Abstract
Background
The beneficial effects of a plant-based diet on gut microbiota diversity are well documented, however, its impact on clinical bowel health and defecation patterns are less well understood. Vegetarian diets have been associated with a higher bowel movement (BM) frequency as well as softer stools in cross-sectional studies. The effects of the de-novo adoption of a vegan diet on bowel health, however, have never been investigated in a randomized-controlled trial.
Materials and Methods
The present study examined bowel health and defecation patterns in relation to diet and nutrient intake in a young and healthy sample of n = 65 physically-active German university students who were randomly assigned to either a vegan or a meat-rich diet for eight weeks. Bowel health assessment included the Bristol Stool Form Scale (BSFS), the Gastrointestinal Quality of Life Index (GIQLI) and the Cleveland Clinic Fecal Incontinence Score (CCFIS). Nutrient intake was assessed using weighed food diaries. The study was prospectively registered at the German Clinical Trial Register (DRKS00031541).
Results
Weekly BM frequency slightly increased in vegans, whereas it remained unaltered in participants assigned to a meat-rich diet. Fiber intake increased significantly in vegans (34.89 (18.46) g/d) whereas it decreased in those assigned to the meat-rich group (22.79 (12.5) g/d). No significant intergroup differences in BSFS and CCFIS patterns were observed. Adoption of a vegan diet neither resulted in a transient increase in abdominal discomfort nor in a decreased gastrointestinal quality of life, which was comparable across the diet groups.
Conclusions
The short-term de-novo adoption of a vegan diet did not negatively affect markers of bowel health in this study.
Introduction
Plant-based diets enjoy increasing popularity in many modern societies for their potential benefits to human and environmental health [Citation1,Citation2]. The adoption of vegetarian and vegan diets has been associated with a lower risk of incident type-2-diabetes [Citation3], cardiovascular disease [Citation4] and cancer [Citation5]. Both diets also offer benefits for gastrointestinal health [Citation6], and have been linked to gut microbiota diversity and improved Short Chain Fatty Acid (SCFA) levels as well as protective effects against cancers of the digestive system [Citation7,Citation8].
While the beneficial effects of plant-based nutrition on the gut microbiota composition are now well documented [Citation9,Citation10], its impact on clinical bowel health, defecation patterns and stool morphology are less well understood [Citation11]. Often attributed to a more optimal fiber intake [Citation11,Citation12], vegetarian diets have been associated with a higher Bowel Movement (BM) frequency as well as softer stools with fewer superficial cracks [Citation11,Citation13]. As such, vegetarians appear to be less frequently affected from chronic constipation [Citation14] – A debilitating condition with a high economic and social burden in many Western societies [Citation15]. Vegetarians also avoid meat, for which an inverse dose relationship with the frequency of defecation has been reported [Citation16].
Bowel health and BM frequency in plant-based populations seem to be strongly associated with fiber intake [Citation17], and our previous work suggested that the overall quality of a plant-based diet plays an important role for both [Citation11]. The number of studies in this field, however, is severely limited [Citation11,Citation13,Citation16–18], and the majority of information was derived from cross-sectional studies.
Randomized-controlled trials in this scientific area are scarce but could greatly enhance our understanding of the role of plant-based nutrition for defecation and bowel health beyond the gut. This applies in particular to the ‘de-novo’ adoption of plant-based diets, e.g. when individuals suddenly transition from a meat-based diet to a diet that restricts or completely avoids animal products and that is more abundant in high-fiber foods.
Here, we report the results of an eight-weeks dietary intervention in healthy German omnivores who were randomized to either a meat-rich diet or a plant-based vegan diet. Defecation patterns, stool morphology and self-perceived gastrointestinal quality of life were assessed with validated tools and compared between both groups.
We hypothesized that the vegan diet would increase BM frequency and soften stools in comparison to the meat-rich diet. In addition to that, we put forward the hypothesis that the expected increase in the intakes of fibers and certain phytochemicals (such as phytic acid) could transiently result in abdominal discomfort, bloating and cramping in vegans, and thus in a temporary reduction of gastrointestinal quality of life during the first weeks of the new diet. Finally, we used dietary intake data to examine potential associations between bowel movement frequency and selected nutrients and foods.
Materials and methods
Study design
The study was conducted between April and June 2023 and extends our series of studies on plant-based nutrition [Citation19–21]. The methodology and design have been adopted from our previous trial [Citation19], but were modified in some relevant aspects and supplemented with new features and study outcomes. The study was a monocentric, randomized-controlled trial performed at the Centre for Complementary Medicine at Freiburg University Medical Center, Germany. This multi-topic study was primarily designed to investigate the impact of a vegan dietary intervention on immune system-related outcomes. The primary study outcome focused on changes in measured neutrophilic granulocytes. Given the comprehensive scale and outlay of the study, however, multiple secondary endpoints were included. Immune-system related outcomes will be discussed at a later stage in another publication specifically dedicated towards blood count alterations subsequent to the dietary modification. Here, we focused on a detailed study description and on multiple secondary endpoints including alterations in nutrient intake patterns, defecation patterns and overall bowel health.
