Abstract
Background
Animal and human studies have shown that exposure to hypoxia can increase brain-derived neurotrophic factor (BDNF) protein transcription and reduce systematic inflammatory cytokine response. Therefore, the aim of this study was to investigate the acute and chronic effects of intermittent hypoxic-hyperoxic exposure (IHHE) prior to aerobic exercise on BDNF, interleukin-6 (IL-6), and C-reactive protein (CRP) blood levels in geriatric patients.
Patients and Methods
Twenty-five geriatric patients (83.1 ± 5.0 yrs, 71.1 ± 10.0 kg, 1.8 ± 0.9 m) participated in a placebo-controlled, single-blinded trial and were randomly assigned to either an intervention (IG) or control group (CG) performing an aerobic cycling training (17 sessions, 20 min·session−1, 3 sessions·week−1). Prior to aerobic cycling exercise, the IG was additionally exposed to IHHE for 30 min, whereas the CG received continuous normoxic air. Blood samples were taken immediately before (pre-exercise) and 10 min (post-exercise) after the first session as well as 48 h (post-training) after the last session to determine serum (BDNFS) and plasma BDNF (BDNFP), IL-6, and CRP levels. Intervention effects were analyzed using a 2 x 2 analysis of covariance with repeated measures. Results were interpreted based on effect sizes with a medium effect considered as meaningful (ηp2 ≥ 0.06, d ≥ 0.5).
Results
CRP was moderately higher (d = 0.51) in the CG compared to the IG at baseline. IHHE had no acute effect on BDNFS (ηp2 = 0.01), BDNFP (ηp2 < 0.01), BDNF serum/plasma-ratio (ηp2 < 0.01), IL-6 (ηp2 < 0.01), or CRP (ηp2 = 0.04). After the 6-week intervention, an interaction was found for BDNF serum/plasma-ratio (ηp2 = 0.06) but not for BDNFS (ηp2 = 0.04), BDNFP (ηp2 < 0.01), IL-6 (ηp2 < 0.01), or CRP (ηp2 < 0.01). BDNF serum/plasma-ratio increased from pre-exercise to post-training (d = 0.67) in the CG compared to the IG (d = 0.51). A main effect of time was found for BDNFP (ηp2 = 0.09) but not for BDNFS (ηp2 = 0.02). Within-group post-hoc analyses revealed a training-related reduction in BDNFP in the IG and CG by 46.1% (d = 0.73) and 24.7% (d = 0.57), respectively.
Conclusion
The addition of 30 min IHHE prior to 20 min aerobic cycling seems not to be effective to increase BDNFS and BDNFP or to reduce IL-6 and CRP levels in geriatric patients after a 6-week intervention.
The study was retrospectively registered at drks.de (DRKS-ID: DRKS00025130).
Introduction
Aging is associated with white and grey matter volume loss as well as a decline in white matter integrity [Citation1]. More specifically, it has been shown that the age-related shrinkage occurs in the medial temporal (i.e. hippocampus) [Citation2] and frontal lobes (i.e. prefrontal cortex) [Citation3]. These age-related neuroanatomical changes are assumed to be associated with the worsening in cognitive performance (e.g. intelligence, memory, processing speed, and executive functioning) [Citation4,Citation5]. In addition to changes in brain structure, a previous study using diffusion tensor imaging tractography showed that age-related changes in cognitive performance might also be mediated by declined integrity of the corpus callosum (i.e. central portion of the genu and splenium-partial fibers in the right hemisphere) [Citation6]. Noteworthy, in patients with neurodegenerative diseases (e.g. dementia), changes in brain structure and function are more pronounced compared to age-matched healthy controls [Citation7].
Among other factors, regular and continuous physical activity (e.g. strength and/or endurance training) can reduce the age-related structural and functional deteriorations [Citation8–11]. Brain-derived neurotrophic factor (BDNF) is considered one key neurotrophin responsible for the beneficial functional and/or structural brain adaptations induced by physical activity (e.g. brain volume and integrity), facilitating socioemotional (e.g. sleep quality and mood) and/or performance-related changes (e.g. memory and executive functioning) [Citation12–14]. In this process, the precursor protein proBDNF is cleaved to BDNF pro-peptide and mature BDNF (mBDNF). While proBDNF predominantly activates the p75-receptor and leads to apoptosis, mBDNF mainly binds to tropomyosin related kinase B (TrkB), which promotes essential processes such as neurogenesis, neuronal differentiation, synaptogenesis, neuronal survival, and long-term potentiation [Citation15,Citation16]. Nevertheless, it appears that there is no clear and strict separation between proBDNF/p75 and mBDNF/TrkB signaling pathways. Actually, it is possible that there are transitions between the two receptors and/or induced signaling pathways that complementarily and synergistically contribute to the formation and remodeling of synaptic spines [Citation14]. Therefore, activity-induced release of BDNF [Citation17] promotes neuroplasticity and thus regulates processes involved in learning and memory formation [Citation16,Citation18–22]. The majority of BDNF is released in the brain with high and usually lifetime expression in the hippocampus and neocortex [Citation23–26]. Since BDNF is assumed to cross the blood-brain barrier in both directions, peripheral BDNF concentration in venous blood, especially in serum (BDNFS) and plasma (BDNFP), is suggested as a suitable surrogate biomarker for brain BDNF concentration [Citation27,Citation28]. This is supported by studies in healthy young adults revealing a positive association between acute exercise-induced improvements in prefrontal- [Citation29] as well as hippocampal-dependent [Citation30] cognitive performance and increases in BDNFS.
However, BDNF blood concentration shows physiological (e.g. activity- and age-dependent) and pathophysiological (e.g. disease-dependent) fluctuations [Citation31,Citation32]. For instance, an exploratory mediation analysis showed that BDNFS, hippocampal volume, and spatial memory function were reduced with increasing age [Citation33]. In addition, BDNFS was found to mediate the age-related decline in hippocampal volume, which in turn promotes the age-related decline in spatial memory function [Citation33]. Further, studies have shown that BDNFP was lower in older people with pre-frailty [Citation34] and frailty [Citation35] compared to those without frailty. Diminished BDNF levels have also been found in patients with neurodegenerative or neuropsychiatric disorders/disease, such as Alzheimer’s disease [Citation36–38] (especially when severity of disease is progressed [Citation39]) and depression [Citation40]. In this context, BDNFS and BDNFP may serve as surrogate biomarkers for assessing the success of interventions to preserve or even improve brain structure and function.
Interestingly, animal studies have found that acute and chronic exposure to intermittent hypoxia increased BDNF protein and the BDNF messenger ribonucleic acid (mRNA) transcription level in various brain areas (e.g. pons, cerebral cortex, primary motor cortex, and brain endothelial cells) [Citation41–44]. Moreover, Ryou et al. [Citation41] showed that daily exposure to 10 cycles of intermittent hypoxia (6 min hypoxia (fraction of inspired oxygen (FiO2) = 0.10) interspersed by 4 min of normoxia) over a period of 21 days increased cerebrocortical erythropoietin (EPO) and BDNF formation in triple-transgenic mouse model of Alzheimer’s disease. These increments were associated with improved spatial learning and memory function. With regard to human studies, exposure to intermittent or prolonged hypoxia for 4 to 8 weeks (3 sessions·week−1) was shown to increase cognitive performance in healthy older adults [Citation45,Citation46] and patients with amnestic mild cognitive impairment [Citation47]. The neuroprotective effect of intermittent hypoxic exposure is thought to be related to the stabilization of hypoxia-inducible factor (HIF, especially HIF-1α) and the generation of reactive oxygen species (ROS) [Citation48,Citation49]. HIF-1α activates, among others, the transcription of genes responsible for angiogenesis [Citation50], vasodilation [Citation51,Citation52], glucose transport, and glucose metabolism [Citation53,Citation54]. In this regard, especially cardiovascular and metabolic adaptations to intermittent hypoxia are assumed to protect against neurodegeneration (e.g. by augmenting cerebral blood flow and vascularization and reducing cardiovascular risk factors) [Citation48,Citation55]. Based on the phenomenon of cross adaptations, ROS-initiated redox signaling induces anti-oxidative and anti-inflammatory genes by activating the transcription factor nuclear factor-erythroid 2-related factor 2 [Citation56,Citation57]. Moreover, exposure to normobaric hypoxia is a valuable strategy to enhance EPO production [Citation58–60]. Besides its function in regulating erythropoiesis [Citation61], EPO also induces anti-oxidative and anti-inflammatory effects [Citation62], which could be beneficial in the context of systemic chronic inflammation associated with a wide range of (age-associated) diseases, such as neurodegenerative disease [Citation48,Citation63,Citation64]. In this regard, Kiers et al. demonstrated that continuous exposure to normobaric hypoxia (3.5 h at an arterial oxygen saturation of 80–85%) enhanced adenosine release, resulting in an adenosine 2B receptor-dependent increase in anti-inflammatory markers (i.e. interleukin (IL)-10) blood concentration and subsequent dampening of endotoxin-induced systematic inflammatory cytokine response (i.e. tumor necrosis factor α (TNFα), IL-6, and IL-8) [Citation65].
A previous study examining BDNF secretion from mouse brain microvascular endothelial cells found that both prolonged and intermittent hypoxia stimulated BDNF expression, with the latter being more potent, possibly mediated by increased ROS formation [Citation44]. Since ROS formation occurs during reoxygenation, it is supposed that replacing normoxia by moderate hyperoxia can increase the neuroprotective effect of intermittent hypoxia by upregulation of redox signaling [Citation63,Citation66,Citation67]. This method, referred to as intermittent hypoxic-hyperoxic exposure (IHHE) [Citation68], has already been shown to be a well applicable and tolerable non-pharmacological intervention strategy for improving cognitive performance [Citation69–71] and to reduce circulating biomarkers of Alzheimer’s disease [Citation71] in geriatric patients and patients with mild cognitive impairment.
However, the acute and chronic effects of IHHE on BDNF blood concentration and inflammatory state in humans remain largely uninvestigated. Therefore, this study was designed to analyze the acute and chronic effects of IHHE prior to aerobic cycling on post-exercise and -training levels of BDNFS and BDNFP as well as C-reactive protein (CRP) and IL-6 in geriatric patients, respectively. It was expected that adding IHHE prior to aerobic cycling would augment the exercise-induced acute increase in BDNFS, BDNFP, and IL-6. Furthermore, it was hypothesized that 6 weeks of training would increase basal BDNFS and BDNFP as well as decrease CRP and IL-6 levels in geriatric patients receiving the IHHE compared with those receiving sham-IHHE (i.e. placebo control group). In addition, BDNF serum/plasma-ratio (BDNFS/P-ratio) was calculated to assess changes in the proportion of BDNF stored in platelets (serum) and freely circulating BDNF (plasma). Since no study has investigated the effect of exercise or IHHE interventions on the BDNFS/P-ratio so far, we applied an exploratory approach for this purpose.
