Abstract
Background
It was well documented that calcium (Ca), phosphorus (P), and magnesium (Mg) participate in many physiological processes. We aimed to study the changing trend of serum levels of Ca, P, and Mg in frequent respiratory tract infections (FRTI) in children.
Methods
A retrospective study was performed in our centre. A total of 213 FRTI cases and 33 controls were enrolled in our study. We analyzed the correlation between serum Ca/P/Mg levels and inflammatory indexes by using Spearman correlation analysis. Standard mean difference (SMD) was applied to test the differences of serum Ca/P/Mg levels between FRTI subgroups and controls. In terms of the findings of SMD between Ca/P/Mg status between FRTI subgroups and controls, receiver operating characteristics (ROC) curve analysis was further applied to test the association between serum Ca level and bronchitis, parainfluenza virus infection, influenza B virus infection and cytomegalovirus infection.
Results
Serum Ca level was significantly associated with white blood cell (WBC), platelet (PLT) and procalcitonin (PCT) (p = 0.006; p < 10−4; p = 0.004). Serum P level was markedly associated with eryhtrocyte sedimentation rate (ESR) and PCT (p = 0.018; p < 10−4). Controls showed significantly lower serum Ca level than that among bronchitis (p = 0.001), parainfluenza virus infection (p = 0.027), influenza B virus infection (p = 0.017), cytomegalovirus infection (p = 0.029), and two pathogens infected (p = 0.020). ROC curve analysis showed that serum Ca level was significantly associated with bronchitis (p = 0.047) and influenza B virus infection (p = 0.049).
Conclusions
Serum levels of Ca and P may reflect the inflammatory status in children with FRTI. Alteration of serum Ca level may predict the risk of bronchitis and influenza B virus infection. Keeping the homeostasis of Ca, P, and Mg may be important for the prevention and treatment of FRTI.
KEY MESSAGES
Serum status of Ca and P was closely associated with the inflammatory status in children with frequent respiratory tract infections.
Changes of serum Ca status may predict the susceptibility to bronchitis and influenza B virus infection in children with frequent respiratory tract infections.
Homeostasis of Ca, P, and Mg status may be important for the prevention and treatment of frequent respiratory tract infections in children.
Introduction
Respiratory tract infections (RTI), most common disorders among children, are likely to occur in cases with low immunity during the epidemic season [Citation1]. RTI are defined as those infections including the respiratory tract, such as the airway, sinuses, throat or lungs [Citation2]. The majority of the RTI are self-limited, some immunodeficient cases are likely to be with high risk of complications [Citation3]. Recurrent and life-threatening respiratory infections are nearly universal in patients with primary immunodeficiency diseases [Citation4]. Recurrent RTI may affect the growth and development of children, leading to an increased economic and social burden [Citation5]. Serious RTI, such as severe pneumonia, may induce poor clinical outcomes, even death [Citation6].
Frequent respiratory tract infections (FRTI), defined as >42 days with symptoms of RTI during a 12-month period prior to study inclusion, are likely to influence normal life, including work and study, and lower the immunity, even lead to certain complications, such as liver and kidney injury [Citation7–9]. Increased severity and possible frequency of RTI causes some medical, educational and social problems [Citation10]. About 18% of children between the ages of one and four years experience frequent acute RTI or recurrent RTI [Citation11]. FRTI adversely affects the development of the children [Citation12]. Vitamin A supplementation is unlikely to improve children’s growth in populations with a high prevalence of respiratory infections [Citation13]. In terms of the harms of FRTI, early intervention should be applied in FRTI, particularly the cases with immunodeficiency. However, overactive or aggressvie therapy may lead to certain side effects, including intestinal microflora disorder and immune dysfunction [Citation14]. Multiple risk factors may be involved in the development of FRTI, an integrated and comprehensive prevention measure is imperative. To lower the risk and harms of FRTI, identification of novel targets for early prevention and appropriate nursing seems important for the health of children.
Trace elements play an important role in many physiological processes [Citation15]. Disorders of trace elements participate in the pathogenesis of a number of diseases, including RTI. For example, iron deficiency is likely to lead to anaemia, and calcium (Ca) deficiency is closely associated with rickets risk [Citation16]. Ca is an essential ion for pathogens survival and virulence and is involved in the inflammatory response regulation [Citation17]. Hypophosphoremia was a prognostic factor of respiratory infections in aged patients [Citation18]. Inadequate magnesium (Mg) intake was likely to lead to a decrease in resistance to RTI [Citation19]. Ca and Mg showed high diagnostic accuracy for the prediction of critical COVID-19 [Citation20]. We also previously reported that Mg/phosphorus (P) disorder may be associated with the susceptibility to suppuration in children with tonsillitis [Citation21]. On the other hand, some trace elements are applied in treating some diseases in children. For instance, Mg is used in asthma by relaxing smooth muscle [Citation22]. P plays a role in protecting against myocardial damage [Citation23]. Mg supplementation prevents and treats some diseases related to respiratory system [Citation24].