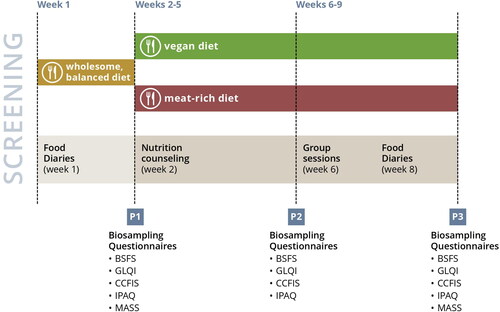
schematically displays the course of the study. After a one-week run-in phase, participants were randomized to either a vegan or meat-rich diet for eight consecutive weeks. The meat-rich diet was defined by a consumption of at least 150 g of meat daily, whereas the vegan diet was defined by the exclusion of all animal-based products. Any kind of meat including poultry, pork or beef were permitted whereas fish and fish products were not considered to fulfill this criterion in the context of our study.
Recruitment
We recruited participants by placing advertisements on notice boards at local universities, in student residences and in public places in Freiburg, Germany. In addition, we posted advertisements on social media platforms. Potentially eligible individuals were screened by phone and subsequently invited for an in-person appointment. In- and exclusion criteria were checked on both occasions.
Eligibility criteria
Healthy individuals aged 18–60 years and with a BMI between 21 and 30 kg/m2 were considered. Criteria of exclusion included: chronic health conditions, a regular intake of medication, pregnancy or lactation, clinically-relevant allergies, eating disorders as well as alcohol (>20 g/d) and daily nicotine intake. Individuals who reported any kind of infection two weeks prior to their screening appointment were not considered. Those who already indicated a plant-based diet prior to their study participation were excluded, as well. The same applied to individuals who considered themselves as ‘flexitarians’ and to individuals who consumed meat less than 3 times a week on average prior to the study. Eligible participants had to be able to speak and understand German as the study included multiple nutrition-related group seminars and dietary counseling sessions.
Course of the study
Individuals who met the inclusion criteria were assigned to a balanced mixed diet for one week (hereafter referred to as ‘run-in phase’). Participants were asked to prepare their own meals in accordance with the recent recommendations of the German Nutrition Association (DGE) for the German general population [Citation22]. For this, participants were instructed in individual coaching sessions and received a handout summarizing the key points. Although these recommendations were considered obvious by many participants, the coaching session was mandatory to partake in the study. The key aspects of these recommendations are summarized in Supplementary Table 1. Moreover, participants were required to complete a weighted food diary at three consecutive days during the run-in phase.
After the first week, participants were invited for a second appointment where randomization and biosampling took place. Prior to that, we captured sociodemographic data and participants were asked to complete several questionnaires as discussed below. An independent person who was unfamiliar with the study aims created a block-wise randomization list (block size: 6) using Stata software. Sealed envelopes were used for implementation.
Blood samples were taken early in the morning between 7 and 9:30 am after a fasting period of at least 10 h. We subsequently instructed all participants on how to implement their assigned diet in individual coaching sessions of at least 30 min. The same group-specific scheme was used for all participants who also received a study- and diet-specific booklet for their assigned diet. The coaching ended with a brief capstone session and participants were invited to a mandatory group seminar for their diet at the end of the first study week (see below). The information in the booklet for the vegan group was mainly compiled of specifically tailored advice for individuals who just recently adopted a plant-based diet. It was mainly based on two sources: (I) the Physician Committee for Responsible Medicine’s literature resource hub and (II) on information from the German website of the Physicians Association of Nutrition [Citation23,Citation24]. The diet thus focused on whole plant-based foods and aimed to minimize processed food items. The information in the booklet for the meat-rich group was based on a brochure published and developed by the German QS GmbH [Citation25].
Blood results were checked on the same day and participants with abnormal values (e.g. due to iron-deficiency anemia or elevated inflammation markers suggesting a recent infection) were excluded despite having indicated good health in the screening examination.
Biosampling was repeated after 4 weeks and 8 weeks, respectively. Weighed food diaries were again collected during the last study week. We asked participants to complete various questionnaires at both stages. Supplementary Table 2 summarizes the collected data for each time point. Participants received a financial remuneration of 400€ upon successful completion of the study (as defined by adhering to the assigned diet for eight weeks, by providing complete nutritional protocols and by partaking in all biosampling sessions and at least one nutrition-related group seminar).
Group seminars, coaching sessions and newsletters
A hallmark of this study was our regular and close contact with the participants to increase motivation and to ensure high dietary adherence in both groups. A study-specific hotline and email-address were installed to allow for a close exchange between the study personnel and the participants throughout the study and with regard to all study aspects, e.g. for diet-related questions or administrative issues.