Materials and methods
Participants
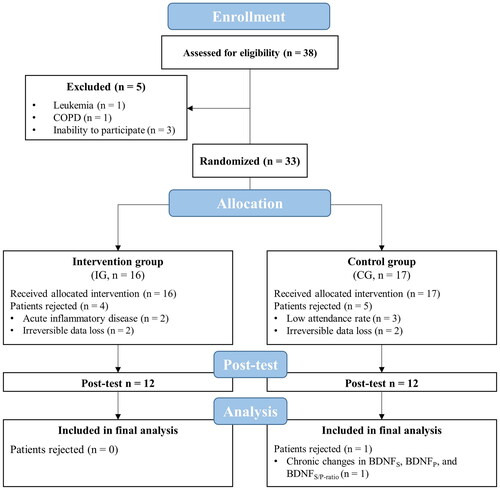
A total of 38 geriatric patients aged over 70 years were recruited from two inpatient full-day care facilities for geriatric patients and assessed for eligibility to participate in this study. All patients and, if required, their legal guardians were informed about the aims and the experimental procedure and have given their written informed consent. Patients were part of a larger trial that investigated the effect of IHHE prior to aerobic cycling on physical and cognitive performance [Citation70] and cardiovascular risk factors [Citation72]. Based on a medium effect size (Cohen’s f = 0.25), a significance level of 0.05, a power of 0.80, and an expected correlation between measures of 0.7, a sample size calculation was performed for the whole trial for a 2 × 2 repeated measures ANOVA. The Dementia Detection Test performance was chosen as the primary outcome. Accordingly, a total sample size of 22 patients was required to detect potential effects [Citation70]. All patients included in the study met the definition of geriatric patients according to the German Society of Geriatrics, the German Society of Gerontology and Geriatrics, as well as the German Group of Geriatric Institutions [Citation73]. Therefore, geriatric patients were defined by geriatric multimorbidity and an increased age (commonly > 70 years, however, multimorbidity is the primary criterion before calendar age) or an age of at least 80 years in combination with increased vulnerability. Patients were excluded if they were current smokers, had untreated or uncontrolled diseases (hypertension, coronary artery disease with unstable angina pectoris (CCS 3-4), severe heart failure (NYHA II-IV), arrhythmia, diabetes mellitus, pulmonary fibrosis), chronic obstructive pulmonary disease, cancer, acute inflammatory disease, need for continuous or intermittent ventilation or oxygenation, arterial oxygenation saturation at rest < 93%, or simultaneously participated in other interventions. After excluding 5 patients (Leukemia (n = 1), chronic obstructive pulmonary disease (n = 1), inability to participate (n = 3)), a total of 33 patients participated in the present study ().
Study design
A randomized, two-armed, controlled, and single-blinded (patients’ allocation) study design was used. Prior to the start of this study, patients’ age, sex, anthropometric data, medical history, previous diagnosed disease, and level of cognitive impairment (Mini-Mental State Examination) were recorded. All patients were randomly assigned to an intervention group (IG, n = 16) or a control group (CG, n = 17) using stratified (Mini-Mental State Examination) and counterbalanced (1:1) randomization (DatInf Randlist v. 1.5, DatInf GmbH, Tübingen, Germany). Blood sample collection was performed immediately before (pre-exercise) and 10 min after the 1st training session (post-exercise) as well as 48 h after the last training session (post-training). All training sessions and data collections were conducted in the respective inpatient care facility.
The experimental protocol was approved by the Otto-von-Guericke University Magdeburg (No. 202/20) confirming the principles of the Declaration of Helsinki on human experimentations. The study was retrospectively registered at drks.de (DRKS-ID: DRKS00025130).
Intervention
Prior to the first training session, patients were familiarized with the environment and interventional procedure. Furthermore, patients’ individual level of resistance for the aerobic cycling training was estimated as described previously [Citation70,Citation74]. The training was conducted on Monday, Wednesday, and Friday for 6 weeks resulting in 17 training sessions. At the beginning of each training session, patients of both groups were connected to an altitude breathing therapy device (ReOxy, Ai Mediq S.A., rue de Bitbourg, Luxemburg) through a face mask, inhaling a gas mixture for 30 min while sitting on an armchair. During this procedure, the IG was breathing intermittent hypoxic-hyperoxic air (IHHE), while the CG was breathing normoxic air (sham-IHHE, FiO2 ∼ 0.209). During the IHHE or sham-IHHE, patients’ peripheral oxygen saturation (SpO2) was continuously monitored. The dose of the IHHE program was individually tailored, depending on the results of a hypoxic test, which was performed prior the first and 10th training session [Citation66], and the patients’ acute responses (i.e. SpO2 and pulse rate) [Citation70]. Based on the ‘SpO2 clamp’ approach [Citation75], the external hypoxic intensity (i.e. FiO2) was automatically adjusted to ensure that patients achieved an SpO2 of 85–88% and 85% in the first and last three weeks, respectively. Thus, patients of the IG intermittently (4–8 cycles) received a hypoxic gas mixture (FiO2 = 0.100 − 0.140) for 1 to 5 min, followed by a 1 to 3.5 min exposure to a hyperoxic gas mixture (FiO2 = 0.300 − 0.400).
Afterwards, patients of both groups participated in a supervised aerobic cycling training (20 min per session) using a motorized cycle ergometer (MOTOmed viva 2 and viva 1, Reck, Betzensweiler, Germany) under normoxic conditions (without a facemask). The training program was designed based on current exercise recommendations for older adults [Citation76] and previous studies [Citation74,Citation77]. In brief, the training consisted of a 2.5 min warm-up (i.e. passive resistance pedaling at 20 rpm), followed by a 15 min main part (i.e. active pedaling at 30–60 rpm), and a 2.5 min cool-down (i.e. passive resistance pedaling at 20 rpm). Patients’ pulse rate (i.e. prior to the warm-up and immediately after the main part) and total work (kJ) were recorded for each session.
Blood sample collection and analysis
Venous blood samples were taken by a physician from a superficial forearm vein under stasis conditions with patients seated at rest at the time points mentioned above. The blood samples were collected in two vacutainers with separated gel and coagulation activator (BD Vacutainer® SSTTM II Advance, 8.5 ml, Franklin Lakes, NJ USA) to determine BDNFS, IL-6, and CRP levels as well as one vacutainer with lithium heparin (BD Vacutainer® LiHeparin, 4.0 ml, Franklin Lakes, NJ USA) to determine BDNFP level. Immediately after blood collection, vacutainers were swirled head down for 10 times. Only for the first blood collection (i.e. pre-exercise), a sample of venous blood from the vacutainer with lithium heparin (∼ 400 µL) was filled into an Eppendorf tube to assess patients’ BDNF genotype (Val66Val, Val66Met, or Met66Met). Thereafter, the serum and plasma samples were rested for 30 min at room temperature and for 10 min on ice, respectively, before serum and plasma was separated by centrifugation at 2000 G for 15 min. For each blood sample, 600 µL of the supernatant serum and plasma fluid were extracted and stored at −80 °C. BDNFS and BDNFP were determined using a commercially available DuoSet ELISA kit (R&D Systems®, Wiesbaden, Germany). Genomic DNA was extracted from anticoagulated venous blood (BD Vacutainer® LiHeparin, 4.0 ml, Franklin Lakes, NJ USA) collected immediately prior to the 1st training session. BDNF genotype was assessed by using conventional polymerase chain reaction (PCR) and restriction fragment length polymorphism analysis. PCR amplification of the BDNF polymorphism Val66Met was performed using the forward primer 5′- GCA TCC CGG TGA AAG AAA GCC CTA AC −3′ and the reverse primer 5′- GCC CCT GCA GCC TTC TTT TGT GTA AC −3′. PCR products were digested using the restriction enzyme Enzym Eco 72i (Thermo Fisher Scientific; Waltham, MA USA). IL-6 and CRP levels were quantified using an electro chemiluminescent assay and particle-enhanced immunological turbidity assay, respectively (COBAS® 2000, Roche Diagnostics, Basel, Switzerland). In addition,BDNFS/P-ratio was calculated to assess changes in the proportion of BDNF stored in platelets (serum) and freely circulating BDNF (plasma) [Citation14].
Statistical analysis
Data analysis was conducted using JASP Statistics (Version 0.16, University of Amsterdam, Amsterdam, Netherlands). The descriptive statistics were presented as means and standard deviations. To compare patients’ characteristics at baseline and training variables between the IG and CG, independent Student’s t-tests were used. Intervention effects regarding acute and chronic changes in BDNF, IL-6, and CRP plasma and/or serum concentration as well as BDNFS/P-ratio were examined using an analysis of covariance (ANCOVA) with repeated measures with time (acute: pre-exercise and post exercise; chronic: pre-exercise and post-training) as a within-subject factor and group (IG and CG) as a between-subject factor. In addition, the following covariates were entered in the statistical analyses: BDNFS and BDNFP (acute: average work performed during the first aerobic cycling session, MMSE-score, age, BMI, and BDNF genotype; chronic: MMSE-score, age, BMI, and BDNF genotype) and serum IL-6 and CRP concentrations (acute: average work performed during the first aerobic cycling session, MMSE-score, age, BMI; chronic: MMSE-score, age, BMI). If the assumption of sphericity was violated, Greenhouse-Geisser correction was applied. In case of significant interactions or main effects, post-hoc analyses with Bonferroni correction were performed. Since it was shown that the ANOVA [Citation78,Citation79] and t-test [Citation80,Citation81] could be used without significant error despite violation of homogeneity or normality assumptions, no alternative nonparametric tests were performed. Differences between groups are presented as mean differences together with 95% confidence intervals. When interpreting the results of intervention studies, it is recommended to consider the effect size in order to clarify the practical and clinical relevance of the results [Citation82–85]. Accordingly, the results of the present study were interpreted based on the effect sizes with a medium effect considered as meaningful. Thus, the effect sizes partial eta squared (ηp2) and Cohen’s d (d) were calculated and classified as small (ηp2 ≥ 0.01, d ≥ 0.20), medium (ηp2 ≥ 0.06, d ≥ 0.50), and large (ηp2 ≥ 0.14, d ≥ 0.80) effect [Citation82,Citation86,Citation87].
Results
To determine the practical relevance and generalizability of the effect of IHHE prior to aerobic cycling of neurotrophic and inflammatory blood markers in geriatric patients, results were interpreted based on effect sizes (not p values) with a medium effect considered as meaningful (i.e. ηp2 ≥ 0.06, d ≥ 0.50).
