusion. It can alleviate the problem of delayed respiratory support during DCC. It can also be performed in some placental abruption cases.
EPT may lead to the same clinical outcome as DCC in singleton pregnancies but based on an abundance of caution it is not recommended for very preterm twins.
Introduction
The first minutes following delivery is a profound and critical period for very preterm infants (VPIs). These infants usually show significant cardiopulmonary failure that can be seen as they are separated from the placenta and the transition from foetal to neonatal breathing and circulation. Lung aeration, which triggers an increase in pulmonary blood flow, plays an important role in the transition from intra- to extra-uterine life. Placental transfusion facilitates the transfer of blood volume from the placenta to the newborn and can improve the venous return to the right heart as well as an increase in pulmonary blood flow, thus protecting the newborn from the transient low cardiac output. For VPIs with gestational age <32 weeks, placental transfusion can maintain cerebral blood flow and reduce severe intra-ventricular haemorrhage (IVH) [Citation1,Citation2]. In addition to placental transfusion, most VPIs require respiratory support at birth to adequately aerate their lungs. Non-invasive respiratory support in the form of nasal continuous positive airway pressure (nCPAP) is recommended in several international guidelines [Citation3,Citation4]. It is also clear that lung aeration and spontaneous inspiration are the most dominant factors which influence blood flow in the umbilical vessels after birth. Some studies assessed the role of respiratory support during DCC (including our research team) [Citation5,Citation6], which found no statistical clinical improvement in respiratory support during DCC.
Caesarean section may be associated with reductions in the possibility of death and of severe IVH in actively resuscitated VPIs, especially for infants < 28 weeks of gestation [Citation7,Citation8]. However, the blood volume has not been shown to be significantly increased by delayed cord clamping (DCC) at cesarean section when compared to vaginal delivery [Citation9]. Umbilical cord milking (UCM) may be a more efficient technique for improving the blood volume in premature infants delivered by caesarean delivery [Citation10]. However, a recent randomized clinical trial concluded that UCM may be related to a significantly higher rate of severe IVH for VPIs, especially at lower gestational ages [Citation11].
We wanted to find an alternative method to DCC apart from UCM. Kuehne and colleagues [Citation12] evaluated a novel extra-uterine placental transfusion (EPT) approach. In their retrospective analysis, they showed no negative effects of EPT on the clinical outcome of the infants. The EPT approach follows this procedure whereby infants born preterm are delivered with the placenta still attached to the infant via the umbilical cord. Then, placental transfusion is performed by holding the placenta above the baby’s heart while respiratory support is initiated simultaneously. In our institution, we performed delayed cord clamping (DCC) on VPIs as a standard procedure from 2018, and performed EPT from 2020.
The objective of this study was to compare the clinical consequences of two different placental transfusion approaches for use in VPIs, born < 32 weeks. We hypothesized that EPT in these infants would not be associated with any adverse effects.
Patients and methods
A retrospective matched pairs study was performed in infants <32 weeks of gestation. All VPIs (<32 weeks) receiving placental transfusion from January 2020 until June 2021 were included. EPT was a new clinical practice in our institution, with the purpose of improving the outcomes of these preterm infants. The local institutional Research Ethics Committee of the Chongqing Health Center for Women and Children(EC-20210309-1028) approved this new practice. A senior neonatologist would routinely communicate with the parents if a mother was at high risk of a preterm birth <32 weeks’ gestation (prenatal counselling). We explained the advantages and disadvantages of placental transfusion including EPT and DCC and obtained consent for placental transfusion and respiratory support in the delivery room during prenatal counselling. The consent obtained from patients is a routine procedure at the hospital. Parents had the option to choose their preferred method of placental transfusion and none of these were specifically for the purposes of the research. All the data were obtained from the medical records. No data were obtained directly from the parents or patients for the study. The ethical approval was obtained from Research Ethics Committee of the Chongqing Health Center for Women and Children for conducting the study and for accessing the data.