Two diet-specific group seminars (a mandatory one in the first week after randomization and an optional one in week 6) of 45 min each were offered. These seminars included important contextual information on the assigned diets, as well as cooking and shopping hints. The small seminar group sizes (6–10 persons) allowed for an interpersonal exchange between participants, who were also encouraged to share both their positive and negative experiences with other members. Potential solutions were discussed in a constructive and open-end style. Each seminar was led by at least two persons from the study personnel and the head of the study was always available for individual queries.
In addition to that, we offered weekly newsletters that covered study-related information in an FAQ-style. A specific micronutrient (e.g. iron, zinc or calcium) was discussed in every newsletter, detailing its relevance for human health and listing potential foods to ensure an adequate intake in both groups (Supplementary Table 3). The newsletter was created by an experienced physician supported by a registered dietician. It also included discounts for selected restaurants in Freiburg, where study participants of both groups received benefits upon showing their individualized certificate of participation (Supplementary Figure 1). Supplementary Table 4 summarizes these discounts and offers.
Bowel health and defecation pattern assessment
Bowel health and defecation pattern assessment have been described previously [Citation11,Citation26], and included the Bristol Stool Form Scale (BSFS) [Citation27], the Gastrointestinal Quality of Life Index (GIQLI) [Citation28], and the Cleveland Clinic Fecal Incontinence Score/Jorge-Wexner Score (CCFIS/JW Score) [Citation29]. The BSFS is a reliable and validated tool to evaluate the stool form of healthy adults [Citation30], and a surrogate marker for its consistency [Citation31]. The BSFS ranges from 1 (separate hard lumps) to 7 (water, no solid pieces) and previous studies suggested that values of 1 or 2 could be used as surrogate for a delayed Colonic Transit Time (CTT) in Western populations [Citation32]. Study participants were asked to define their stool by recording the number type that corresponded to their usual/most common stool type within the last week. Following Wilson’s method, BSFS stool types 1 and 2 were defined as constipation, whereas stool types 6 (fluffy pieces with ragged edges) and stool type 7 (described above) were defined as diarrhea [Citation33].
We assessed stool frequency with the question: ‘How many times do you have a BM per week?’ [Citation11,Citation26]. To screen for potential anal incontinence leakage, we used the CCFIS (Cleveland Clinic Fecal Incontinence Score)/Jorge-Wexner (JW) score [Citation29], which is a widely used and accepted instrument that applies a type x frequency matrix to obtain a participant’s perception of symptom. The overall scores ranges from 0 (indicating perfect continence) to 20 (suggesting complete incontinence) and was mainly used for the assessment of gas leakage in our study [Citation34].
Finally, we used the GIQLI developed and validated by Eypasch et al. in Germany [Citation28] to assess alterations in gastrointestinal life quality subsequent to dietary modifications. This reliable and validated questionnaire encompasses 36 items covering 5 sub-domains, such as abdominal symptoms (e.g. bloating and fullness) as well as physical and mental functioning associated with bowel health. There are 5 answer options for each item and scoring ranges from 0 points (the least desirable answer option) to 4 points (the most desirable answer option). Following the instructions of Eypasch et al. [Citation28], the modified (and validated) version for healthy individuals was used [Citation28].
Nutrient intake assessment and weighed food diaries
Dietary intake was estimated based on a 3-day weighed food diary. We instructed participants to quantify all consumed foods and beverages (including tap water) to the nearest 0.1 g or ml, respectively. The participants received detailed instructions in filling in the food diaries by qualified medical personnel and completed protocols were provided as templates. We provided participants with a calibrated precision scale (Wedo Elektronische Universalwaage Optimo5000, Dieburg, Germany or a comparable model) for a security deposit of 20 Euros.
Participants were asked to document the following information for all foodstuffs and beverages: time and place of consumption, brand name, exact product name, packaging details, condition at the time of purchase, weighed quantity and remaining quantity. In case an exact weighing was impossible, semi-quantitative household recording with measures (e.g. spoon, cups, etc.) was allowed following an established approach by Alexy et al. [Citation35]. NutriGuide® plus software (Version 4.9, Nutri-Science GmbH, Hausach, Germany) was used to analyze the protocols.
Biosampling details
We obtained 130 mL of blood from each participant by venipuncture in the early morning hours. Blood samples were used to measure various laboratory parameters, including a differential blood count, a standard biochemistry profile, a lipid panel as well as for peripheral blood mononuclear cell isolation. For this sub-analysis, we restricted ourselves to reporting high-sensitive C-reactive protein (hs-CRP) and albumin levels, which were deemed to be potentially relevant when discussing nutrient and bowel health related outcomes, and which were measured by the accredited Central Laboratory of the University Medical Center of Freiburg.
Covariates
We measured social status using the German version of the MacArthur Scale of Subjective Social Status (MASSS) [Citation36], which is a pictorial representation that employs a symbolic ladder developed to capture the common sense of social status based on usual socioeconomic status indicators [Citation37]. Physical activity was assessed with the International Physical Activity Questionnaire - Short Form (IPAQ-SF), a reliable method that showed associations with an individual’s cardiorespiratory fitness but that also has been reported to overestimate physical activity [Citation38,Citation39]. Supplementation behavior was meticulously captured and participants were asked to bring supplements taken within the last 12 months prior to the baseline examination as described by Kristensen et al. [Citation40]. Supplement names and content as well as intake frequency and quantity were noted. Participants were requested not to take any supplements during the study. Nevertheless, we inquired about supplement/medication usage during the study period at week 8 to assess for all potentially relevant confounders.