Five out of 33 enrolled patients were excluded from the final analysis due to a low attendance rate (< 80%, CG: n = 3) or acute inflammatory disease (IG: n = 2). In addition, there were irreversible data losses due to measurement errors in 4 patients (e.g. blood sampling or genotyping, IG: n = 2, CG: n = 2). Thus, data of 24 patients (average attendance rate: IG = 99 ± 2%; CG = 94 ± 8%) were included in the final analysis. Concerning the chronic effects on BDNFS, BDNFP, and BDNFS/P-ratio, data of one patient could not be included in the analysis due to irreversible data loss (CG: n = 1). No differences in patients’ cognitive ability (MMSE Score), number of diseases, and demographic as well as anthropometric characteristics were found between IG and CG (d ≤ 0.45), except the body mass index (T22 = 1.438, p = .164, d = 0.59; MD = 1.27 kg/m2 (95% CI = −4.46 to 0.81 kg/m2); shown in ). The minimum SpO2 was lower in the IG (84.3 ± 2.3%) during the IHHE than in the CG (94.0 ± 1.8%) during the sham-IHHE (T22 = 11.473, p < .001, d = 4.68; MD = 9.71% (95% CI = 0.84 to 7.95%)), whereas there were no differences between groups for maximum SpO2 (d = 0.37; IG = 96.8%, CG = 96.2%). With respect to the aerobic cycling training, there were no differences between groups for average work generated during the 20 min cycling sessions (IG = 99.3 ± 37.0 kJ, CG = 117.9 ± 51.4 kJ, d = 0.42) and the average pulse rate before (IG = 71.5 ± 9.9 min−1, CG = 72.6 ± 8.4 min−1, d = 0.12) and after (IG = 74.0 ± 9.7 min−1, CG = 75.3 ± 10.8 min−1, d = 0.13) these sessions. By retrospectively calculating the percentage of the estimated heart rate (208 − 0.7 · age [Citation88]), patients from the IG and CG achieved an average of 51 ± 8% and 49 ± 6% of their estimated maximum heart rate at the end of each aerobic cycling session, respectively. During the intervention period, there were no injuries or adverse side effects associated with the IHHE, sham-IHHE, or aerobic cycling training except for some reports of mild dizziness and uncomfortable feeling caused by wearing the facemask. The IG (Val66Val = 70%, Val66Met = 20%, and Met66Met = 10%) and CG (Val66Val = 73%, Val66Met = 18%, and Met66Met = 9%) showed similar distribution of BDNF genotypes.
Table 1. Patients’ characteristics at baseline split by group (intervention group (IG), control group (CG)). Values are presented as means ± standard deviations.
shows the BDNFS, BDNFP, IL-6, and CRP blood concentrations as well as BDNFS/P-ratio before (pre-exercise) and 10 min after (post-exercise) the first intervention session as well as 48 h after the last intervention session (post-training) together with the results of the ANCOVA with repeated measures. Descriptive data are shown in . Results of the post-hoc tests are shown in Supplementary Tables 1 and 2.
Figure 2. Acute and chronic effects of intermittent hypoxic-hyperoxic exposure (IHHE, intervention group) and sham-IHHE (control group) prior to aerobic cycling on (A) brain-derived neurotrophic factor serum blood concentration (BDNFS), (B) brain-derived neurotrophic factor plasma blood concentration (BDNFP), (C) brain-derived neurotrophic factor serum/plasma-ratio (BDNFS/P-ratio), (D) interleukin-6 (IL-6), and (E) C-reactive protein (CRP). Values are presented as individual data points and medians (horizontal lines) with the 25th and 75th percentiles. * d > 0.50 vs. pre-exercise, # d > 0.50 vs. intervention group.
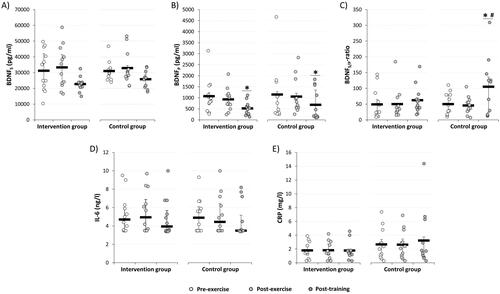
Table 2. Acute and chronic effects of intermittent hypoxic-hyperoxic exposure (IHHE, intervention group (IG)) and sham-IHHE (control group (CG)) prior to aerobic cycling on brain-derived neurotrophic factor serum blood concentration (BDNFS), brain-derived neurotrophic factor plasma blood concentration (BDNFP), brain-derived neurotrophic factor serum/plasma-ratio (BDNFS/P-ratio), interleukin-6 (IL-6), and C-reactive protein (CRP). Values are presented as means ± standard deviations.
With regard to the acute effects, the statistical analyses revealed no interaction or main effect of group but a main effect of time for BDNFS and BDNFP. However, post-hoc analyses indicated no within-group differences from pre- to post-exercise for BDNFS (IG = 2588.3 pg/ml (95% CI = −3074.4 to 8250.9 pg/ml), p = 1.000, d = 0.23; CG = 1303.4 pg/ml (95% CI = −4359.0 to 6966.0 pg/ml), p = 1.000, d = 0.12) and BDNFP (IG: −148.2 pg/ml (95% CI = −1107.1 to 810.6 pg/ml), p = 1.000, d = 0.18; CG: −88.8 pg/ml (95% CI = −1047.6 to 870.1 pg/ml), p = 1.000, d = 0.11). No interaction or main effects were found for acute changes in BDNFS/P-ratio and IL-6. Furthermore, no interaction or main effect of time but a main effect of group was found for CRP. Between-group post-hoc analyses showed a moderate effect for higher CRP blood concentrations in the CG at pre-exercise (0.96 mg/l (95% CI = −1.50 to 3.42 mg/l), p = 1.000, d = 0.51), whereas there was a trend for a moderate effect post-exercise (0.84 mg/l (95% CI = −1.62 to 3.29 mg/l), p = 1.000, d = 0.45).
Concerning the chronic effects, no interaction or main effects were found for BDNFS. While there was no interaction or main effect of group, a main effect of time was found for training-related changes in BDNFP. Within-group post-hoc analyses for the IG and CG indicated a reduction in BDNFP by 46.1 ± 33.5% (-577.7 pg/ml (95% CI = −1268.3 to 112.9 pg/ml), p = .139, d = 0.73) and 24.7 ± 72.9% (-451.5 pg/ml (95% CI = −1174.4 to 271.3 pg/ml), p = .478, d = 0.57), respectively. For the training-related changes in BDNFS/P-ratio, there was an interaction but no main effects. Within-group post-hoc analysis for the CG revealed an increase in BDNFS/P-ratio from pre-exercise to post-training by 91.3 ± 128.8 (48.7 (95% CI = −16.8 to 114.2), p = .243, d = 0.67), whereas no changes occurred in the IG (16.9 (95% CI = −45.7 to 79.5), p = 1.000, d = 0.23). In addition, between-group post-hoc analysis indicated a greater BDNFS/P-ratio in CG compared to IG at post-training (31.1 (95% CI = −40.3 to 102.39), p = 1.000, d = 0.54). There was no interaction or main effect for CRP. Furthermore, there was no interaction or main effect of group, but a main effect of time for IL-6. However, within-group post-hoc analyses revealed no training-related changes in IL-6 concentration for the IG (-0.43 ng/l (95% CI = −1.39 to 0.52 ng/l), p = 1.000, d = 0.22) and CG (-0.46 ng/l (95% CI = −1.41 to 0.49 ng/l), p = 1.000, d = 0.23).
Discussion
The results of the present study indicate that the addition of 30 min IHHE prior to 20 min aerobic cycling has no acute or chronic effect on BDNFS, BDNFP, IL-6, and CRP levels in geriatric patients. Interestingly, while the BDNFS/P-ratio was not affected by a single session, there was a training-related effect after the 6-week intervention. As a further result, it was shown that regardless of the IHHE treatment, BDNFP was reduced in both groups after the 6-week intervention period.
Based on the findings of animal [Citation41] and human [Citation89] studies, hypoxia-induced secretion and/or expression of neurotrophic factors, such as BDNF, has been proposed as a possible mechanism to explain the beneficial neurocognitive effects of interventions applying intermittent hypoxia [Citation48,Citation49,Citation90]. There is currently no study available that has examined the acute and/or chronic effects of IHHE prior to aerobic exercise on BDNFS and BDNFP in humans. However, the data of the present study indicate that the previously observed hypoxia-induced increase in BDNF expression [Citation89] does not seem to be possible with the applied IHHE protocol performed prior to aerobic cycling in our sample of geriatric patients.
Regarding the acute effects, previous studies have examined the influence of a single aerobic exercise session under normobaric hypoxia (i.e. continuous hypoxic training) on BDNF blood concentration [Citation91,Citation92]. Piotrowicz et al. showed that BDNFP increased immediately after an incremental cycling test until exhaustion by 29.3% and 50.0% under normoxia and normobaric hypoxia (FiO2 = 0.147), respectively, in young cyclists [Citation91]. Although the relative increase in BDNFP seemed to be higher in the hypoxia session, there were no differences between conditions. Interestingly, blood lactate concentration was higher, while maximal power output and oxygen consumption were lower under hypoxia compared to normoxia. Similar results were reported by Van Cutsem et al. examining the effect of hypoxia and ambient temperature (i.e. exercising under (i) normoxia at 15 °C, (ii) normoxia at 25 °C, (iii) hypoxia (3800 m, FiO2 ∼ 0.132) at 15 °C, and (iv) hypoxia (3800 m, FiO2 ∼ 0.132) at 25 °C) on BDNFS during a self-paced 30 min time trial in young healthy trained males [Citation92]. The authors have found that BDNFS increased immediately after exercise, regardless of hypoxia or temperature. Noteworthy, subjects exhibited increased blood lactate concentration and decreased power output in hypoxia compared to normoxia. Thus, it appears that hypoxia can modulate metabolic and molecular responses to aerobic exercise, as evidenced by equal (i.e. BDNFS or BDNFP) and enhanced (i.e. blood lactate concentration) physiological responses at lower levels of mechanical work (i.e. reduced power output) compared to normoxia. In this regard, studies suggested that blood lactate is involved in the regulation of BDNF blood concentration [Citation93–96] and associated with greater improvements in executive functions [Citation97,Citation98]. Considering that blood lactate is an indicator of exercise intensity, the absence of an acute increase in BDNFS and/or BDNFP in the present study could be due to the low intensity of the used aerobic cycling exercise [Citation99,Citation100] and/or the characteristics of the hypoxic stimulus administered (i.e. the hypoxic dose (e.g. intensity/severity, duration, density, frequency) or method (e.g. passive vs. active application)) [Citation101]. Indeed, previous studies have shown that BDNFS increased after acute continuous aerobic cycling of 30 min at 60% of individuals heart rate reserve [Citation102] and 40 min at 65–75% of individuals maximum heart rate [Citation103] in older adults. Meta-analyses indicate that the exercise intensity [Citation104] and duration [Citation105] are potential factors influencing the effect of acute exercise on BDNF blood concentration. In particular, it was suggested that acute high-intensity exercise increases BDNFS compared with non-exercise or low-intensity exercise but not with moderate-intensity exercise [Citation104]. In addition, exercise sessions with a duration of > 30 min lead to a greater acute increase in BDNF blood concentration than exercise sessions lasting ≤ 30 min [Citation105]. The aerobic exercise sessions in the current study lasted 20 min and patients from the IG and CG achieved an average of 51 ± 8% and 49 ± 6% of their estimated maximum heart rate, which corresponds to a low exercise intensity [Citation106]. Therefore, it can be assumed that the exercise intensity and/or duration was not sufficient to induce a detectable increase in BDNF blood concentration.