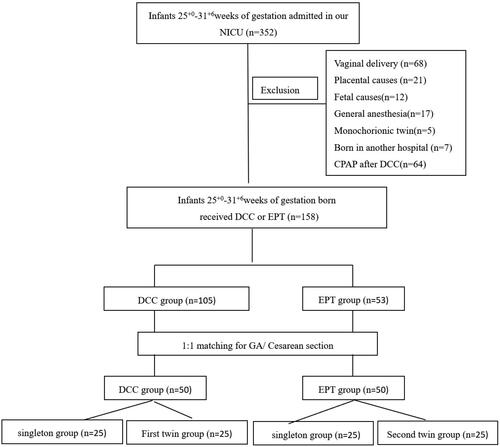
All infants <32 weeks were eligible for placental transfusion, unless they met one of the following exclusion criteria: use of general anaesthesia, monochorionic twins, severe maternal illness that prompted immediate delivery and either placental causes for delivery (such abruption or previa) or fetal causes (major congenital anomalies or hydrops fetalis). For single births, infants were retrospectively matched using IBM statistics SPSS version 22 (IBM Software, Chicago, Illinois, 60 USA, 2016) on a 1:1 basis using gestational age (±5days) and cesarean section as the comparators. For twin births, the infants were matched with each other and the first twin was subjected to DCC and the second twin to EPT.
After delivery, the infants in the DCC group were left attached to the placenta. They were covered with a pre-warmed and disinfected towel, while the neonatologist applied the nCPAP technique on the infant. The cord was clamped and cut within 60 s, and then the infant was transferred to the incubator (Giraffe Omnibed) to continue the transition support.
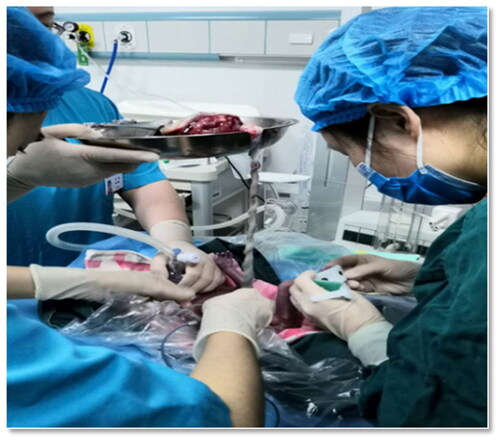
The infants in the EPT group were delivered with an intact amniotic sac, or if the membranes ruptured prior to or during caesarean section, only with the placenta and intact umbilical cord. When either the amniotic sac or the placenta was delivered, the infant was transferred to the incubator (Giraffe Omnibed) to initiate the transition support directly. The placenta was held approximately 20–30 cm above the baby, according to the length of the cord. Then nCPAP bi-nasal prongs or masks were placed simultaneously on the infant. The umbilical cord was then clamped when the umbilical vessels were observed to have collapsed ().
The respiratory support strategy used was the same for both the two groups. NCPAP support was given via bi-nasal prongs or masks, with initial nCPAP at 6 to 8 cm H2O and an initial inspired fraction of oxygen (FiO2) of 0.30 for babies < 28 weeks of gestation and 0.21–0.30 for those at 28–31 weeks. Two pulse oximeter probes were placed on the right hand and lower limbs of the infants. The FiO2 could be increased to 1.0 based on the 25th percentile of the Dawson criteria [Citation13]. If the infant was apnoeic, then positive pressure ventilation (PPV, with the peak inspiratory pressure set at 20 cm H2O and PEEP at 6 cm H2O) was provided.
When feasible, video recordings were obtained by fixed camera (GoPro, Cardiff, CA). All the recordings were reviewed by a researcher (WY) and if in doubt, another researcher (OJF or CGX) would review the recording again. Data collected included maternal demographics, obstetric complications, antenatal steroid and magnesium administration and postpartum haemorrhage in first 24 h. The gestational age (GA), birth weight, gender, Apgar scores at 1, 5 and 10 min, umbilical cord blood haemoglobin levels, mode of delivery were also collected to describe their baseline characteristics.
The primary study outcome was either death or major morbidities. These were initially defined as any of the following conditions: IVH on postnatal ultrasonography, retinopathy of prematurity requiring treatment, necrotizing enterocolitis (NEC) Bell’s stage greater than 2 stage [Citation14], early-onset (less than 72 h) or late-onset (more than 72 h) sepsis, bronchopulmonary dysplasia (BPD), spontaneous intestinal perforation (SIP), patent ductus arteriosus (PDA), polycythemia and pulmonary haemorrhage. Severe IVH was defined as graded 3 to 4 based on the criteria developed by Papile et al. [Citation15]. Diagnosis of BPD was made at 36 weeks post-menstrual age if there was any supplemental oxygen requirement.