RTI are likely to be complicated with multisystem diseases [Citation25], the dysregulation of trace elements may participate in this process or influence the prognosis of RTI indirectly. We speculated that the disorder of trace elements may be involved in the development of RTI in children.
Ca, P and Mg are the main minerals which must be absorbed through the dietary way [Citation26].
They are involved in many biological functions, and vital for the growth and development of children [Citation27]. Dysregulation of Ca/P/Mg is involved in many respiratory disorders. For instance, patients with lower level of Ca demonstrated higher mortality and more complications [Citation28]. Vitamin D supplementation was safe and reduced the risk of acute respiratory infections [Citation29]. lower status of P aggravates the chronic obstructive pulmonary disease and increases the period of ventilation process [Citation30]. Mg deficiency is associated with airway hyperreactivity, wheezing, and impaired lung function [Citation31]. Hence, Ca/P/Mg levels may be associated with the severity of RTI. Multiple pathogens, such as bacteria, viruses and mycoplasma, are involved in inducing RTI risk. Inflammatory reaction is also an important risk factor for the progression of RTI [Citation32]. Excessive inflammation is a postulated cause of severe symptoms in respiratory virus infections [Citation33].
An important link was also observed between the upper and lower respiratory tracts whereby inflammation in one environment can influence the other [Citation34]. For example, pathogen exposures are the primary driver for inflammation in the nose in acute rhinosinusitis [Citation34]. Modulating the inflammatory response may be an important aspect of definitive therapy for RTI [Citation35]. Excessive inflammatory response damages the airways [Citation36]. Ca/P/Mg disorder may also interact with the inflammation in the development of RTI. An in-depth analysis of these issues seems imperative.
To further understand the potential role of Ca/P/Mg in the children with FRTI, we made a correlation analysis of inflammatory indexes with serum Ca/P/Mg levels in children with FRTI. We also compared the differences of serum Ca/P/Mg levels between FRTI subgroups and controls. Furthermore, we tried to calculate the cut-off point of serum Ca level in bronchitis and influenza B virus infection for providing more specific guiding significance.
Methods
Patient population
The study population was recruited from the Paediatrics Department of Shanghai Sixth People’s Hospital between January 2016 to April 2018. The FRTI cases (defined as >42 days with symptoms of RTI during a 12-month period prior to study inclusion [Citation7]) were enrolled according to the following criteria: children with FRTI aged 0-14 years, all the subjects did not receive any Ca, P or Mg components before admission to our hospital. The cases with systemic diseases (defined by the disorders targeting the intestine, kidney and bone, such as hyperparathyroidism, malignancy, reduced gastrointestinal absorption, and kidney diseases [Citation37]) that may influence the status of trace elements were excluded. The enrolled FRTI cases were all hospitalized children. We also enrolled healthy check-up as controls with age and gender-matched to FRTI cases. The healthy check-up controls were all outpatient children without the FRTI history. The study was approved by ethics committee of Shanghai Sixth People’s Hospital (No. 2018-106). The study was performed in accordance with the Declaration of Helsinki (as revised in 2013). The requirement of informed consent was waived due to the retrospective nature of the study. All the recruited data was de-identified. We performed the anonymous data analysis in the retrospective style.
Data collection
The clinical and laboratory data were earnestly extracted from the electronical medical records of the participants. We reviewed the data of age, gender, clinical diagnosis and pathogens infected.
The clinical diagnosis included upper respiratory tract infections (defined as nasopharyngitis, pharyngitis, tonsillitis, or otitis media), bronchitis (defined as cough due to acute inflammation of the trachea and large airways without evidence of pneumonia), ordinary pneumonia, and severe pneumonia (severe pneumonia defined as pneumonia plus inability to drink, persistent vomiting, convulsions, lethargy, stridor at rest, severe malnutrition or signs of respiratory failure, while ordinary pneumonia defined as pneumonia without these plus symptoms) [Citation38]. The recorded pathogens included Epstein-Barr (EB) virus, Herpes simplex virus, parainfluenza virus, influenza A virus, influenza B virus, cytomegalovirus, coxsackie virus, chlamydia, and mycoplasma. Meanwhile, the laboratory data acquired were all tested within three days after the admission. The laboratory data included serum levels of white blood cell (WBC), erythrocyte sedimentation rate (ESR), platelet (PLT), procalcitonin (PCT), calcium (Ca), phosphorus (P), and magnesium (Mg).