Statistical analysis
We used Stata 14 software (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP) for the statistical analysis. Box plots and histograms were used in conjunction with Stata’s Shapiro-Wilk Test to test for normality. Normally distributed variables were described with their mean and standard deviation whereas categorical variables were described as follows: number of observations (percentage).
Depending on the data distribution, parametric (paired and unpaired two-tailed t-tests) and non-parametric tests (Wilcoxon rank-sum test, Mann–Whitney-U-Test) were used to test for statistically significant intergroup differences in continuous variables. For the intergroup comparison of the proportions of categorical variables, the chi-squared test, Fisher’s exact test, McNemar-test, and McNemar-Bowker test were used where applicable. Statistical significance was determined at α = 0.05. We used scatterplots, Pearson’s correlation coefficients, and Spearman’s correlations coefficients to examine potential associations between the estimated fiber intakes and BM frequency as well as the GIQLI. Stripplots were used to visualize the relationship between macronutrient distributions and diet groups.
To control the family-wise error rate when performing multiple hypothesis tests, we used Stata’s command ‘wyoung’. The command enables the user to calculate adjusted p-values using the free step-down resampling methodology of Westfall and Young [Citation41]. A precursor to the Romano-Wolf procedure, this command allows for dependence amongst p-values. The Westfall-Young correction was performed using 1000 and 10,000 bootstraps where applicable. Familywise error rate-adjusted Westfall-Young p-values were reported for all significant crude outcomes and may be obtained from the legend of the respective tables.
Finally, a repeated measures analysis of variance (ANOVA) was performed for selected outcomes of interest to test for the between-subject factor of diet (two levels: meat-rich diet vs vegan diet), and the within-subject factor of time (three levels: week 0, week 4 and week 8).
Results
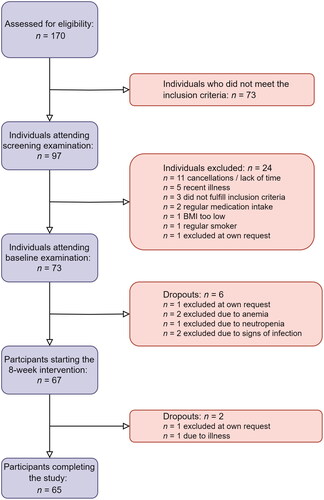
The sample encompassed n = 65 individuals who completed the study. displays a participant inclusion flowchart, providing an explanation for inclusion and exclusion of participants in our study.
shows baseline sample characteristics. The sample included 60 % males and 40% females. Participants had a median age of 23 years and a median BMI of 23.45 kg/m2. Approximately 2/3 of the sample were highly active in terms of physical activity (health enhancing physical activity, HEPA). The sample was mainly composed of students, and all participants had finished secondary education with the German Abitur (highest national high-school diploma). No significant differences in baseline sample characteristics were observed between those participants randomized to the vegan diet and those randomized to the meat-rich diet.
Table 1. Sample characteristics including sociodemographic, anthropometric and physical activity data at baseline, after 4 weeks and after 8 weeks of the dietary intervention.
Weight slightly decreased in both groups over the course of the study. We observed no significant intergroup differences in the median/mean weight change scores when comparing baseline to week 8 (−1.25 (1.7) kg vs. −1.08 ± 1 kg; p = .295). Likewise, participants did not significantly alter their physical activity levels over the course of the study.
shows bowel health outcomes at baseline and over the course of the study. Median weekly BM frequency was 9 for the entire sample and no significant baseline differences between the two groups were detected. The BSFS type 4 was the most prevalent type in both groups at baseline. The mean GIQLI score was slightly higher in those randomized to the vegan group, yet again no significant intergroup differences were found at baseline. Over the course of the study, median BM frequency slightly increased in the vegan group whereas it remained almost unchanged in the meat-rich group (Electronic Supplementary Figure 2). The differences, however, were not statistically significant. No significant intergroup differences were observed in the overall BSFS patterns at week 4 and 8, respectively. Four weeks into the study, vegan participants scored significantly better on the GIQLI emotional subscale. Although the intergroup difference remained significant after adjustments, it appeared marginal from a clinical viewpoint. Gas leakage did not appear to be an issue in any of the groups.
Table 2. Bowel movement frequency, defecation patterns and gastrointestinal life quality at baseline, after 4 weeks and after 8 weeks of the dietary intervention.