The other study outcomes included the time of cord clamping, the use of PPV and intubation in the delivery room and the temperature on admission to the neonatal intensive care unit (NICU). Urine output over the first 24 h, haemoglobin/haematocrit (Hb/HCT) of umbilical cord, on admission (obtained in the NICU within first 1 h of life) and within 12–24 h, red blood cell (RBC) transfusion on the first 7 and 28 days of life and mechanical ventilation within the first 72 h were also collected. In addition, the heart rate (HR) and oxygen saturation (SpO2) were noted at 1, 2, 3, 5, 7 and 10 min after delivery.
The maternal and neonatal demographics were compared between the EPT and DCC groups.
The recordings comprised of pulse oximetric data (SpO2 levels) which were measured by using Masimo Radical pulse oximeters (Masimo Corporation, Irvine, CA, USA) that were placed on the right hand within the first 10 min of life and compared between the two cohorts. The records of HR were assessed by electrocardiograph (Philips, Japan) within the first 10 min. Before birth, these were detected by ECG and auscultation.
Statistical analysis
Categorical data are presented as n (%) and continuous variables are presented either as mean ± SD for normally distributed values or median (IQR) for non-normal data. The Shapiro–Wilk test was used to determine normality of the data. The comparisons were analysed using the McNemar test (for dichotomous variables), the paired sample t-test (for normally distributed continuous variables) and the related samples Wilcoxon Rank test (for non-normally distributed values). Conditional logistic regression was used to analyse the odds ratio (OR) for any possible complications of pregnancy.
A mixed model with a random intercept per individual and matched pairs was used to analyse the HR and SpO2 over a 10-min period. The indicator variable was added to the model which showed a linear trend. To compare HR and SpO2 over the time periods, these are reported at time points as medians (IQR). The differences between groups over the first 10 min periods were evaluated using the group covariate of the mixed model and these are reported as mixed model P-values. All analyses were conducted with SPSS (version 22). A P-value < 0.05 was considered statistically significant.
Results
During the study period, 352 VPIs were admitted into our unit. One hundred and fifty-eight infants were excluded, 68 were delivered vaginally, 55 either had their cords clamped immediately after birth or were subjected to UCM due to placental causes, foetal causes, general anaesthesia or being monochorionic twins, seven were born in another hospital and 64 were subjected to CPAP after DCC. Using the matching criteria, we were able to match 50 infants receiving DCC with 50 infants receiving EPT as shown in . In the EPT group, there were three cases of placental abruption and the placenta was delivered with the infants, so EPT was performed.
There were no significant differences in the maternal demographics (). Antenatal steroid administration (full dose), maternal magnesium therapy, incidence of obstetric complications and postpartum haemorrhage in first 24 h were similar for both groups.
Table 1. The maternal demographics of the subjects in this study.
The time of cord clamping was longer in the EPT group when compared to the DCC group. The placenta was held (33.06 ± 6.42) cm above the infant’s heart in the EPT group. There were no significant differences in neonatal demographics, including gestational age, birth weight and gender between the two groups. The Apgar scores at 1, 5 and 10 min, the incidence of PPV with either a mask or intubation in the delivery room (DR) and the incidence of mechanical ventilation within the first 72 h of delivery were also similar in both groups. The temperature at admission, the Hb/HCT of the umbilical cord on admission and in the first 12–24 h and the urine output over the first 24 h, did not differ significantly in the two groups ().
Table 2. The neonatal demographics and delivery room outcomes of the infants in this study.
The data for the neonatal primary and other outcomes of the infants in this study are shown in . There was no significant difference in neonatal outcomes between the two groups. However, in the twins group, EPT(the second twin) was associated with a significantly higher rate of NEC (greater than II stage) when compared with DCC (the first twin) (28% [7/25] vs 4% [1/25], respectively;[odds ratio = 7 (95% CI, 1.06 to 56.89));p = 0.031]. The rate of NEC (greater than II stage) was similar between the twin EPT group and the singleton EPT group (28% [7/25] vs 8% [2/25], p = 0.141). Although there was no significant difference in death and other outcomes between the two twin groups, three patients in the EPT group (the second twin) had a pulmonary hemorrhage, as compared to none of the infants in the DCC group (the first twin).