Statistical analysis
Spearman correlation analysis was applied to test the association between the serum levels of Ca/P/Mg and WBC/PLT/PCT/ESR status among enrolled FRTI cases. Variables of serum Ca/P/Mg status were expressed as means ± standard deviation (SD), Standard mean deviation (SMD) was used to measure the differences in serum Ca/P/Mg levels between various FRTI groups (grouped in terms of the type of clinical diagnosis and pathogens infected) and healthy controls. In terms of the findings of SMD between Ca/P/Mg status between FRTI subgroups and controls, receiver operating characteristics (ROC) curve analysis was used to measure the association between serum Ca level and bronchitis, parainfluenza virus infection, influenza B virus infection and cytomegalovirus infection. All the analyses were performed by using SPSS version 19. p < 0.05 was considered statistically significant, except where otherwise specified.
Results
Patients characteristics
A total of 213 FRTI cases and 33 healthy controls were involved in our study. The cases involved 41 upper respiratory tract infections, 33 bronchitis, 111 ordinary pneumonia, and 28 severe pneumonia (). We also recorded the data of some respiratory pathogens. There are 49 Epstein-Barr (EB) virus infection, 21 Herpes simplex virus infection, 14 parainfluenza virus infection, 23 influenza A virus infection, 31 influenza B virus infection, 11 cytomegalovirus infection, 10 coxsackie virus infection, 11 chlamydia infection, and 113 mycoplasma infection.
Table 1. Baseline characteristics of the recruited subjects.
85 cases were infected with single pathogen, 57 cases were infected with two pathogens, 23 cases were infected with three pathogens, and 17 cases were infected with no less than four pathogens.
Correlation between Ca/P/Mg levels and inflammatory indexes among FRTI cases
Serum Ca level was positively associated with WBC and PLT (r = 0.183, p = 0.006; r = 0.239, p < 10−4, ). Ca status was negatively associated with PCT (r= −0.193, p = 0.004, ). No marked association between Ca level and ESR was observed (r = 0.063, p = 0.354, ). P status was negatively associated with ESR and PCT (r= −0.163, p = 0.018; r= −0.242, p < 10−4, ). No significant association between P level and WBC/PLT was noted (r= −0.103, p = 0.131; r = 0.059, p = 0.389, ). No marked association between Mg level and WBC/ESR/PLT/PCT was observed (r = 0.058, p = 0.393; r = 0.088, p = 0.203; r=-0.094, p = 0.167; r= −0.032, p = 0.640, ).
Table 2. Correlation between Ca/P/Mg status and WBC/ESR/PLT/PCT in cases with respiratory diseases.
Differences of serum Ca/P/Mg levels between FRTI subgroups and controls
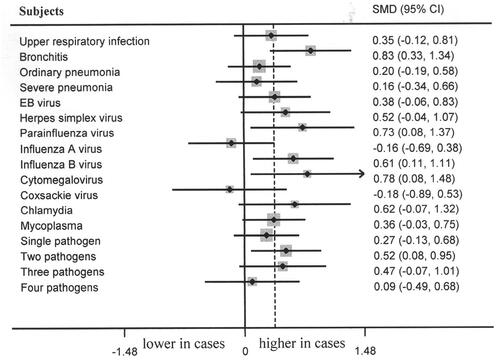
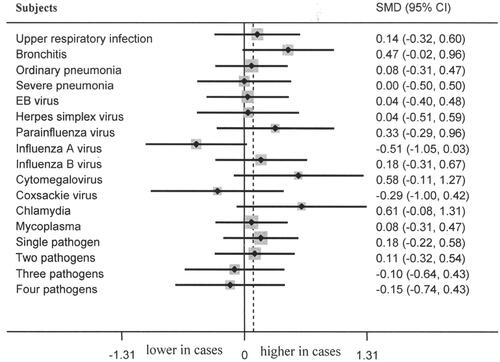
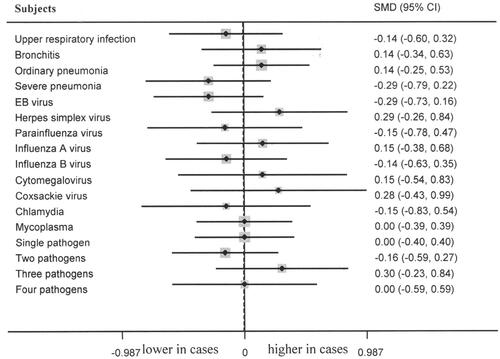
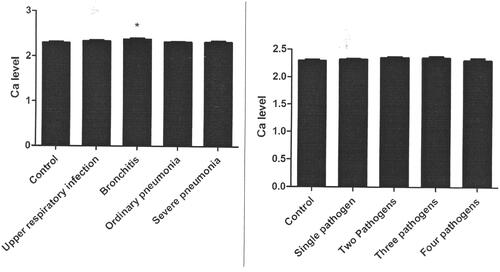
Healthy controls showed significantly lower serum level of Ca than that among bronchitis (p = 0.001, 95% CI 0.33–1.34, , ), parainfluenza virus infections (p = 0.027, 95% CI 0.08–1.37, , ), influenza B virus infection (p = 0.017, 95% CI 0.11–1.11, , ), cytomegalovirus infection (p = 0.029, 95% CI 0.08–1.48, , ) and two pathogens infections (p = 0.020, 95% CI 0.08–0.95, , ). No marked differences of serum Ca level between controls and cases of upper respiratory infection, ordinary pneumonia, severe pneumonia, EB virus infection, herpes simplex virus infection, influenza A virus infection, coxsackie virus infection, chlamydia infection, mycoplasma infection, single pathogen infection, three pathogens infection and four pathogens infection (, Citation4) were observed. No marked difference of P/Mg levels between controls and cases of upper respiratory infection, bronchitis, ordinary pneumonia, severe pneumonia, EB virus infection, herpes simplex virus infection, parainfluenza virus infection, influenza A virus infection, influenza B virus infection, cytomegalovirus infection, coxsackie virus infection, chlamydia infection, mycoplasma infection, single pathogen infection, two pathogens infection, three pathogens infection, and four pathogens infection were noted (, Citation3).