Nutrient intakes at baseline and at week 8 are displayed in . We observed no significant intake differences at baseline, except for total moisture intake which was significantly higher in those assigned to the vegan group (p = .017). Following the eight-week dietary intervention, significant intergroup intake differences were observed for the following nutrients: protein, fiber, calcium, sodium, phosphorus and zinc. Significant changes in comparison to the baseline-values are marked with an asterisks in . This table also lists the local dietary recommendations published by the Societies for Nutrition in Germany (DGE), Austria (Österreichische Gesellschaft für Ernährung, ÖGE) and Switzerland (Schweizerische Gesellschaft für Ernährung, SGE), the so-called D-A-CH (D—Deutschland, Germany, A—Austria, CH—Confederation Helvetica, Switzerland) [Citation42].
Table 3. Nutrient intakes at baseline and after 8 weeks of the dietary intervention.
At baselines no significant differences in mean energy-adjusted fiber intakes were found between the meat-rich and the vegan group (12.75 ± 4.59 g/1000 kcal vs 11.12 ± 4.25 g/1000 kcal). At week 8, significant differences were found (10.88 ± 3.46 g/1000 kcal vs 15.96 ± 4.40 g/1000 kcal) at a p-value <.001.
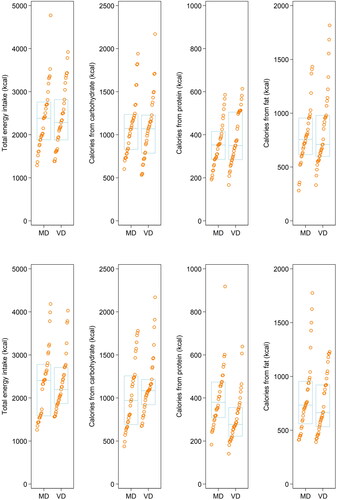
A pre-post comparison of macronutrient-intake profiles may be obtained from .
Figure 3. Macronutrient profile in both groups before and after the intervention. Stripplot showing macronutrient profiles for both groups at baseline (top) and after eight weeks (bottom). MD: Meat-rich diet; VD: Vegan diet.
shows the intake of selected food groups that have known associations with BM frequency (e.g. coffee, legumes) over the course of the study. Consumption of legumes as well as tofu and other soy products was significantly more prevalent in the vegan group as compared to the meat-rich diet group after 8 weights.
Table 4. Intakes of selected food groups related to bowel health and dietary group at baseline and after 8 weeks of the dietary intervention.
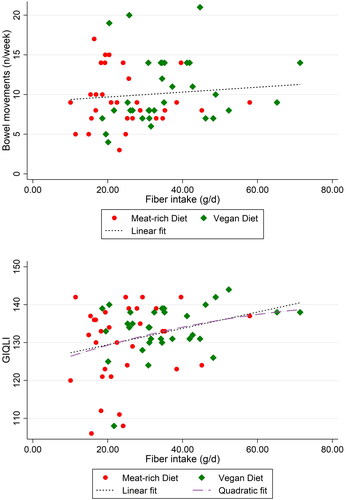
displays scatterplots visualizing the relationship between BM frequency and fiber intake (top) and GIQLI and fiber intake (bottom). While no significant association between fiber intake and BM frequency was detected in this particular sample, the Spearman’s correlation coefficient for fiber intake and GIQLI was significant (p = .03), revealing a weak correlation (Spearman’s rho = 0.27) between both variables.
Figure 4. Scatterplots visualizing the relationship between BM frequency and fiber intake (top) and GIQLI and fiber intake (bottom). BM: Bowel Movement; GIQLI: Gastrointestinal Quality of Life Index. One outlier (fiber intake per day: 120 g) in the vegan group was removed to highlight full cohort behavior over the casuality of one outlier. Spearman’s rho for fiber intake and GIQLI was 0.27 (p = .03), whereas it was not significant for fiber and BM frequency.
shows the repeated measures ANOVA results. No significant diet × time interactions were found for the three investigated endpoints. A significant effect of ‘diet’ on GIQLI was found (p = .039), yet a closer inspection of the pooled within-subject covariance matrix casted doubt on the validity of the required compound symmetry assumption. Thus, the results must be discussed with great caution. Moreover, the overall effect appeared clinically irrelevant and the p-value was no longer significant after adjustment for multiple testing.
Table 5. Comparison of BM frequency, BSFS, and GIQLI across dietary groups.
The electronic Supplementary Table 5 shows hs-CRP levels and albumin levels over the course of the study. Although hs-CRP levels increased in the meat-rich group, no significant differences were detected between both groups. Finally, electronic Supplementary Table 6 summarizes bowel health-related adverse events which participants reported over the course of the study. Events occurred more frequently in the meat-rich diet group and largely included postprandial abdominal pain, constipation and two gastrointestinal infections with self-limiting diarrhea and vomiting.
Discussion
The present study investigated bowel health and defecation patterns related to diet and nutrient intake in a young and healthy sample of German university students who were randomly assigned to either a vegan or a meat-rich diet for eight weeks. Our results did not support the hypothesis of a significant increase in BM frequency in vegans. The de-novo adoption of a vegan diet neither resulted in a transient increase in abdominal discomfort nor in a decreased gastrointestinal quality of life, which could have occurred subsequent to the sudden increase in dietary fibers and selected phytochemicals (mainly phytic acid). These results require a thorough contextual discussion which considers previous publications on bowel health in plant-based diets.