Table 3. The neonatal outcomes of the infants in this study.
There was no significant difference in HR and SpO2 in the first 10 min of delivery of all the infants ( and ; ). However, in the twin subgroup, the HRs of the infants in the EPT group [120 (100, 136) and 132 (103, 147)] were lower than in the DCC group [131(120, 146) and147 (133, 152)] at the second and third minutes after delivery, respectively. At the first, fifth, seventh and tenth minutes, the HRs did not show any significant differences between the two groups (; ). SpO2 levels did not differ at any time points during the first 10 min of life (p > 0.05 in all cases; ; ).
Figure 3. (A) Heart rate. All the presented data were based on all matched pairs n = 50. (B) Oxygen saturation. data of infants who were subjected to either DCC or EPT were calculated based on n = 12 at t = 1min, n = 34 at t = 2 min, n = 49 at t = 3 min, respectively. All the other presented data were based on n = 50. (C) Heart rate. The presented data were based on all the twins recruited, n = 25. (D) Oxygen saturation. The data on infants who were subjected to either DCC or EPT were calculated based on n = 2 at t = 1min, n = 11 at t = 2 min and n = 24 at t = 3 min, respectively. All the other presented data were based on n = 25.
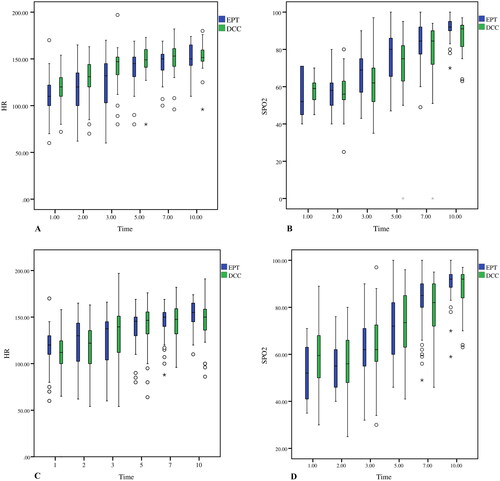
Table 4. The heart rate during transition of the infants.
Table 5. Oxygen saturation during transition of the infants.
Discussion
In this retrospective matched pairs study, we compared EPT with DCC among preterm infants of less than 32 weeks of gestation and there was no significant difference in the rate of death and the complications between the two groups. The delivery room (DR) outcomes included the Apgar scores, rate of PPV or intubation, admission temperature and rate of mechanical ventilation over the first 72 h and these were also similar between the two groups. The levels of Hb/HCT in the first 24 h of delivery in the EPT group were slightly higher than that in the DCC group, but did not differ significantly. There was no significant difference in HR and SpO2 in the first 10 min of birth in all the infants. However, in the twin subgroup, there was a significantly higher rate of NEC in the EPT group of the twin infants, the HRs of the infants in the EPT group were lower than in the DCC group. Therefore, EPT in VPIs of twin births was subsequently terminated in our hospital.
EPT was first described by Kuehne and his colleagues in 201812. The approach comprised of the simultaneous initiation of nCPAP support and placental transfusion. In addition, the infants were placed in an incubator and pulse oximeter probes were placed on the right hand and lower limbs. This was a more convenient way for us to evaluate the infants, and even perform resuscitation such as intubation or chest compression. In some clinical situations such as placental detachment when DCC was contra-indicated, EPT may be a preferred alternative. We have successfully performed EPT in 3 cases of placental detachment. In other cases, that involved the manual removal of the placenta in the EPT group, the increased maternal blood loss was a concern. However, in this study, there was no significant difference in the change of maternal blood loss between the two groups.
As reported by Kuehne [Citation12], although EPT had no significant negative effects on the outcome of the infants, there were five patients in the EPT group who had spontaneous intestinal perforations. This did not occur in any of the infants in the DCC/UCM group. In this study, the rate of spontaneous intestinal perforation was similar in both groups (both in the overall study and in the twin subgroup). However, there was a significantly higher rate of NEC stage 2 or higher in the EPT twin group (the second twin) in comparison to the DCC twin group (the first twin; 28 vs 4%, respectively). This was the major factor that led to the death of five babies in the EPT twin group compared to a single death in the DCC twin group.