Table 3. Characteristics of Ca/P/Mg levels in respiratory .diseas es.
Association between serum Ca level and bronchitis/influenza B virus infection
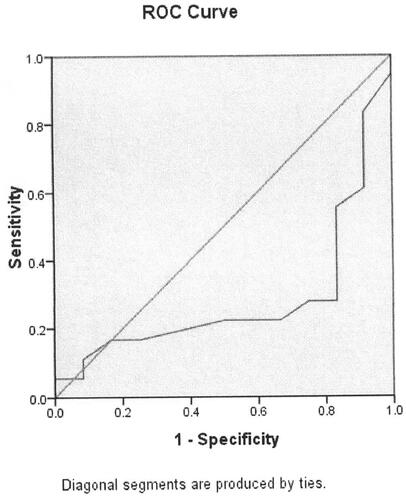
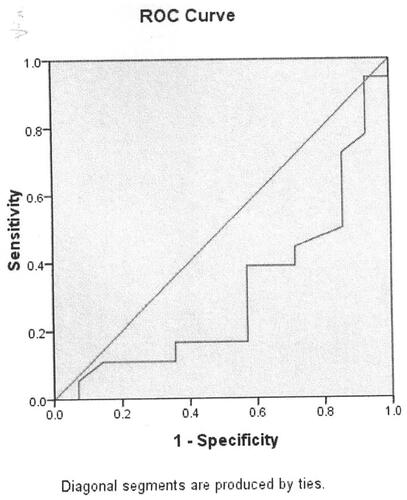
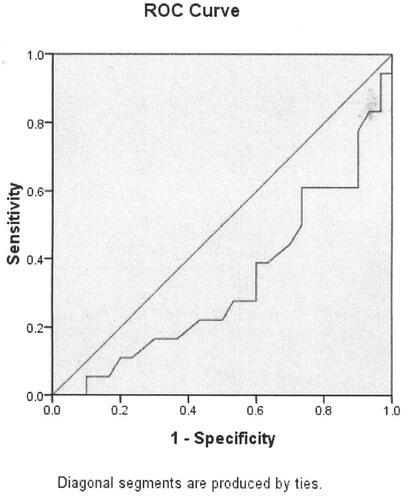
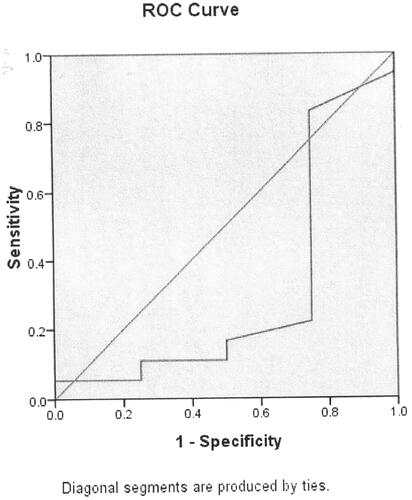
In terms of the results of the significant differences of serum Ca level in healthy controls compared with that among bronchitis, parainfluenza virus infections, influenza B virus infection, cytomegalovirus infection. We used ROC curve analysis to measure the association between serum Ca level and bronchitis, parainfluenza virus infection, influenza B virus infection and cytomegalovirus infection. Serum Ca level was significantly associated with bronchitis (p = 0.047, , ). The cut-off point is 2.33 mmol/L. Serum Ca level was not associated with parainfluenza virus infection (p = 0.068, , ). Serum Ca level was significantly associated with influenza B virus infection (p = 0.049, , ). Serum Ca level was not associated with cytomegalovirus infection (p = 0.250, , ).
Table 4. Association between Ca level and respiratory diseases.