Hubold et al. [Citation107] and Chroboczek et al. [Citation108] examined the effect of a brief hypoxic exposure (30 min of continuous normobaric hypoxia) on BDNFP levels in young healthy males in comparison to normoxic control. Interestingly, the results from Hubold et al. indicate that brief hypoxia (SpO2 = 75%) alleviate the time-dependent decrease in BDNFP resulting in a higher BDNFP concentration 90 min after the acute exposure when plasma glucose was held stable between 4.5 and 5.5 mmol/l by euglycemic clamp [Citation107]. Chroboczek et al. showed an increase in BDNFP level 5–6 min after acute exposure to hypoxia (FiO2 = 0.130) compared to baseline, which was not seen in the normoxic control condition [Citation108]. Furthermore, blood cortisol concentration was decreased after the normoxic control compared to the hypoxia condition [Citation108]. Nevertheless, it must be taken into account that the order in which the subjects performed the conditions was not randomized (i.e. all subjects started with the control condition) and that all subjects had performed a cognitive task immediately before blood collecting, which could have influenced BDNFP levels [Citation108]. Studies investigating the effect of prolonged hypoxic exposure compared to normoxic control for at least 72 h on BDNF have shown contrasting results [Citation89,Citation109]. For instance, Li et al. have found a decrease in BDNFS on the fifth day after arrival to high altitude (3900 m, average arterial blood oxygenation saturation = 88.7 to 89.5%) in highly trained and untrained individuals [Citation109], while Helan et al. reported an increase in BDNFS following the exposure to an FiO2 of 0.150 (∼ 2800 m) for 72 h in healthy volunteers [Citation89]. Furthermore, Becke et al. demonstrated that 2 weeks of intermittent hypoxic exposure (12 sessions, 60 min·session−1, 5 min·period−1, SpO2 = 85–80% (simulated altitude was manually adjusted between 4000 and 5000 m)) resulted in decreased BDNFP but not BDNFS levels compared to a normoxic control group in healthy young adults [Citation110]. In a further study, performing prolonged hypoxic exposure (90 min, SpO2 = progressively decreased from 90 to 80%) prior to aerobic cycling (30 min, load = progressively increased from 65 to 75% of maximum heart rate) over 4 weeks (3 sessions·week−1), no additive effect on BDNFS were observed compared to a normoxic control group in healthy older adults aged 60 to 75 years [Citation45]. Previous studies revealed that the magnitude and even the direction (i.e. harmful or beneficial) of the effects elicited by hypoxia largely depend on the hypoxic dose [Citation58,Citation101,Citation111,Citation112]. Moderate- to low-dose hypoxia triggers redox signaling, which activates transcription factors initiating beneficial effects (e.g. expression of anti-inflammatory enzymes and reduction of the pro-inflammatory response [Citation65,Citation113]), whereas a more intense hypoxic dose provokes toxic levels of ROS formation causing harmful oxidative stress and inflammation [Citation63,Citation114]. In this regard, increased IL-1 receptor antagonist, IL-6, and CRP blood levels have been observed in healthy humans exposed to sustained hypobaric hypoxia at high altitudes (3458 to 8848 m) for 3 days to 8 weeks [Citation115,Citation116]. Moreover, the results of an animal study suggest that injection of high doses of lipopolysaccharide, an endotoxin and proinflammatory agent, results in an increased level of inflammatory markers in the hippocampus and a decrease in long-term potentiation and BDNF expression in adult male mice [Citation117]. Indeed, Becke et al. and Li et al. observed an increase in lymphocytes, granulocytes, and IL-1β, respectively, leading to the assumption that the applied hypoxic dose was too high, and thus caused systemic inflammation that possibly decreased BDNF expression [Citation109,Citation110]. In the current study, no within- or between-group differences were found for IL-6 and CRP blood levels, suggesting that hypoxia-induced systemic inflammation may not have been occurred. Perhaps, these contradictory results could be explained by differences in the hypoxic dose. In this regard, Li et al. used prolonged exposure to hypobaric hypoxia (i.e. arrival to high altitude), whereas patients in the present study were exposed to intermittent normobaric hypoxia and hyperoxia (i.e. IHHE) [Citation109]. Furthermore, Becke et al. performed 12 sessions of intermittent hypoxic exposure within 2 weeks, while inter-session frequency (i.e. number of sessions across a distinct time interval) and density (i.e. distribution of sessions across a distinct time interval with regard to recovery time in-between the sessions) were lower in the current study (i.e. 3 sessions·week−1) [Citation110]. However, other factors such as genotype, physical capacity, nutritional status, metabolic or neurological impairments and/or age [Citation58,Citation118–122] are thought to influence the individual acute responses and/or adaptations to hypoxia (i.e. dose-response relationship) in addition to the intensity, duration, frequency, density, and type of hypoxic stimulus.
Interestingly, the present study has found that the BDNFS/P-ratio increased in the CG compared to the IG after the 6-week intervention. While BDNFS reflects to > 95% the amount of BDNF stored in platelets, which is released due to degranulation during the clotting process [Citation123], BDNFP mirrors the freely circulating portion of peripheral BDNF. Although the origin of BDNFP is not completely understood, potential sources are vascular endothelial [Citation124] and skeletal muscle cells [Citation125], macrophages, lymphocytes [Citation126,Citation127], and, to an substantial extent, neurons and glia cells of the central nervous system [Citation23,Citation24,Citation27]. An increased BDNFS/P-ratio could indicate that (i) the relative amount of BDNF stored in platelets was increased and/or (ii) the relative amount of freely circulating BDNF was slightly decreased in the CG. Although somewhat surprising, the BDNFS/P-ratio was not affected in the IG suggesting that IHHE might have influenced the effect of an aerobic training on the relative proportion of stored to freely circulating BDNF in peripheral blood. These results might be partially related to potential hematologic changes (e.g. reduced plasma volume [Citation128]) that may have occurred as a result of the IHHE intervention. However, to the best of the authors’ knowledge, no study has investigated the effect of physical activity or intermittent hypoxia on BDNFS/P-ratio so far. Therefore, the exact reasons for the change in BDNFS/P-ratio and its potential implications in the context of the effect of IHHE on health-related outcomes in geriatric patients cannot be clearly elucidated at this time. As a consequence, further investigations should be carried out to clarify the potential underlying mechanisms of intra- and inter-individual molecular responses (in particular BDNF secretion and/or expression) to intermittent hypoxia and hyperoxia.
With regard to CRP and IL-6 blood concentrations, the effect of intermittent hypoxia on inflammatory biomarkers in older adults has been scarcely investigated [Citation68,Citation129]. Timon et al. reported that a 24-week intervention using prolonged hypoxic exposure (FiO2 = 0.161 for 45 min, 3 sessions·week−1) decreased CRP blood concentration in healthy older adults aged 65 to 75 years compared to a normoxic control group, with no changes in IL-8 and IL-10 [Citation113]. In contrast, no changes in CRP and other inflammatory biomarkers (e.g. TNFα) were observed after 8 weeks of intermittent hypoxic exposure (7 cycles, 5 min normobaric hypoxia (SpO2 ∼ 75%) interspersed with 3 min normoxia, 3 sessions·week−1) in overweight/obese individuals [Citation130] or 3 weeks of IHHE (4 cycles, 5 min normobaric hypoxia (FiO2 = 0.120) interspersed with 3 min of normobaric hyperoxia (FiO2 = 0.330)) in healthy older adults aged 65 to 75 years and those with mild cognitive impairment [Citation71]. Furthermore, when combining normobaric hypoxia with resistance exercise, no additive effects on IL-8, IL10, and CRP blood concentration as well as the IL-6/IL-10-ratio were observed after 24 weeks (3 sessions·week−1, 30 min·session−1, SpO2 ∼ 90%) in older adults aged 65 to 75 years [Citation131]. However, after the intervention, IL-8 and CRP blood concentrations were reduced in participants who performed resistance training either in normoxia or normobaric hypoxia compared with an inactive control group [Citation131]. The anti-inflammatory effect of physical training in older people is well known, although the exact mechanisms are complex and not yet fully understood [Citation132,Citation133]. This training-related effect is partly mediated by the acute expression of IL-6 from contracting skeletal muscle fibers, as physiological concentrations of IL-6 stimulates the expression of anti-inflammatory cytokines (e.g. IL-10) and inhibit the formation of pro-inflammatory cytokines (e.g. TNFα) [Citation134,Citation135]. The acute exercise-induced release of IL-6 depends on the type of exercise, the intensity, the duration, and the mass of recruited skeletal muscles [Citation136]. In the present study, no acute increase in IL-6 was detected after the first intervention session. Therefore, it can be assumed that the aerobic exercise program was not sufficient to elicit anti-inflammatory effects, which might explain the unchanged IL-6 and CRP blood concentrations after the 6-week intervention period. With regard to the anti-inflammatory effect of IHHE, exposure to intermittent hypoxia can trigger the transcription and production of anti-inflammatory and neuroprotective genes, such as EPO. Wojan et al. demonstrated that eight 4-min cycles of intermittent normobaric hypoxia (FiO2 = 0.104 ± 0.02, corresponding to a total duration of 32 min at an SpO2 of ≤ 83%) represent the shortest protocol to increase serum EPO level in healthy individuals [Citation59]. Certainly, the IHHE program used in the present study did not correspond to the lowest dose necessary to induce a hypoxia-related increase in serum EPO level. Therefore, it might be possible that the selected IHHE dose was too low to sufficiently increase transcription and production of anti-inflammatory genes.