In previous studies, there was a markedly higher incidence of NEC amongst twins and although this was different to our study, NEC was observed to develop universally in the first twins [Citation16,Citation17]. In another retrospective cohort study of 1377 twin pregnancies, no association between the birth order and the risk of death among infants born before 36 weeks of gestation was observed [Citation18]. We considered that the increased rate of NEC in the EPT group cannot be explained by the order of twins.
There is little information on either the safety or efficacy of placental transfusion in multiple pregnancies. The 2020 American College of Obstetricians and Gynaecologists (ACOG) Committee Opinion, No. 814, did not make a recommendation of either for or against DCC in twin pregnancies [Citation19]. The Italian recommendation for placental transfusion strategies suggested performing DCC in vaginal- and caesarean-delivered twin newborns for dichorionic, but not for monochorionic twins [Citation20]. A few studies focused on very preterm twins and compared DCC with immediate cord clamping (ICC). However, the incidence of NEC and other neonatal outcomes was not found to be significantly different in these VPIs [Citation21,Citation22].
There are some possible explanations for the adverse effects observed with EPT that may be related to different mechanisms, which occur during EPT and DCC. Before umbilical cord clamping, several factors including lung aeration (spontaneous inspirations or crying), uterine contractions, time of clamping and gravity will influence the umbilical venous blood flow [Citation23]. In this study, all the infants were delivered by caesarean section, so uterine contractions would have played only a minor role during placental transfusion. With regards to the Apgar score, the incidence of PPV with mask and the incidence of intubation were similar in both groups, and therefore lung aeration probably played a similar role in the two groups. In the EPT group in this study, the umbilical cord was clamped later than in the DCC group (244.36 ± 137.04 vs 63.00 ± 16.35 s).
Infants in the DCC group were usually placed on the mother’s abdomen or less than 5 cm below the placenta, and therefore, the effect of gravity would have been minimal, or it could even have been reversed [Citation24]. When the placenta was high enough and after lung aeration, there was a positive hydrostatic pressure which promoted the blood flow to the infant [Citation25]. As mentioned by Yao and Lind [Citation26], in the infants delivered vaginally, the hydrostatic pressure generated as a result of keeping the infant at 40 cm below the introitus, hastened placental transfusion to almost completion within approximately 30 s. In this study, the placenta was held at an average of 33 cm above the infant without uterine contractions in the EPT group. It is unclear whether the blood transfusion velocity from placenta was similar between this study that of Yao and Lind, although the twins included in this study were dichorionic twins and the two placentas were almost always fused. When performing EPT, we held the two placentas together above the second twin, which may have increased the positive hydrostatic pressure. It is likely that the velocity of placental transfusion during EPT was faster than that in the DCC group after lung aeration in the first 60 s.
Combining the effects of gravity and time, there was probably more blood transfer from the placenta to the infants before cord clamping in the EPT group when compared to the DCC group. Haemoglobin at 12–24 hours were higher in the EPT than in the DCC group (191.52 ± 25.86 vs 186.58 ± 28.41g/L). However, this finding was not statistically significant. The improved faster and greater placental transfusion may be associated with a higher incidence of PDA (68 vs 48%, respectively), although these findings were also not statistically significant. The resultant PDA may have led to a greater amount of left-to-right shunting which could have resulted in increased pulmonary blood flow an diminished gastrointestinal blood flow, which may have proceeded to either NEC or pulmonary haemorrhage.
The HR was lower in infants in the EPT twin group than that in the DCC twin group in the first 3 min of life, which was also the mean time of cord clamping in the EPT group (3 min and 29 s). Similar observations were also described in the preterm or term infants receiving DCC compared to ICC in other studies [Citation27,Citation28]. In the previous studies, the preterm infants were always left without respiratory support during DCC, and therefore, the lower HR observed may have been associated within adequate lung aeration. However, in this study, both of the two groups received respiratory support while undergoing either DCC or EPT. The infants’ HR decreased due to the increased venous return after EPT when compared to DCC in the twin subgroup. After 3 min, the HRs were found to be similar in both the groups.