Discussion
Ca, P and Mg play an important role in a lot of physiological processes. Sufficient intake of Ca/P/Mg is vital for the growth and development of children. Meanwhile, Ca/P/Mg are important components of various enzymes, participating in many pathological processes [Citation39]. Hence, monitoring the serum levels of Ca/P/Mg is of great significances for children, which is also helpful for the appropriate nursing of children, guiding the nutrient intake. We found that serum Ca level was significantly associated with WBC, PLT and PCT; serum P level was markedly associated with ESR and PCT; controls showed significantly lower serum level of Ca than that among the FRTI with bronchitis, parainfluenza virus infection, influenza B virus infection, cytomegalovirus infection, and two pathogens infection; and further ROC curve analysis showed that serum Ca level was significantly associated with FRTI with bronchitis and influenza B virus infection. Our findings indicated that serum Ca and P levels may reflect the inflammatory status, and serum Ca level may be an indicator of the risk of bronchitis and influenza B virus infection.
Several mechanisms may account for our findings. First, inflammation is involved in the progression of FRTI. WBC, ESR, PLT and PCT are important markers of inflammation [Citation40].
Patients with COVID-19 showed significantly higher values of total WBC, CRP and ESR compared with controls [Citation41]. PCT status reflects the infection, inflammation and organ failure [Citation42]. Elevated CRP and PCT were associated with the severity and prognosis of COVID-19 [Citation43]. CRP can activate the inflammatory cells and induce the secretion of inflammatory cytokines [Citation44]. Refractory mycoplasma pneumonia demonstrated significantly increased levels of CRP, ESR and PCT [Citation45]. These above-mentioned evidence showed that WBC/ESR/PLT/PCT level increased obviously during the status of inflammation. Ca and P participate in a lot of inflammatory signal transductions, and are components of some enzymes, which may account for that serum Ca level was correlated with WBC, PLT and PCT, and P level was correlated with ESR and PCT. Notably, serum Ca status was negatively associated with PCT, which may be due to the fact that calcitonin can decrease the level of Ca, and PCT is the propeptide substance of calcitonin without hormone activity [Citation46]. The null association between Mg and inflammatory markers may be due to the facts that Mg exerts its effects mainly on relaxing the smooth muscle and constituting various enzymes, and Mg does not directly play a role in the inflammation-associated signal pathways. Second, the serum levels of Ca/P/Mg were obtained during the early stage of FRTI. Hence, Ca/P/Mg status may mainly reflect the risk, not the progression of FRTI. We also noted that there were no differences of serum levels of P/Mg between healthy controls and FRTI subgroups. Interestingly, we observed that serum level of Ca was significantly higher among FRTI cases with bronchitis, parainfluenza virus infection, influenza B virus infection, cytomegalovirus infection, and two pathogens infection than that in healthy controls. Lung is one of the most vulnerable target organs for Ca deposition [Citation47]. Ca deposition in the alveolar epithelial or endothelial cells may destroy the alveolar-epithelial-endothelial barrier, resulting in the lung injury, even acute pulmonary edoema [Citation48]. Ca oxalate deposits increased the risk of urinary tract infection and tubulointerstitial injury, with bacterial inducing increased presence of Ca oxalate deposition in a renal allograft [Citation49]. In the meantime, hypercalcemia increases tumour necrosis factor, interleukin-1 and PCT in plasma and lung perfusate [Citation50]. The above-mentioned data indicated that high level of Ca can lead to the lung injury and induce inflammation. Finally, ROC curve analysis yielded that serum Ca level was significantly associated with FRTI with bronchitis and influenza B virus. It may be due to the fact that influenza B virus infection is a common respiratory disorder in children [Citation51]. The high incidence of bronchitis and influenza B virus infection may be associated with the dysregulation of Ca. Multiple factors may affect the alterations of Ca/P/Mg among children. The growth and development of children requires sufficient intake of trace elements.
Our findings showed that multiple pathogens infection did not display differences of Ca/P/Mg levels from single pathogens infection. We speculated that specific pathogen did not affect the metabolism of trace elements, and the testing of Ca/P/Mg levels was performed in the early stage of diseases, which made the null differences. Meanwhile, severe pneumonia cases did not show marked difference of serum level of Ca from ordinary pneumonia and bronchitis, which may be due to the facts that severe cases were likely to have poor appetite and lower intake of trace elements, which offset the possible deposition of Ca. Notably, primary immunodeficiencies in children may present with recurrent pneumonia, bronchitis and suppurative otitis [Citation52]. The dysfunction of immune system is likely to result in recurrent infections [Citation52]. Immunodeficiency is a state of impaired immunity balance in the body, which usually predispose to infections [Citation52]. About 65% of recurrent RTI were underlain by disorders of humoral immunity [Citation53]. RTI in children are also often underlain by both IgA and IgM abnormal states [Citation53]. Further studies should be performed to investigate the impact of immunodeficiency.