As a further result, BDNFP decreased after the 6-week intervention in both groups regardless of IHHE. In contrast, a meta-analysis suggested that low- to moderate-intensity aerobic training does not evoke a change in BDNF blood concentration in older healthy adults aged ≥ 60 years [Citation137]. However, due a limited number of studies concerning the impact of aerobic training on BDNF blood concentration, specifically in older adults, no clear conclusion can be drawn. Indeed, previous randomized controlled trials in this field showed contradictory findings demonstrating increased [Citation138], unchanged [Citation139–141], and decreased [Citation142] BDNF blood levels. The discrepancy between these results indicates that BDNF blood concentration probably dependent on multiple factors modulating BDNF expression and release in a complex manner [Citation17]. For instance, BDNFS level was lower in females with anorexia nervosa, whereas it was higher in obese females [Citation143] and female patients newly diagnosed with type 2 diabetes mellitus [Citation122] compared to healthy normal-weight females. Furthermore, previous cross-sectional studies indicated that the basal BDNF level is negatively correlated with maximal oxygen uptake [Citation144,Citation145], heart rate reserve [Citation145], and daily physical activity level [Citation146,Citation147], whereas it is positively correlated with body mass index, total cholesterol level, triglyceride level [Citation145], and sedentary activities (e.g. average television time [Citation147]). In summary, these results suggest that basal blood BDNF concentration may be related to, e.g. a person’s metabolic status and disorders, fitness level, and level of daily physical activity. In this regard, there is convincing evidence that aerobic training can improve glucose metabolism and aerobic capacity in adults aged ≥ 70 years [Citation76,Citation148]. Although the duration of intervention in these studies was at least 9 weeks, it can be assumed that the patients in the current study improved their glucose metabolism and/or aerobic capacity, which could be related to the reduction in basal blood BDNF concentration. However, the limited number and discrepancy between previous results as well as the lack of evidence regarding intermittent hypoxia-hyperoxia induced effects in the present study emphasize the necessity for further research on the effect of exercise, training, and intermittent hypoxia and/or hyperoxia on BDNF blood concentration and the potential underlying mechanisms.
This study is not without limitations that must be considered. The first limitation is the small sample size (n = 12 per group). In this regard, large variations in basal BDNFP can be observed, evidenced by the large standard deviations of BDNFP blood concentration in the current and previous studies [Citation149]. In addition, evidence suggested that the BDNF met allele is recognized to be associated with lower activity-dependent secretion of BDNF [Citation150]. Indeed, the BDNF genotype distribution among patients from the IG and CG is consistent with previous cross-sectional studies investigating Caucasian populations [Citation151,Citation152]. However, due to the small sample size a subgroup analysis to investigate the effect of the BDNF genotype on changes in BNDFS and BNDFP was not feasible. Second, we did not measure further potentially relevant pro- and anti-inflammatory blood markers, such as TNFα and IL-10. It was shown that aerobic exercise (cycling for 3 h) and recombinant human IL-6 infusion inhibited endotoxin-induced TNFα expression [Citation153]. Furthermore, it was previously shown that short-term hypoxia dampens the pro-inflammatory response during systemic inflammation due to stimulatory effect of adenosine on IL-10 mediated through the adenosine 2B receptor [Citation65]. This should be considered in future studies. Hence, no conclusions can be drawn regarding the effect on other potential pro- and anti-inflammatory markers that were not analyzed in this study. Third, all patients in this study had several chronic diseases and most of the patients had cognitive impairments/diseases (e.g. dementia, mild cognitive impairment). In this context, although we included patients’ cognitive status (i.e. MMSE-score) as a covariate in the statistical analyses, it cannot be ruled out that different neurodegenerative processes could have influenced the results. Therefore, the current results cannot be generalized to healthy individuals and/or individuals without cognitive impairment and/or multimorbidity.
Conclusion
In conclusion, the current study showed that the addition of 30 min IHHE prior to 20 min aerobic cycling is not effective to increase BDNFS or BDNFP in geriatric patients, either immediately after a single session or after the 6-week training period. Furthermore, the applied intervention did not increase anti-inflammatory and/or decrease pro-inflammatory markers as indicated by no acute and chronic changes in IL-6 and CRP blood concentration. Therefore, other hypoxia-induced mechanisms might have been responsible for the improvements in cognitive function demonstrated in previous studies examining the effect of 3- to 8-week intermittent hypoxic interventions [Citation45–47,Citation69–71]. Nevertheless, the results show that IHHE was well tolerated by geriatric patients without showing adverse effects. As previous studies have shown that IHHE can improve physical and cognitive performance and reduce cardiometabolic risk factors in older adults, this method could be a promising adjuvant intervention strategy especially for geriatric patients. In addition, this study reports for the first time that IHHE prior to aerobic cycling might affect training-induced changes in the BDNFS/P-ratio in geriatric patients. However, the underlying mechanisms and potential clinical effects are still unknown and require further investigation. Therefore, further studies are needed to explain the mechanisms underlying this finding, such as (i) augmented cerebral blood flow (i.e. by increased endothelial nitric oxide production and angiogenesis), (ii) reduced oxidative stress (i.e. by Nrf2-activated antioxidant genes), and/or (iii) improved energy metabolism (i.e. by glycolytic enzymes and glycose transporters) [Citation48,Citation55,Citation63].
Author contributions
TB, OG, and LS contributed to conception and design of the study. TB, JI, and LS contributed to the implementation of the study. TB performed the data analysis. TBR and VL designed and performed the BDNF blood analysis. All authors contributed to the acquisition and interpretation of the data. TB and JI contributed to the manuscript drafting. RB, MB, OG, TBR, VL, and LS contributed to the review of the drafted manuscript. All authors have read and approved the submitted version of the manuscript.
Ethical approval
The experimental protocol was approved by the Otto-von-Guericke University Magdeburg (No. 202/20) confirming the principles of the Declaration of Helsinki on human experimentations. The study was retrospectively registered at drks.de (DRKS-ID: DRKS00025130).
Supplemental Material
Download Zip (24.3 KB)Acknowledgement
The authors thank AiMediqu S. A. Company Luxembourg for providing the ReOxy equipment and the software for implementing the intermittent hypoxic-hyperoxic exposure program. The authors also thank Alexey Platonenko for his great support in obtaining the ReOxy equipment and Margit Schmidt (Institute of Physiology, OVGU) for excellent technical assistance.
Disclosure statement
The authors declare that the research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.
Data availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on reasonable request.
Additional information
Funding
References
- Harada CN, Natelson Love MC, Triebel KL. Normal cognitive aging. Clin Geriatr Med. 2013;29(4):1–17. doi:10.1016/j.cger.2013.07.002.
- Raz N, Rodrigue KM, Head D, et al. Differential aging of the medial temporal lobe: a study of a five-year change. Neurology. 2004;62(3):433–438. doi:10.1212/01.wnl.0000106466.09835.46.
- Fjell AM, Walhovd KB. Structural brain changes in aging: courses, causes and cognitive consequences. Rev Neurosci. 2010;21(3):187–221. doi:10.1515/revneuro.2010.21.3.187.
- Oschwald J, Guye S, Liem F, et al. Brain structure and cognitive ability in healthy aging: a review on longitudinal correlated change. Rev Neurosci. 2019;31(1):1–57. doi:10.1515/revneuro-2018-0096.
- O’Shea A, Cohen RA, Porges EC, et al. Cognitive aging and the hippocampus in older adults. Front Aging Neurosci. 2016;8:298. doi:10.3389/fnagi.2016.00298.
- Madden DJ, Spaniol J, Costello MC, et al. Cerebral white matter integrity mediates adult age differences in cognitive performance. J Cogn Neurosci. 2009;21(2):289–302. doi:10.1162/jocn.2009.21047.
- Tartaglia MC, Rosen HJ, Miller BL. Neuroimaging in dementia. Neurotherapeutics. 2011;8(1):82–92. doi:10.1007/s13311-010-0012-2.
- Colcombe SJ, Erickson KI, Scalf PE, et al. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61(11):1166–1170. doi:10.1093/gerona/61.11.1166.
- Wood KN, Nikolov R, Shoemaker JK. Impact of long-term endurance training vs. Guideline-based physical activity on brain structure in healthy aging. Front Aging Neurosci. 2016;8:155. doi:10.3389/fnagi.2016.00155.
- Jonasson LS, Nyberg L, Kramer AF, et al. Aerobic exercise intervention, cognitive performance, and brain structure: results from the physical influences on brain in aging (PHIBRA) study. Front Aging Neurosci. 2016;8:336. doi:10.3389/fnagi.2016.00336.
- Herold F, Törpel A, Schega L, et al. Functional and/or structural brain changes in response to resistance exercises and resistance training lead to cognitive improvements - a systematic review. Eur Rev Aging Phys Act. 2019;16(1):10. doi:10.1186/s11556-019-0217-2.
- Stillman CM, Cohen J, Lehman ME, et al. Mediators of physical activity on neurocognitive function: a review at multiple levels of analysis. Front Hum Neurosci. 2016;10:626. doi:10.3389/fnhum.2016.00626.
- Stimpson NJ, Davison G, Javadi A-H. Joggin’ the noggin: towards a physiological understanding of exercise-induced cognitive benefits. Neurosci Biobehav Rev. 2018;88:177–186. doi:10.1016/j.neubiorev.2018.03.018.
- Brigadski T, Leßmann V. BDNF: a regulator of learning and memory processes with clinical potential. e-Neuroforum. 2014;5(1):1–11. doi:10.1007/s13295-014-0053-9.
- Eggert S, Kins S, Endres K, et al. Brothers in arms: proBDNF/BDNF and sAPPα/Aβ-signaling and their common interplay with ADAM10, TrkB, p75NTR, sortilin, and sorLA in the progression of alzheimer’s disease. Biol Chem. 2022;403(1):43–71. doi:10.1515/hsz-2021-0330.
- Edelmann E, Lessmann V, Brigadski T. Pre- and postsynaptic twists in BDNF secretion and action in synaptic plasticity. Neuropharmacology. 2014;76 Pt C:610–627. doi:10.1016/j.neuropharm.2013.05.043.
- Brigadski T, Leßmann V. The physiology of regulated BDNF release. Cell Tissue Res. 2020;382(1):15–45. doi:10.1007/s00441-020-03253-2.
- Leal G, Afonso PM, Salazar IL, et al. Regulation of hippocampal synaptic plasticity by BDNF. Brain Res. 2015;1621:82–101. doi:10.1016/j.brainres.2014.10.019.
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24(1):677–736. doi:10.1146/annurev.neuro.24.1.677.