Limitations of the study
Firstly, this study was a small sample retrospective trial and the data are reported from a single center, However, all our obstetricians and paediatricians received unified training before performing placental transfusion on VPIs in 2018. The decision for which placental transfusion strategies to use was at the discretion of the caregivers. We reported only on twins, and not triplets and higher-order multiple pregnancies, as we did not have any born during the DCC study period. The twins were assigned to the different groups according to the birth order. Secondly, the HR was assessed through auscultation before being detected by ECG, which may lead to an underestimation of the HR in the first few minutes of birth. Thirdly, we planned to measure the amount of blood transfusion in relation to the placental weight. However, the placental weight was affected by the movements of the infant and caregiver before the cord was clamped. Despite these limitations, we believe it is important to share our observations, in the practice of placental transfusion in preterm twin infants. Fourthly, there were numerous contributing factors for NEC, such as the feeding mode (breastfeeding or formula milk), the speed of increasing milk volume and use of antibiotics. Because of the high incidence of NEC is in the twin subgroup, we did not analyze these factors separately. When the new technique may be associated with an increase in adverse complications, we should conduct it with caution.
Conclusions
However, in the subgroup with twins, the incidence of NEC stage 2 or more was higher in the EPT group(the second twin) than in the DCC group. With an abundance of caution, we terminated the use of EPT in very preterm twins. A prospective study on twins may be needed to determine if there really is a risk from the procedure itself, and further studies are warranted to investigate the safety and efficacy of using EPT in singleton pregnancies.
Authors contributions
Yan Wu performed the data analyses and wrote the manuscript;
Xiaoyun Zhong contributed to the conception of the study;
JiangfengOu helped perform the analysis with constructive discussions.
Gongxue Chen contributed to data collection
Yefang Zhu contributed significantly to analysis and manuscript preparation;
All authors agree to be accountable for all aspects of the work.
Acknowledgements
The authors thank Professors Gerhard Jorch and Hui Ma for establishing the protocol for extra-uterine placental transfusion and guiding the writing of the manuscript. We also thank Dr Dev Sooranna of Imperial College London for editing the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets generated during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Fogarty M, Osborn DA, Askie L, et al. Delayed vs early umbilical cord clamping for preterm infants:a systematic review and meta-analysis. Am J Obstet Gynecol. 2018;218(1):1–11. doi: 10.1016/j.ajog.2017.10.231.
- Vesoulis ZA, Liao SM, Mathur AM. Delayed cord clamping is associated with improved dynamic cerebral autoregulation and decreased incidence of intraventricular hemorrhage in preterm infants. J Appl Physiol (1985). 2019;127(1):103–110. doi: 10.1152/japplphysiol.00049.2019.
- Wyckoff MH, Wyllie J, Aziz K, et al. Neonatal life support: 2020 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation. 2020;142(16_suppl_1): s 185–S221. doi: 10.1161/CIR.0000000000000895.
- Sweet DG, Carnielli V, Greisen G, et al. European consensus guidelines on the management of respiratory distress syndrome - 2019 update. Neonatology. 2019;115(4):432–450. Epub 2019 Apr 11. doi: 10.1159/000499361.
- Katheria A, Poeltler D, Durham J, et al. Neonatal resuscitation with an intact cord: a randomized clinical trial. J Pediatr. 2016;178:75–80.e3. doi: 10.1016/j.jpeds.2016.07.053.
- Deng R, Wu Y, Xiao G, et al. With or without nasal continuous positive airway pressure during delayed cord clamping in premature infants <32 weeks: a randomized controlled trial using an Intention-To-Treat analysis. Front Pediatr. 2022;10:843372. doi: 10.3389/fped.2022.843372.
- Grabovac M, Karim JN, Isayama T, et al. What is the safest mode of birth for extremely preterm breech singleton infants who are actively resuscitated? A systematic review and meta-analyses. BJOG. 2018;125(6):652–663. doi: 10.1111/1471-0528.14938.
- Jarde A, Feng YY, Viaje KA, et al. Vaginal birth vs caesarean section for extremely preterm vertex infants: a systematic review and meta-analyses. Arch Gynecol Obstet. 2020;301(2):447–458. doi: 10.1007/s00404-019-05417-0.
- Aladangady N, McHugh S, Aitchison TC, et al. Infants’ blood volume in a controlled trial of placental transfusion at preterm delivery. Pediatrics. 2006;117(1):93–98. doi: 10.1542/peds.2004-1773.
- Katheria AC, Truong G, Cousins L, et al. Umbilical cord milking versus delayed cord clamping in preterm infants. Pediatrics. 2015;136(1):61–69. doi: 10.1542/peds.2015-0368.