Our investigation had obvious strengthes that we further analyzed the association between serum Ca/P/Mg levels and FRTI subgroups, and we also clarified the impact of different pathogens infected on the serum status of Ca/P/Mg. The homeostasis of Ca/P/Mg levels is helpful for the prevention and treatment of FRTI. Too higher or lower levels of serum Ca/P/Mg are not appropriate for the body, which indicates that the nutrient nursing merits consideration, and appropriate nutrient is important for the paediatric health.
Some limitations in our study also merit attention. First, the ongoing monitoring of the serum Ca/P/Mg levels may be helpful for understanding the impact of Ca/P/Mg on the progression and prognosis of RTI. On the other hand, the follow-up of the recurrence of FRTI is helpful for the knowledge of the long-term influence of Ca/P/Mg levels on FRTI. Regrettably, due to the time limit, further studies may be performed to elucidate this issue. Second, the in-depth mechanism of the association between Ca/P/Mg levels and different pathogens should be studied in the future, which will offer the insight for the therapy of the specific pathogen infection. Nevertheless, our study had important clinical implications that serum Ca level may be a biomarker of specific FRTI.
In conclusion, our investigation indicates that serum levels of Ca and P may reflect the inflammatory status in FRTI. High level of serum Ca was associated with bronchitis and influenza B virus infection in FRTI. Early monitoring and intervention of Ca/P may be needed for children with FRTI, appropriate nutrient nursing is helpful for the paediatric health.
Adherence to national and international ethical regulations
This investigation was conducted according to the Declaration of Helsinki. This study was conducted in the retrospective style. Hence, the informed consent of guardians of participants was waived. All the recruited data was de-identified. We conducted the anonymous data analysis in the retrospective style.
Author contributions
Wenjing Shi performed the study design, Liangxia Wu performed the data collection, Song Mao performed the data analyses and the writing of manuscript.
Acknowledgements
Not applicable.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data will be shared by the corresponding author upon the scientific request.
Additional information
Funding
References
- Kenmoe S, Vernet MA, Miszczak F, et al. Genetic diversity of human respiratory syncytial virus isolated among children with acute respiratory infections in Southern Cameroon during three consecutive epidemic seasons, 2011-2013. Trop Med Health. 2018;46(1):1. doi: 10.1186/s41182-018-0088-7.
- Yang Z, Feng T, Guan W, et al. Chinese expert consensus on immunoprophylaxis of common respiratory pathogens in children (2021 edition). J Thorac Dis. 2022;14(3):749–11. doi: 10.21037/jtd-21-1613.
- Schuster Bruce C, Hoare C, Mukherjee A, et al. Managing acute respiratory tract infections in children. Br J Nurs. 2017;26(11):602–609.
- Heather KL, Karl OAY, Christopher TT, et al. Respiratory infections in patients with primary immunodeficiency. J Allergy Clin Immunol Pract. 2022;10(3):683–691.
- Trandafir LM, Moscalu M, Diaconu G, et al. The impact of respiratory tract infections on the nutritional state of children with cystic fibrosis. Rev Med Chir Soc Med Nat Iasi. 2013;117(4):863–869.
- Champatiray J, Satapathy J, Kashyap B, et al. Clinico-aetiological study of severe and very severe pneumonia in two months to five years children in a tertiary health care centre in Odisha, India. J Clin Diagn Res. 2017;11(9):SC06–SC10. doi: 10.7860/JCDR/2017/26027.10595.
- Bergman P, Norlin AC, Hansen S, et al. Vitamin D3 supplementation in patients with frequent respiratory tract infections: a randomised and double-blind intervention study. BMJ Open. 2012;2(6):e001663. doi: 10.1136/bmjopen-2012-001663.
- Kamin W, Adams O, Kardos P, et al. Liver involvement in acute respiratory infections in children and adolescents - results of a non-interventional study. Front Pediatr. 2022;10:840008.
- Breidthardt T, Christ-Crain M, Stolz D, et al. A combined cardiorenal assessment for the prediction of acute kidney injury in lower respiratory tract infections. Am J Med. 2012;125(2):168–175. doi: 10.1016/j.amjmed.2011.07.010.
- Litzman J, Lokaj J, Krejcí M, et al. Isoprinosine does not protect against frequent respiratory tract infections in childhood. Eur J Pediatr. 1999;158(1):32–37.
- Su G, Chen X, Liu Z, et al. Oral astragalus (Huang qi) for preventing frequent episodes of acute respiratory tract infection in children. Cochrane Database Syst Rev. 2016; doi: 10.1002/14651858.CD011958.pub2.;12(12):CD011958.
- Lewandowicz-Uszyńska A, Pasternak G, Pentoś K. Immunoglobulin G deficiency in children with recurrent respiratory infections with and without history of allergy. Adv Exp Med Biol. 2021;1289:63–70. doi: 10.1007/5584_2020_541.