- Park H, Poo M. Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci. 2013;14(1):7–23. doi:10.1038/nrn3379.
- Yamada K, Nabeshima T. Brain-derived neurotrophic factor/TrkB signaling in memory processes. J Pharmacol Sci. 2003;91(4):267–270. doi:10.1254/jphs.91.267.
- Edelmann E, Cepeda-Prado E, Franck M, et al. Theta burst firing recruits BDNF release and signaling in postsynaptic CA1 neurons in Spike-Timing-dependent LTP. Neuron. 2015;86(4):1041–1054. doi:10.1016/j.neuron.2015.04.007.
- Das K, Chao S, White L, et al. Differential patterns of nerve growth factor, brain-derived neurotrophic factor and neurotrophin-3 mRNA and protein levels in developing regions of rat brain. Neuroscience. 2001;103(3):739–761. doi:10.1016/S0306-4522(01)00011-2.
- Conner JM, Lauterborn JC, Yan Q, et al. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J Neurosci. 1997;17(7):2295–2313. doi:10.1523/JNEUROSCI.17-07-02295.1997.
- Webster MJ, Herman MM, Kleinman JE, et al. BDNF and trkB mRNA expression in the hippocampus and temporal cortex during the human lifespan. Gene Expr Patterns. 2006;6(8):941–951. doi:10.1016/j.modgep.2006.03.009.
- Rasmussen P, Brassard P, Adser H, et al. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp Physiol. 2009;94(10):1062–1069. doi:10.1113/expphysiol.2009.048512.
- Klein AB, Williamson R, Santini MA, et al. Blood BDNF concentrations reflect brain-tissue BDNF levels across species. Int J Neuropsychopharmacol. 2011;14(3):347–353. doi:10.1017/S1461145710000738.
- Pan W, Banks WA, Fasold MB, et al. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology. 1998;37(12):1553–1561. doi:10.1016/S0028-3908(98)00141-5.
- Hwang J, Brothers RM, Castelli DM, et al. Acute high-intensity exercise-induced cognitive enhancement and brain-derived neurotrophic factor in young, healthy adults. Neurosci Lett. 2016;630:247–253. doi:10.1016/j.neulet.2016.07.033.
- Griffin ÉW, Mullally S, Foley C, et al. Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiol Behav. 2011;104(5):934–941. doi:10.1016/j.physbeh.2011.06.005.
- Erickson KI, Miller DL, Roecklein KA. The aging hippocampus: interactions between exercise, depression, and BDNF. Neuroscientist. 2012;18(1):82–97. doi:10.1177/1073858410397054.
- Miranda M, Morici JF, Zanoni MB, et al. Brain-Derived neurotrophic factor: a key molecule for memory in the healthy and the pathological brain. Front Cell Neurosci. 2019;13:363. doi:10.3389/fncel.2019.00363.
- Erickson KI, Prakash RS, Voss MW, et al. Brain-derived neurotrophic factor is associated with age-related decline in hippocampal volume. J Neurosci. 2010;30(15):5368–5375. doi:10.1523/JNEUROSCI.6251-09.2010.
- Coelho FM, Pereira DS, Lustosa LP, et al. Physical therapy intervention (PTI) increases plasma brain-derived neurotrophic factor (BDNF) levels in non-frail and pre-frail elderly women. Arch Gerontol Geriatr. 2012;54(3):415–420. doi:10.1016/j.archger.2011.05.014.
- Roh E, Hwang SY, Song E, et al. Association of plasma brain-derived neurotrophic factor levels and frailty in community-dwelling older adults. Sci Rep. 2022;12(1):18605. doi:10.1038/s41598-022-19706-3.
- Laske C, Stransky E, Leyhe T, et al. BDNF serum and CSF concentrations in alzheimer’s disease, normal pressure hydrocephalus and healthy controls. J Psychiatr Res. 2007;41(5):387–394. doi:10.1016/j.jpsychires.2006.01.014.
- Du Y, Wu H-T, Qin X-Y, et al. Postmortem brain, cerebrospinal fluid, and blood neurotrophic factor levels in alzheimer’s disease: a systematic review and Meta-Analysis. J Mol Neurosci. 2018;65(3):289–300. doi:10.1007/s12031-018-1100-8.
- Ng TKS, Ho CSH, Tam WWS, et al. Decreased serum brain-derived neurotrophic factor (BDNF) levels in patients with alzheimer’s disease (AD): a systematic review and Meta-Analysis. Int J Mol Sci. 2019;20(2):257. doi:10.3390/ijms20020257.
- Kim BY, Lee SH, Graham PL, et al. Peripheral brain-derived neurotrophic factor levels in alzheimer’s disease and mild cognitive impairment: a comprehensive systematic review and meta-analysis. Mol Neurobiol. 2017;54(9):7297–7311. doi:10.1007/s12035-016-0192-9.
- Karege F, Perret G, Bondolfi G, et al. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res. 2002;109(2):143–148. doi:10.1016/s0165-1781(02)00005-7.
- Ryou M-G, Chen X, Cai M, et al. Intermittent hypoxia training prevents deficient learning-memory behavior in mice modeling alzheimer’s disease: a pilot study. Front Aging Neurosci. 2021;13:674688. doi:10.3389/fnagi.2021.674688.
- Vermehren-Schmaedick A, Jenkins VK, Knopp SJ, et al. Acute intermittent hypoxia-induced expression of brain-derived neurotrophic factor is disrupted in the brainstem of methyl-CpG-binding protein 2 null mice. Neuroscience. 2012;206:1–6. doi:10.1016/j.neuroscience.2012.01.017.
- Satriotomo I, Nichols NL, Dale EA, et al. Repetitive acute intermittent hypoxia increases growth/neurotrophic factor expression in non-respiratory motor neurons. Neuroscience. 2016;322:479–488. doi:10.1016/j.neuroscience.2016.02.060.
- Wang H, Ward N, Boswell M, et al. Secretion of brain-derived neurotrophic factor from brain microvascular endothelial cells. Eur J Neurosci. 2006;23(6):1665–1670. doi:10.1111/j.1460-9568.2006.04682.x.
- Schega L, Peter B, Brigadski T, et al. Effect of intermittent normobaric hypoxia on aerobic capacity and cognitive function in older people. J Sci Med Sport. 2016;19(11):941–945. doi:10.1016/j.jsams.2016.02.012.
- Schega L, Peter B, Törpel A, et al. Effects of intermittent hypoxia on cognitive performance and quality of life in elderly adults: a pilot study. Gerontology. 2013;59(4):316–323. doi:10.1159/000350927.
- Wang H, Shi X, Schenck H, et al. Intermittent hypoxia training for treating mild cognitive impairment: a pilot study. Am J Alzheimers Dis Other Demen. 2020;35:1533317519896725. doi:10.1177/1533317519896725.
- Burtscher J, Mallet RT, Burtscher M, et al. Hypoxia and brain aging: neurodegeneration or neuroprotection? Ageing Res Rev. 2021;68:101343. doi:10.1016/j.arr.2021.101343.
- Rybnikova EA, Nalivaeva NN, Zenko MY, et al. Intermittent hypoxic training as an effective tool for increasing the adaptive potential, endurance and working capacity of the brain. Front Neurosci. 2022;16:941740. doi:10.3389/fnins.2022.941740.
- Hickey MM, Simon MC. Regulation of angiogenesis by hypoxia and hypoxia-inducible factors. In:: Elsevier; 2006. p. 217–257. doi:10.1016/S0070-2153(06)76007-0.
- Katayama K, Fujita O, Iemitsu M, et al. The effect of acute exercise in hypoxia on flow-mediated vasodilation. Eur J Appl Physiol. 2013;113(2):349–357. doi:10.1007/s00421-012-2442-5.
- Muangritdech N, Hamlin MJ, Sawanyawisuth K, et al. Hypoxic training improves blood pressure, nitric oxide and hypoxia-inducible factor-1 alpha in hypertensive patients. Eur J Appl Physiol. 2020;120(8):1815–1826. doi:10.1007/s00421-020-04410-9.
- Chen C, Pore N, Behrooz A, et al. Regulation of glut1 mRNA by hypoxia-inducible factor-1. Interaction between H-ras and hypoxia. J Biol Chem. 2001;276(12):9519–9525. doi:10.1074/jbc.M010144200.
- Soo J, Raman A, Lawler NG, et al. The role of exercise and hypoxia on glucose transport and regulation. Eur J Appl Physiol. 2023;123(6):1147–1165. doi:10.1007/s00421-023-05135-1.
- Manukhina EB, Downey HF, Shi X, et al. Intermittent hypoxia training protects cerebrovascular function in alzheimer’s disease. Exp Biol Med (Maywood). 2016;241(12):1351–1363. doi:10.1177/1535370216649060.
- Smith KA, Waypa GB, Schumacker PT. Redox signaling during hypoxia in mammalian cells. Redox Biol. 2017;13:228–234. doi:10.1016/j.redox.2017.05.020.
- Sies H, Jones DP. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol. 2020;21(7):363–383. doi:10.1038/s41580-020-0230-3.
- Törpel A, Peter B, Hamacher D, et al. Dose-response relationship of intermittent normobaric hypoxia to stimulate erythropoietin in the context of health promotion in young and old people. Eur J Appl Physiol. 2019;119(5):1065–1074. doi:10.1007/s00421-019-04096-8.
- Wojan F, Stray-Gundersen S, Nagel MJ, et al. Short exposure to intermittent hypoxia increases erythropoietin levels in healthy individuals. J Appl Physiol (1985). 2021;130(6):1955–1960. doi:10.1152/japplphysiol.00941.2020.
- Knaupp W, Khilnani S, Sherwood J, et al. Erythropoietin response to acute normobaric hypoxia in humans. J. Appl. Physiol. 1992;73:937–840.
- Beckman B, Silberstein P, Aldoss IT. Erythropoiesis. In: Enna SJ, Bylund DB, editors. xPharm: the comprehensive pharmacology reference. Elsevier; 2010. p. 1–4. doi:10.1016/B978-008055232-3.60269-7.
- Rey F, Balsari A, Giallongo T, et al. Erythropoietin as a neuroprotective molecule: an overview of its therapeutic potential in neurodegenerative diseases. ASN Neuro. 2019;11:1759091419871420. doi:10.1177/1759091419871420.
- Mallet RT, Burtscher J, Manukhina EB, et al. Hypoxic–hyperoxic conditioning and dementia. In: Martin CR, Preedy VR, editors. Diagnosis and management in dementia. Elsevier; 2020. p. 745–760. doi:10.1016/B978-0-12-815854-8.00047-1.
- Furman D, Campisi J, Verdin E, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25(12):1822–1832. doi:10.1038/s41591-019-0675-0.