- Katheria A, Reister F, Essers J, et al. Association of umbilical cord milking vs delayed umbilical cord clamping with death or severe intraventricular hemorrhage among preterm infants. JAMA. 2019;322(19):1877–1886. doi: 10.1001/jama.2019.16004.
- Kuehne B, Kirchgaessner C, Becker I, et al. Mask continuous positive airway pressure therapy with simultaneous extrauterine placental transfusion for resuscitation of preterm infants- a preliminary study. Biomed Hub. 2018;3(2):1–10. doi: 10.1159/000488926.
- Dawson JA, Kamlin CO, Vento M, et al. Defining the reference range for oxygen saturation for infants after birth. Pediatrics. 2010;125(6):e1340-7–e1347. doi: 10.1542/peds.2009-1510.
- Bell MJ, Ternberg JL, Feigin RD, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187(1):1–7. doi: 10.1097/00000658-197801000-00001.
- Papile LA, Burstein J, Burstein R, et al. Incidence and evolution of Sub-ependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1500 g. J Pediatr. 1978;92(4):529–534. doi: 10.1016/s0022-3476(78)80282-0.
- Burjonrappa SC, Shea B, Goorah D. NEC in twin pregnancies: incidence and outcomes. J Neonatal Surg. 2014; 3(4):45.
- Samm M, Curtis-Cohen M, Keller M, et al. Necrotizing enterocolitis in infants of multiple gestation. Am J Dis Child. 1986;140(9):937–939. doi: 10.1001/archpedi.1986.02140230107045.
- Smith GC, Fleming KM, White IR. Birth order of twins and risk of perinatal death related to delivery in England, Northern Ireland, and Wales, 1994-2003: retrospective cohort study. BMJ. 2007; 334(7593):576. doi: 10.1136/bmj.39118.483819.55.
- Delayed umbilical cord clamping after birth: ACOG committee opinion, number 814. Obstet Gynecol. 2020;136(6):e100–e106. doi: 10.1097/AOG.0000000000004167.
- Ghirardello S, Di Tommaso M, Fiocchi S, et al. Italian recommendations for placental transfusion strategies. Front Pediatr. 2018;6:372. doi: 10.3389/fped.2018.00372.
- Chiruvolu A, Daoud Y, Inzer RW. Effect of delayed cord clamping on very preterm twins. Early Hum Dev. 2018;124:22–25. doi: 10.1016/j.earlhumdev.2018.08.002.
- Grabovac M, Beltempo M, Lodha A, et al. Impact of deferred cord clamping on mortality and severe neurological injury in twins born at <30 weeks of gestation. J Pediatr. 2021;238:118–123.e3. doi: 10.1016/j.jpeds.2021.07.058.
- Katheria AC, Lakshminrusimha S, Rabe H, et al. Placental transfusion: a review. J Perinatol. 2017;37(2):105–111. doi: 10.1038/jp.2016.151.
- Vain NE, Satragno DS, Gorenstein AN, et al. Effect of gravity on volume of placental transfusion: a multicentre, randomised, non-inferiority trial. Lancet. 2014;384(9939):235–240. doi: 10.1016/S0140-6736(14)60197-5.
- Hooper SB, Crossley KJ, Zahra VA, et al. Effect of body position and ventilation on umbilical artery and venous blood flows during delayed umbilical cord clamping in preterm lambs. Arch Dis Child Fetal Neonatal Ed. 2017;102(4):F312–F319. doi: 10.1136/archdischild-2016-311159.
- Yao AC, Lind J. Effect of gravity on placental transfusion. Lancet. 1969; 2(7619):505–508. doi: 10.1016/s0140-6736(69)90213-x.
- Pichler G, Baik N, Urlesberger B, et al. Cord clamping time in spontaneously breathing preterm neonates in the first minutes after birth: impact on cerebral oxygenation - a prospective observational study. J Matern Fetal Neonatal Med. 2016;29(10):1570–1572. doi: 10.3109/14767058.2015.1054275.
- Smit M, Dawson JA, Ganzeboom A, et al. Pulse oximetry in newborns with delayed cord clamping and immediate skin-to-skin contact. Arch Dis Child Fetal Neonatal Ed. 2014;99(4):F309–14. doi: 10.1136/archdischild-2013-305484.