- Hadi H, Stoltzfus RJ, Moulton LH, et al. Respiratory infections reduce the growth response to vitamin a supplementation in a randomized controlled trial. Int J Epidemiol. 1999;28(5):874–881. doi: 10.1093/ije/28.5.874.
- Yallapragada SG, Nash CB, Robinson DT. Early-life exposure to antibiotics, alterations in the intestinal microbiome, and risk of metabolic disease in children and adults. Pediatr Ann. 2015;44(11):e265-9–e269. doi: 10.3928/00904481-20151112-09.
- Rostampour N, Almasi T, Rostampour M, et al. Impact of low level radiation on concentrations of some trace elements in radiation workers. J Exp Ther Oncol. 2018;12(3):187–192.
- Ramírez-Luzuriaga MJ, Larson LM, Mannar V, et al. Impact of double-fortified salt with iron and iodine on hemoglobin, anemia, and iron deficiency anemia: a systematic review and Meta-Analysis. Adv Nutr. 2018;9(3):207–218. doi: 10.1093/advances/nmy008.
- Torres B, Alcubilla P, González-Cordón A, et al. COVID19 hospital clínic infectious diseases research group. Int J Infect Dis. 2021;104:164–168. doi: 10.1016/j.ijid.2020.11.207.
- Adoue D, Arlet P, Vilain C, et al. Hypophosphoremia, prognostic factor of respiratory infections in aged patients. Apropos of 10 cases. Rev Med Interne. 1988;9(1):73–77.
- Calder PC, Carr AC, Gombart AF, et al. Optimal nutritional status for a well-functioning immune system is an important factor to protect against viral infections. Nutrients. 2020;12(4):1181. doi: 10.3390/nu12041181.
- Fatemeh Y, Meshkat A, Atefeh A, et al. The role of electrolyte imbalances in predicting the severity of COVID-19 in the hospitalized patients: a cross-sectional study. Sci Rep. 2022;12(1):14732.
- Mao S, Liu F, Wang XP, et al. Association between Ca/Mg/P status and suppurative tonsillitis in children. J Biol Regul Homeost Agents. 2018;32(4):881–885.
- Khadilkar A, Khadilkar V, Chinnappa J, et al. Prevention and treatment of vitamin D. Indian Pediatr. 2017;54(7):567–573. doi: 10.1007/s13312-017-1070-x.
- Kew KM, Kirtchuk L, Michell CI. Intravenous magnesium sulfate for treating adults with acute asthma in the emergency department. Cochrane Database Syst Rev. 2014;4(4):CD011050. doi: 10.1002/14651858.CD011050.pub2.
- Tang CF, Ding H, Jiao RQ, et al. Possibility of magnesium supplementation for supportive treatment in patients with COVID-19. Eur J Pharmacol. 2020;886:173546. doi: 10.1016/j.ejphar.2020.173546.
- Chen Q, Zhang Y, Ding D, et al. Associations between serum calcium, phosphorus and mortality among patients with coronary heart disease. Eur J Nutr. 2017;57(7):2457–2467. doi: 10.1007/s00394-017-1518-8.
- Hawke K, van Driel ML, Buffington BJ, et al. Homeopathic medicinal products for preventing and treating acute respiratory tract infections in children. Cochrane Database Syst Rev. 2018;9(9):CD005974. doi: 10.1002/14651858.CD005974.pub5.
- El Mallah C, Ghattas H, Shatila D, et al. Urinary magnesium, calcium, and phosphorus to creatinine ratios of healthy elementary school Lebanese children. Biol Trace Elem Res. 2016;170(2):264–270. doi: 10.1007/s12011-015-0484-3.
- Alemzadeh E, Alemzadeh E, Ziaee M, et al. The effect of low serum calcium level on the severity and mortality of covid patients: a systematic review and meta-analysis. Immun Inflamm Dis. 2021;9(4):1219–1228. doi: 10.1002/iid3.528.
- Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. doi: 10.1136/bmj.i6583.
- Chowdhury B, Hoque MA, Hossain MA, et al. Serum zinc, copper, magnesium & phosphorus level in children with severe acute malnutrition (SAM). Mymensingh Med J. 2016;25(4):635–640.
- Fiaccadori E, Coffrini E, Ronda N, et al. Hypophosphatemia in course of chronic obstructive pulmonary disease. Prevalence, mechanisms, and relationships with skeletal muscle phosphorus content. Chest. 1990;97(4):857–868.
- Rolla G, Bucca C. Hypomagnesemia and bronchial hyperreactivity. A case report. Allergy. 1989;44(7):519–521.
- Dutta A, Hung CY, Chen TC, et al. An IL-17-EGFR-TRAF4 axis contributes to the alleviation of lung inflammation in severe influenza. Commun Biol. 2023;6(1):600.