- Kiers D, Wielockx B, Peters E, et al. Short-Term hypoxia dampens inflammation in vivo via enhanced adenosine release and adenosine 2B receptor stimulation. EBioMedicine. 2018;33:144–156. doi:10.1016/j.ebiom.2018.06.021.
- Glazachev O. Optimization of clinical application of interval hypoxic training. Biomed Eng. 2013;47(3):134–137. doi:10.1007/s10527-013-9352-7.
- Sazontova TG, Bolotova AV, Bedareva IV, et al. Adaptation to intermittent hypoxia/hyperoxia enhances efficiency of exercise training. In: xi L, Serebrovskaya TV, editors. Intermittent hypoxia and human diseases. London: springer London; 2012. p. 191–205. doi:10.1007/978-1-4471-2906-6_16.
- Behrendt T, Bielitzki R, Behrens M, et al. Effects of intermittent hypoxia-hyperoxia on performance- and Health-Related outcomes in humans: a systematic review. Sports Med Open. 2022;8(1):70. doi:10.1186/s40798-022-00450-x.
- Bayer U, Likar R, Pinter G, et al. Intermittent hypoxic-hyperoxic training on cognitive performance in geriatric patients. Alzheimers Dement (N Y). 2017;3(1):114–122. doi:10.1016/j.trci.2017.01.002.
- Behrendt T, Bielitzki R, Behrens M, et al. Effects of intermittent hypoxia-hyperoxia exposure prior to aerobic cycling exercise on physical and cognitive performance in geriatric patients—a randomized controlled trial. Front Physiol. 2022;13:899096. doi:10.3389/fphys.2022.899096.
- Serebrovska ZO, Serebrovska TV, Kholin VA, et al. Intermittent hypoxia-hyperoxia training improves cognitive function and decreases circulating biomarkers of alzheimer’s disease in patients with mild cognitive impairment: a pilot study. Int J Mol Sci. 2019;20(21):5405. doi:10.3390/ijms20215405.
- Behrendt T, Altorjay A-C, Bielitzki R, et al. Influence of acute and chronic intermittent hypoxic-hyperoxic exposure prior to aerobic exercise on cardiovascular risk factors in geriatric patients—a randomized controlled trial. Front Physiol. 2022;13:1043536. doi:10.3389/fphys.2022.1043536.
- Deutsche Gesellschaft für Geriatrie e. V. (DGG). Deutsche Gesellschaft für Geriatire (DGG). https://www.dggeriatrie.de/.
- Holthoff VA, Marschner K, Scharf M, et al. Effects of physical activity training in patients with alzheimer’s dementia: results of a pilot RCT study. PLoS One. 2015;10(4):e0121478. doi:10.1371/journal.pone.0121478.
- Soo J, Girard O, Ihsan M, et al. The use of the SpO2 to FiO2 ratio to individualize the hypoxic doxe in sport science, exercise, and health settings. Front Physiol. 2020;11:570472. doi:10.3389/fphys.2020.570472.
- Izquierdo M, Merchant RA, Morley JE, et al. International exercise recommendations in older adults (ICFSR): expert consensus guidelines. J Nutr Health Aging. 2021;25(7):824–853. doi:10.1007/s12603-021-1665-8.
- Yang H-C, Lee C-L, Lin R, et al. Effect of biofeedback cycling training on functional recovery and walking ability of lower extremity in patients with stroke. Kaohsiung J Med Sci. 2014;30(1):35–42. doi:10.1016/j.kjms.2013.07.006.
- Schmider E, Ziegler M, Danay E, et al. Is it really robust? Methodology. 2010;6(4):147–151. doi:10.1027/1614-2241/a000016.
- Blanca MJ, Alarcón R, Arnau J, et al. Non-normal data: is ANOVA still a valid option? Psicothema. 2017;29(4):552–557. doi:10.7334/psicothema2016.383.
- Havlicek LL, Peterson NL. Robustness of the T test: a guide for researchers on effect of violations of assumptions. Psychol Rep. 1974;34(3_suppl):1095–1114. doi:10.2466/pr0.1974.34.3c.1095.
- Pagano RR. Understanding statistics in the behavioral sciences. 9th ed. Belmont: wadsworth Cengage Learning; 2009.
- Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol. 2013;4:863. doi:10.3389/fpsyg.2013.00863.
- Abbott EF, Serrano VP, Rethlefsen ML, et al. Trends in P value, confidence interval, and power analysis reporting in health professions education research reports: a systematic appraisal. Acad Med. 2018;93(2):314–323. doi:10.1097/ACM.0000000000001773.
- Lee DK. Alternatives to P value: confidence interval and effect size. Korean J Anesthesiol. 2016;69(6):555–562. doi:10.4097/kjae.2016.69.6.555.
- Ranstam J. Why the P-value culture is bad and confidence intervals a better alternative. Osteoarthritis Cartilage. 2012;20(8):805–808. doi:10.1016/j.joca.2012.04.001.
- Cohen J. Quantitative methods in psychology: a power primer. Psychol Bull. 1992;112(1):155–159. doi:10.1037//0033-2909.112.1.155.
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Routledge; 1988.
- Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. J Am Coll Cardiol. 2001;37(1):153–156. doi:10.1016/S0735-1097(00)01054-8.
- Helan M, Aravamudan B, Hartman WR, et al. BDNF secretion by human pulmonary artery endothelial cells in response to hypoxia. J Mol Cell Cardiol. 2014;68:89–97. doi:10.1016/j.yjmcc.2014.01.006.
- Ehrenreich H, Gassmann M, Poustka L, et al. Exploiting moderate hypoxia to benefit patients with brain disease: molecular mechanisms and translational research in progress. Neuroprotection. 2023;1(1):9–19. doi:10.1002/nep3.15.
- Piotrowicz Z, Chalimoniuk M, Płoszczyca K, et al. Exercise-Induced elevated BDNF level does not prevent cognitive impairment due to acute exposure to moderate hypoxia in well-trained athletes. Int J Mol Sci. 2020;21(15):5569. doi:10.3390/ijms21155569.
- van Cutsem J, Pattyn N, Vissenaeken D, et al. The influence of a mild thermal challenge and severe hypoxia on exercise performance and serum BDNF. Eur J Appl Physiol. 2015;115(10):2135–2148. doi:10.1007/s00421-015-3193-x.
- Ferris LT, Williams JS, Shen C-L. The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Med Sci Sports Exerc. 2007;39(4):728–734. doi:10.1249/mss.0b013e31802f04c7.
- El Hayek L, Khalifeh M, Zibara V, et al. Lactate mediates the effects of exercise on learning and memory through SIRT1-Dependent activation of hippocampal brain-derived neurotrophic factor (BDNF). J Neurosci. 2019;39(13):2369–2382. doi:10.1523/JNEUROSCI.1661-18.2019.
- Schiffer T, Schulte S, Sperlich B, et al. Lactate infusion at rest increases BDNF blood concentration in humans. Neurosci Lett. 2011;488(3):234–237. doi:10.1016/j.neulet.2010.11.035.
- Brooks GA, Osmond AD, Arevalo JA, et al. Lactate as a myokine and exerkine: drivers and signals of physiology and metabolism. J Appl Physiol (1985). 2023;134(3):529–548. doi:10.1152/japplphysiol.00497.2022.
- Hashimoto T, Tsukamoto H, Takenaka S, et al. Maintained exercise-enhanced brain executive function related to cerebral lactate metabolism in men. Faseb J. 2018;32(3):1417–1427. doi:10.1096/fj.201700381RR.
- Hashimoto T, Tsukamoto H, Ando S, et al. Effect of exercise on brain health: the potential role of lactate as a myokine. Metabolites. 2021;11(12):813. doi:10.3390/metabo11120813.
- Rojas Vega S, Strüder HK, Vera Wahrmann B, et al. Acute BDNF and cortisol response to low intensity exercise and following ramp incremental exercise to exhaustion in humans. Brain Res. 2006;1121(1):59–65. doi:10.1016/j.brainres.2006.08.105.
- Hötting K, Schickert N, Kaiser J, et al. The effects of acute physical exercise on memory, peripheral BDNF, and cortisol in young adults. Neural Plast. 2016;2016:6860573–6860512. doi:10.1155/2016/6860573.
- Navarrete-Opazo A, Mitchell GS. Therapeutic potential of intermittent hypoxia: a matter of dose. Am J Physiol Regul Integr Comp Physiol. 2014;307(10):R1181–97. doi:10.1152/ajpregu.00208.2014.
- Behrendt T, Kirschnick F, Kröger L, et al. Comparison of the effects of open vs. closed skill exercise on the acute and chronic BDNF, IGF-1 and IL-6 response in older healthy adults. BMC Neurosci. 2021;22(1):71. doi:10.1186/s12868-021-00675-8.
- Máderová D, Krumpolec P, Slobodová L, et al. Acute and regular exercise distinctly modulate serum, plasma and skeletal muscle BDNF in the elderly. Neuropeptides. 2019;78:101961. doi:10.1016/j.npep.2019.101961.
- Fernández-Rodríguez R, Álvarez-Bueno C, Martínez-Ortega IA, et al. Immediate effect of high-intensity exercise on brain-derived neurotrophic factor in healthy young adults: a systematic review and meta-analysis. J Sport Health Sci. 2022;11(3):367–375. doi:10.1016/j.jshs.2021.08.004.
- Dinoff A, Herrmann N, Swardfager W, et al. The effect of acute exercise on blood concentrations of brain-derived neurotrophic factor in healthy adults: a meta-analysis. Eur J Neurosci. 2017;46(1):1635–1646. doi:10.1111/ejn.13603.
- Coates AM, Joyner MJ, Little JP, et al. A perspective on high-intensity interval training for performance and health. Sports Med. 2023;53(Suppl 1):85–96. doi:10.1007/s40279-023-01938-6.
- Hubold C, Lang UE, Gehring H, et al. Increased serum brain-derived neurotrophic factor protein upon hypoxia in healthy young men. J Neural Transm (Vienna). 2009;116(10):1221–1225. doi:10.1007/s00702-009-0257-2.
- Chroboczek M, Kujach S, Łuszczyk M, et al. Acute normobaric hypoxia lowers executive functions among young men despite increase of BDNF concentration. Int J Environ Res Public Health. 2022;19(17):10802. doi:10.3390/ijerph191710802.
- Li P, Zhang G, You H-Y, et al. Training-dependent cognitive advantage is suppressed at high altitude. Physiol Behav. 2012;106(4):439–445. doi:10.1016/j.physbeh.2012.03.002.