Fiber-rich diets influence gut motility and defecation patterns [Citation43]. Davies et al. reported more frequent bowel movements and softer stools in vegans when compared to vegetarians and omnivores [Citation13]. In their cross-sectional study, vegans had, on average, 1.7 BM per day, whereas vegetarians and omnivores had 1.2 and 1.0, respectively. The authors reported that increasing fiber was associated with a shorter transit time, and – unsurprisingly – vegans had the highest intake (47 g), followed by vegetarians (37 g) and omnivores (23 g). Vegans in the Davies study consumed substantially more fiber than vegans in our study (47 g vs. 34.89 g/d), who barely met the minimum fiber intake recommendations of 14 g/1000 kcal [Citation44]. This could be one of many possible factors explaining the lack of an increase in BM frequency in vegan participants in our study.
Another study that examined BM frequency in vegans used data from the European Prospective Investigation into Cancer and Nutrition Oxford cohort [Citation17]. Sanjoaquin et al. reported a higher BM frequency in vegans, with sex-specific differences. Vegan men had on average 11.6 BM per week, as compared to 9.5 in male meat eaters. Vegan women on the other hand reported 10.5 BM per week, as compared to 8.2 in female meat eaters. The authors reported significant associations with fiber and moisture intake (restricted to non-alcoholic beverages). Vigorous exercise was also positively associated with BM frequency in women in this study, while results for men were less clear.
All of these factors play a pivotal role for bowel health and must be considered simultaneously, particularly because an adequate intake of fluid is necessary to enhance the positive effects of dietary fibers in stool frequency regulation [Citation45]. Studies demonstrated that even a relatively short period of fluid deprivation may decrease BM frequency and could be an etiological factor for constipation [Citation46,Citation47]. While liquid deprivation was not an issue in our study cohort, it must be emphasized that the proportion of physically active participants was high. This could have partially offset the effects of a mild fiber reduction in the meat-rich diet group, as physical activity has been repetitively associated with BM frequency [Citation48].
The unaltered BM frequency in vegans in our study still remains surprising, and, taking into account all potential etiological factors, might be the result of the only moderate increase in dietary fiber intake over the course of the study. While fiber intake increased in the vegan group compared to baseline, it still remained somewhat pale in comparison to other vegan cohorts across Europe. Clarys et al. reported a mean fiber intake of 41 g in Belgian vegans [Citation49], while Kristensen et al. reported an intake of 56 g/d in vegan Danish men [Citation40]. This again demonstrates that not all plant-based diets are created equal [Citation1]. While vegans in our study consumed substantially less fiber, a reservation must be made that they were all naïve to the vegan diet, which requires considerable time and knowledge to adopt and master [Citation50]. Another important difference with previously published studies is that we purposely selected participants who had been on a healthy omnivore diet at baseline, with an overall nutrient intake and level of physical activity that were health-promoting, prior to the dietary intervention. This may explain the relatively small effect size recorded for measures of bowel health, defecation patterns and gastrointestinal life-quality investigated in our study.
Particularly the regular intake and consumption of fiber-rich plant-foods, such as legumes and whole grains, requires habituation and practice [Citation51]. While legume consumption increased in our study, vegans also often consumed fiber-depleted meat analogues and wheat gluten-based replacement products, which did not necessarily contribute to their overall fiber intake. Optimizing diet quality on a vegan diet for beginners is challenging and requires substantial nutritional knowledge and cooking skills. Acquiring these in a mere eight weeks seems unfeasible and could have theoretically contributed to the non-significant changes in BM frequency. While vegans optimized their intake of magnesium and fiber, their potassium intake remained worthy of improvement. The latter is an important indicator of fruit and vegetable intake [Citation52], and reiterates that diet quality in vegans improved as part of the study but could have overall been much better.
It must also be emphasized that gastrointestinal quality of life did not decrease in the vegan group. This is of paramount importance, since abdominal discomfort may be expected upon a sudden fiber intake increase when transitioning from an omnivorous to a vegan plant-based diet. Often used as an argument against plant-based dietary patterns, this phenomenon was not observed in our study. Significant improvements in the GIQLI though were not observed in either group, potentially because the baseline scores were already very high and because we exclusively recruited healthy young individuals.
Our study has strengths and limitations that warrant further discussion. As for the strengths, we compiled a homogenous study sample of young and healthy individuals. Rigorous exclusion criteria and a thorough consideration of relevant covariates and confounders (including physical activity, subjective social status and educational level) are an additional asset. Weighed food diaries are considered the ‘gold standard’ and the most precise method to quantify food intake, particularly in vegans who frequently consume foods that may not be found in commonly used food frequency questionnaires [Citation53]. Participants of both groups received the same amount of attention and support in terms of group seminars, newsletters and individual interaction to reduce bias. To the best of our knowledge, we report the first randomized-controlled trial investigating the effects of a de-novo adoption of a vegan diet on selected parameters of bowel health.