- Staudacher AG, Stevens WW. Sinus infections, inflammation, and asthma. Immunol Allergy Clin North Am. 2019;39(3):403–415. doi: 10.1016/j.iac.2019.03.008.
- Stockley RA. Role of inflammation in respiratory tract infections. Am J Med. 1995;99(6B):8S–13S.
- Chmiel JF, Konstan MW. Anti-inflammatory medications for cystic fibrosis lung disease: selecting the most appropriate agent. Treat Respir Med. 2005;4(4):255–273. doi: 10.2165/00151829-200504040-00004.
- Moe SM. Disorders involving calcium, phosphorus, and magnesium. Prim Care. 2008;35(2):215–237. doi: 10.1016/j.pop.2008.01.007.
- Nascimento-Carvalho CM, Souza-Marques HH. Recommendation of the Brazilian society of pediatrics for antibiotic therapy in children and adolescents with community-acquired pneumonia. Rev Panam Salud Publica. 2004;15(6):380–387. doi: 10.1590/s1020-49892004000600003.
- Wronski S, Dannenmaier J, Schild S, et al. Engystol reduces onset of experimental respiratory syncytial virus-induced respiratory inflammation in mice by modulating macrophage phagocytic capacity. PLOS One. 2018;13(4):e0195822. doi: 10.1371/journal.pone.0195822.
- Gassling V, Douglas TE, Purcz N, et al. Magnesium-enhanced enzymatically mineralized platelet-rich fibrin for bone regeneration applications. Biomed Mater. 2013;8(5):055001. doi: 10.1088/1748-6041/8/5/055001.
- Sabir FNK, Ameen EM. Hematological, immunological, and inflammation markers in patients of COVID-19. Cell Mol Biol (Noisy-le-Grand). 2023;69(2):79–83. doi: 10.14715/cmb/2023.69.2.13.
- Paliwal A, Nawal CL, Meena PD, et al. A study of procalcitonin as an early predictor of severity in acute pancreatitis. J Assoc Physicians India. 2022;70(4):11–12.
- Elouadani M, Konzi C, Addi Y, et al. C-reactive protein, ferritin, and procalcitonin in 300 moroccan patients with COVID 19. Clin Lab. 2022;68(10):2158–2163. doi: 10.7754/Clin.Lab.2022.220124.
- Mahajan N, Bahl A, Dhawan V. C-reactive protein (CRP) up-regulates expression of receptor for advanced glycation end products (RAGE) and its inflammatory ligand EN-RAGE in THP-1 cells: inhibitory effects of atorvastatin. Int J Cardiol. 2010;142(3):273–278. doi: 10.1016/j.ijcard.2009.01.008.
- Zhai YY, Wu SZ, Yang Y, et al. An analysis of 20 clinical cases of refractory mycoplasma pneumonia in children. Ann Palliat Med. 2020;9(5):2592–2599. doi: 10.21037/apm-19-497.
- Matowicka-Karna J. Markers of inflammation, activation of blood platelets and coagulation disorders in inflammatory bowel diseases. Postepy Hig Med Dosw (Online). 2016;70:305–312. doi: 10.5604/17322693.1199305.
- Wada-Mihara C, Seto H, Ohba H, et al. Local administration of calcitonin inhibits alveolar bone loss in an experimental periodontitis in rats. Biomed Pharmacother. 2018;97:765–770. doi: 10.1016/j.biopha.2017.10.165.
- Hsu YH, Chen HI. Acute respiratory distress syndrome associated with hypercalcemia without parathyroid disorders. Chin J Physiol. 2008;51(6):414–418.
- Holmes F, Harlan J, Felt S, et al. Pulmonary oedema in hypercalcaemic crisis. Lancet. 1974;1(7852):311–312.
- Özdemir BH, Ayva Ş, Özdemir G, et al. Renal allograft with calcium oxalate deposition: association with urinary tract infection and development of interstitial fibrosis. Exp Clin Transplant. 2018;16 Suppl 1(Suppl 1):126–130.
- Piralla A, Lunghi G, Ruggiero L, et al. Molecular epidemiology of influenza B virus among hospitalized pediatric patients in Northern Italy during the 2015-16 season. PLOS One. 2017;12(10):e0185893.
- Lewandowicz-Uszyńska A, Pasternak G, Świerkot J, et al. Primary immunodeficiencies: diseases of children and adults - a review. Adv Exp Med Biol. 2021;1289:37–54. doi: 10.1007/5584_2020_556.
- Pasternak G, Lewandowicz-Uszyńska A, Pentoś K. Disorders of humoral immunity in children with IgG subclass deficiency and recurrent respiratory infections. Adv Exp Med Biol. 2018;1108:99–106. doi: 10.1007/5584_2018_263.