- Becke A, Müller P, Dordevic M, et al. Daily intermittent normobaric hypoxia over 2 weeks reduces BDNF plasma levels in young adults – a randomized controlled feasibility study. Front Physiol. 2018;9:1337. doi:10.3389/fphys.2018.01337.
- Wilber RL, Stray-Gundersen J, Levine BD. Effect of hypoxic "dose" on physiological responses and sea-level performance. Med Sci Sports Exerc. 2007;39(9):1590–1599. doi:10.1249/mss.0b013e3180de49bd.
- Serebrovska T, Serebrovska Z, Egorov E. Fitness and therapeutic potential of intermittent hypoxia training: a matter of dose. Fiziol Zh (1994). 2016;62(3):78–91. doi:10.15407/fz62.03.078.
- Timon R, González-Custodio A, Vasquez-Bonilla A, et al. Intermittent hypoxia as a therapeutic tool to improve health parameters in older adults. Int J Environ Res Public Health. 2022;19(9):5339. doi:10.3390/ijerph19095339.
- Burtscher J, Mallet RT, Pialoux V, et al. Adaptive responses to hypoxia and/or hyperoxia in humans. Antioxid Redox Signal. 2022;37(13-15):887–912. doi:10.1089/ars.2021.0280.
- Hartmann G, Tschöp M, Fischer R, et al. High altitude increases circulating interleukin-6, interleukin-1 receptor antagonist and C-reactive protein. Cytokine. 2000;12(3):246–252. doi:10.1006/cyto.1999.0533.
- Siervo M, Riley HL, Fernandez BO, et al. Effects of prolonged exposure to hypobaric hypoxia on oxidative stress, inflammation and gluco-insular regulation: the not-so-sweet price for good regulation. PLoS One. 2014;9(4):e94915. doi:10.1371/journal.pone.0094915.
- Golia MT, Poggini S, Alboni S, et al. Interplay between inflammation and neural plasticity: both immune activation and suppression impair LTP and BDNF expression. Brain Behav Immun. 2019;81:484–494. doi:10.1016/j.bbi.2019.07.003.
- Rivard A, Berthou-Soulie L, Principe N, et al. Age-dependent defect in vascular endothelial growth factor expression is associated with reduced hypoxia-inducible factor 1 activity. J Biol Chem. 2000;275(38):29643–29647. doi:10.1074/jbc.M001029200.
- Richalet J-P, Lhuissier FJ. Aging, tolerance to high altitude, and cardiorespiratory response to hypoxia. High Alt Med Biol. 2015;16(2):117–124. doi:10.1089/ham.2015.0030.
- Wang Y, Lu H, Chen Y, et al. The association of angiotensin-converting enzyme gene insertion/deletion polymorphisms with adaptation to high altitude: a meta-analysis. J Renin Angiotensin Aldosterone Syst. 2016;17(1):1470320315627410. 1470320315627410. doi:10.1177/1470320315627410.
- Asgarzadeh A, Fouladi N, Asghariazar V, et al. Serum brain-derived neurotrophic factor (BDNF) in COVID-19 patients and its association with the COVID-19 manifestations. J Mol Neurosci. 2022;72(9):1820–1830. doi:10.1007/s12031-022-02039-1.
- Suwa M, Kishimoto H, Nofuji Y, et al. Serum brain-derived neurotrophic factor level is increased and associated with obesity in newly diagnosed female patients with type 2 diabetes mellitus. Metabolism. 2006;55(7):852–857. doi:10.1016/j.metabol.2006.02.012.
- Fujimura H, Altar CA, Chen R, et al. Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation. Thromb Haemost. 2002;87(4):728–734. doi:10.1055/s-0037-1613072.
- Nakahashi T, Fujimura H, Altar C, et al. Vascular endothelial cells synthesize and secrete brain-derived neurotrophic factor. FEBS Lett. 2000;470(2):113–117. doi:10.1016/S0014-5793(00)01302-8.
- Matthews VB, Aström M-B, Chan MHS, et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia. 2009;52(7):1409–1418. doi:10.1007/s00125-009-1364-1.
- Kerschensteiner M, Gallmeier E, Behrens L, et al. Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation? J Exp Med. 1999;189(5):865–870. doi:10.1084/jem.189.5.865.
- Gielen A, Khademi M, Muhallab S, et al. Increased brain-derived neurotrophic factor expression in white blood cells of Relapsing-Remitting multiple sclerosis patients. Scand J Immunol. 2003;57(5):493–497. doi:10.1046/j.1365-3083.2003.01260.x.
- Tobin B, Costalat G, Renshaw GMC. Intermittent not continuous hypoxia provoked haematological adaptations in healthy seniors: hypoxic pattern may hold the key. Eur J Appl Physiol. 2020;122(2):395–407. doi:10.1007/s00421-020-04310-y.
- Timon R, Martinez-Guardado I, Brocherie F. Effects of intermittent normobaric hypoxia on Health-Related outcomes in healthy older adults: a systematic review. Sports Med Open. 2023;9(1):19. doi:10.1186/s40798-023-00560-0.
- Chacaroun S, Borowik A, Doutreleau S, et al. Cardiovascular and metabolic responses to passive hypoxic conditioning in overweight and mildly obese individuals. Am J Physiol Regul Integr Comp Physiol. 2020;319(2):R211–R222. doi:10.1152/ajpregu.00311.2019.
- Timon R, Martínez-Guardado I, Camacho-Cardeñosa A, et al. Effect of intermittent hypoxic conditioning on inflammatory biomarkers in older adults. Exp Gerontol. 2021;152:111478. doi:10.1016/j.exger.2021.111478.
- Woods JA, Wilund KR, Martin SA, et al. Exercise, inflammation and aging. Aging Dis. 2012;3(1):130–140.
- Bautmans I, Salimans L, Njemini R, et al. The effects of exercise interventions on the inflammatory profile of older adults: a systematic review of the recent literature. Exp Gerontol. 2021;146:111236. doi:10.1016/j.exger.2021.111236.
- Pedersen BK, Fischer CP. Beneficial health effects of exercise–the role of IL-6 as a myokine. Trends Pharmacol Sci. 2007;28(4):152–156. doi:10.1016/j.tips.2007.02.002.
- Petersen AMW, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol (1985). 2005;98(4):1154–1162. doi:10.1152/japplphysiol.00164.2004.
- Fischer CP. Interleukin-6 in acute exercise and training: what is the biological relevance? Exerc Immunol Rev. 2006;12:6–33.
- Marinus N, Hansen D, Feys P, et al. The impact of different types of exercise training on peripheral blood brain-derived neurotrophic factor concentrations in older adults: a Meta-Analysis. Sports Med. 2019;49(10):1529–1546. doi:10.1007/s40279-019-01148-z.
- Kim J-H, Kim D-Y. Aquarobic exercises improve the serum blood irisin and brain-derived neurotrophic factor levels in elderly women. Exp Gerontol. 2018;104:60–65. doi:10.1016/j.exger.2018.01.024.
- Pereira DS, Queiroz B D, Miranda AS, et al. Effects of physical exercise on plasma levels of brain-derived neurotrophic factor and depressive symptoms in elderly women–a randomized clinical trial. Arch Phys Med Rehabil. 2013;94(8):1443–1450. doi:10.1016/j.apmr.2013.03.029.
- Maass A, Düzel S, Brigadski T, et al. Relationships of peripheral IGF-1, VEGF and BDNF levels to exercise-related changes in memory, hippocampal perfusion and volumes in older adults. Neuroimage. 2016;131:142–154. doi:10.1016/j.neuroimage.2015.10.084.
- Matura S, Fleckenstein J, Deichmann R, et al. Effects of aerobic exercise on brain metabolism and grey matter volume in older adults: results of the randomised controlled SMART trial. Transl Psychiatry. 2017;7(7):e1172–e1172. doi:10.1038/tp.2017.135.
- Gmiąt A, Jaworska J, Micielska K, et al. Improvement of cognitive functions in response to a regular nordic walking training in elderly women - A change dependent on the training experience. Exp Gerontol. 2018;104:105–112. doi:10.1016/j.exger.2018.02.006.
- Monteleone P, Tortorella A, Martiadis V, et al. Opposite changes in the serum brain-derived neurotrophic factor in anorexia nervosa and obesity. Psychosom Med. 2004;66(5):744–748. doi:10.1097/01.psy.0000138119.12956.99.
- Cho H, Kim J, Kim S, et al. The concentrations of serum, plasma and platelet BDNF are all increased by treadmill VO2max performance in healthy college men. Neurosci Lett. 2012;519(1):78–83. doi:10.1016/j.neulet.2012.05.025.
- Jung SH, Kim J, Davis JM, et al. Association among basal serum BDNF, cardiorespiratory fitness and cardiovascular disease risk factors in untrained healthy korean men. Eur J Appl Physiol. 2011;111(2):303–311. doi:10.1007/s00421-010-1658-5.
- Nofuji Y, Suwa M, Moriyama Y, et al. Decreased serum brain-derived neurotrophic factor in trained men. Neurosci Lett. 2008;437(1):29–32. doi:10.1016/j.neulet.2008.03.057.
- Chan KL, Tong KY, Yip SP. Relationship of serum brain-derived neurotrophic factor (BDNF) and health-related lifestyle in healthy human subjects. Neurosci Lett. 2008;447(2-3):124–128. doi:10.1016/j.neulet.2008.10.013.
- Bouaziz W, Vogel T, Schmitt E, et al. Health benefits of aerobic training programs in adults aged 70 and over: a systematic review. Arch Gerontol Geriatr. 2017;69:110–127. doi:10.1016/j.archger.2016.10.012.
- Knaepen K, Goekint M, Heymann EM, et al. Neuroplasticity - Exercise-induced response of peripheral brain-derived neurotrophic factor: a systematic review of experimental studies in human subjects.
- Kim J-M, Stewart R, Bae K-Y, et al. Role of BDNF val66met polymorphism on the association between physical activity and incident dementia. Neurobiol Aging. 2011;32(3):551.e5-12–551.12. doi:10.1016/j.neurobiolaging.2010.01.018.
- Petryshen TL, Sabeti PC, Aldinger KA, et al. Population genetic study of the brain-derived neurotrophic factor (BDNF) gene. Mol Psychiatry. 2010;15(8):810–815. doi:10.1038/mp.2009.24.
- Vulturar R, Chiş A, Hambrich M, et al. Allelic distribution of BDNF Val66Met polymorphism in healthy romanian volunteers. Transl Neurosci. 2016;7(1):31–34. doi:10.1515/tnsci-2016-0006.
- Starkie R, Ostrowski SR, Jauffred S, et al. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-alpha production in humans. Faseb J. 2003;17(8):884–886. doi:10.1096/fj.02-0670fje.