Weaknesses include the modest sample size and potential reporting bias. Participants of both groups were meticulously instructed to maintain their weight as this parameter is known to influence other primary outcomes of the study (e.g. serum cholesterol levels, gut microbiota). While a slight weight loss occurred in both groups, the mean weight loss difference was not significant. It must be noted though that vegan diets are usually characterized by a higher nutrient density and reduced caloric density which both promote earlier satiety and could lead to subsequent weight loss [Citation20]. To maintain their weight, some participants in the vegan group reported the consumption of granola bars, nuts and sweets to comply with their daily energy intake target of approximately 2000 kcal/d. Minimizing the odds for weight reduction was important for the study, as weight loss per se might have changed metabolomics parameters or well-being. Then again, energy intake in vegans in our study was slightly higher as expected compared to free-living vegans. The lack of gut microbiota richness analyses, which are associated with stool consistency, is another weakness that will be subject to future analyses [Citation54].
Ultimately, we acknowledge that this randomized-controlled study was performed in free-living individuals who were allowed to prepare their foods for themselves. No meals were provided and participants were free to select the foods they liked as long as it was in accordance with the study protocol and the requirements of the assigned dietary group. This lack of control may have introduced bias and confounders, which we intended to minimize with regular and close participant contact to ensure a high dietary adherence. Nevertheless, we cannot guarantee 100% dietary adherence in both groups. Then again, the sample was almost exclusively composed of students who preponderantly disclosed to have eaten at the local university cafeteria that provided both a hot meat-based and a hot vegan lunch. After all, controlling all meals is generally impossible in such a study design. While limiting the generalizability of our study, an inpatient setting was also deemed unfeasible in light of the particular study characteristics (study duration, extent, etc.) and the large amount of resources required for infrastructure, personnel and expense allowances in such cases.
To facilitate the recruiting process for a study with a potentially challenging dietary regimen, participants with a BMI between 21 and 30 kg/m2 were considered. While a BMI > 25 kg/m2 may no longer be considered normal or healthy as per definition [Citation55], we decided to include such individuals to ensure continuous recruiting. We acknowledge that this could have introduced some important bias and limitations; then again the share of participants with a BMI ≥ 26 kg/m2 was ‘only’ 15% in our study.
Conclusion
The short-term de-novo adoption of a vegan diet did not negatively affect bowel health. No clinically relevant differences in BM frequency and BSFS patterns were observed in vegan participants with a moderate increase in fiber intake.
Consent form
Written consent was obtained from all participants to publish the results of this study.
Ethics approval
The ethical committee of the University Medical Center of Freiburg approved this trial in January 2023 (approval number: 22-1474-S1). The study was prospectively registered at the German Clinical Trial Register (DRKS00031541). We obtained written and oral informed consent from all study participants.
Authors contributions
J. H. planned and conducted the study, supported the data analysis, performed PBMC isolation, and served as the main contact person for study participants.
F. S. conducted the study, analyzed the weighed food diaries, performed PBMC isolation, and was involved in the in-field organization.
V. L. was responsible for all laboratory-related aspects, performed and supervised the PBMC isolation and managed biosampling.
J.Z. supported the study related to organizational aspects and helped analyzing the weighed food diaries.
A.K.L. supported the study planning and the statistical analysis.
L.H. participated in experimental planning and data interpretation.
R. H. conceived, planned and supervised the clinical trial. R. H. was involved in funding acquisition and helped in the preparation of the manuscript.
M.A.S. served as the principal investigator, conceived, planned, conducted and supervised the clinical trial, analyzed data, analyzed the weighed food diaries, acquired funding and wrote the manuscript.
Supplemental Material
Download Zip (926.9 KB)Acknowledgements
Maximilian Storz would like to express his deep gratitude to the Karl und Veronica Carstens Foundation for their continuous support.
Disclosure statement
M.A.S. and the study were funded by the Karl und Veronika Carstens Foundation in Essen, Germany. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. The remaining authors have no conflict of interest to disclose. This work has been approved by all co-authors.
Data availability statement
The datasets used and analyzed for the current study are available from the corresponding author on reasonable request.
References
- Storz MA. What makes a plant-based diet? a review of current concepts and proposal for a standardized plant-based dietary intervention checklist. Eur J Clin Nutr. 2022;76(6):1–16. doi: 10.1038/s41430-021-01023-z.
- Clem J, Barthel B. A look at plant-based diets. Mo Med. 2021;118:233–238.
- Jardine MA, Kahleova H, Levin SM, et al. Perspective: plant-based eating pattern for type 2 diabetes prevention and treatment: efficacy, mechanisms, and practical considerations. Adv Nutr. 2021;12(6):2045–2055. doi: 10.1093/advances/nmab063.
- Kim H, Caulfield LE, Garcia‐Larsen V, et al. Plant‐based diets are associated With a lower risk of incident cardiovascular disease, cardiovascular disease mortality, and all‐cause mortality in a general population of Middle‐aged adults. J Am Heart Assoc. 2019;8(16):e012865. doi: 10.1161/JAHA.119.012865.
- Dinu M, Abbate R, Gensini GF, et al. Vegetarian, vegan diets and multiple health outcomes: a systematic review with meta-analysis of observational studies. Crit Rev Food Sci Nutr. 2017;57(17):3640–3649. doi: 10.1080/10408398.2016.1138447.
- Mazzocchi S, Visaggi P, Baroni L. Plant-based diets in gastrointestinal diseases: which evidence? Best Pract Res Clin Gastroenterol. 2023;62-63:101829. doi: 10.1016/j.bpg.2023.101829.
- Grosse CSJ, Christophersen CT, Devine A, et al. The role of a plant-based diet in the pathogenesis, etiology and management of the inflammatory bowel diseases. Expert Rev Gastroenterol Hepatol. 2020;14(3):137–145. doi: 10.1080/17474124.2020.1733413.
- Zhao Y, Zhan J, Wang Y, et al. The relationship Between plant-based diet and risk of digestive system cancers: a Meta-Analysis based on 3,059,009 subjects. Front Public Health. 2022;10:892153. doi: 10.3389/fpubh.2022.892153.
- Tomova A, Bukovsky I, Rembert E, et al. The effects of vegetarian and vegan diets on gut microbiota. Front Nutr. 2019;6:47. doi: 10.3389/fnut.2019.00047.
- Kohnert E, Kreutz C, Binder N, et al. Changes in gut microbiota after a Four-Week intervention with vegan vs. meat-rich diets in healthy participants: a randomized controlled trial. Microorganisms. 2021;9(4):727. doi: 10.3390/microorganisms9040727.
- Storz MA, Rizzo G, Müller A, et al. Bowel health in U.S. vegetarians: a 4-year data report from the national health and nutrition examination survey (NHANES). Nutrients. 2022;14(3):681. doi: 10.3390/nu14030681.
- Dressler J, Storz MA, Müller C, et al. Does a plant-based diet stand Out for its favorable composition for heart health? Dietary intake data from a randomized controlled trial. Nutrients. 2022;14(21):4597. doi: 10.3390/nu14214597.
- Davies GJ, Crowder M, Reid B, et al. Bowel function measurements of individuals with different eating patterns. Gut. 1986;27(2):164–169. doi: 10.1136/gut.27.2.164.
- Nath P, Singh SP. 26 - Defecation and stools in vegetarians: implications in health and disease. In: Mariotti F, editor. Vegetarian and plant-based diets in health and disease prevention [internet]. London: Academic Press; 2017. p. 473–481. https://www.sciencedirect.com/science/article/pii/B9780128039687000265
- Nag A, Martin SA, Mladsi D, et al. The humanistic and economic burden of chronic idiopathic constipation in the USA: a systematic literature review. Clin Exp Gastroenterol. 2020;13:255–265. doi: 10.2147/CEG.S239205.
- Gilsing AM, Weijenberg MP, Goldbohm RA, et al. The Netherlands cohort study – meat investigation cohort; a population-based cohort over-represented with vegetarians, pescetarians and low meat consumers. Nutr J. 2013;12(1):156. doi: 10.1186/1475-2891-12-156.
- Sanjoaquin MA, Appleby PN, Spencer EA, et al. Nutrition and lifestyle in relation to bowel movement frequency: a cross-sectional study of 20 630 men and women in EPIC–oxford. Public Health Nutr. 2004;7(1):77–83. doi: 10.1079/phn2003522.
- Panigrahi MK, Kar SK, Singh SP, et al. Defecation frequency and stool form in a coastal Eastern indian population. J Neurogastroenterol Motil. 2013;19(3):374–380. doi: 10.5056/jnm.2013.19.3.374.
- Lederer A-K, Hannibal L, Hettich M, et al. Vitamin B12 status Upon Short-Term intervention with a vegan diet-A randomized controlled trial in healthy participants. Nutrients. 2019;11(11):2815. doi: 10.3390/nu11112815.
- Müller A, Zimmermann-Klemd AM, Lederer A-K, et al. A vegan diet Is associated with a significant reduction in dietary acid load: post hoc analysis of a randomized controlled trial in healthy individuals. Int J Environ Res Public Health. 2021;18(19):9998. doi: 10.3390/ijerph18199998.
- Storz MA, Müller A, Niederreiter L, et al. A cross-sectional study of nutritional status in healthy, young, physically-active german omnivores, vegetarians and vegans reveals adequate vitamin B12 status in supplemented vegans. Ann Med. 2023;55(2):2269969. doi: 10.1080/07853890.2023.2269969.
- DGE. Vollwertig essen und trinken nach den 10 Regeln der DGE [Internet]. 2023 [cited 2023 Jul 21]. http://www.dge.de/gesunde-ernaehrung/dge-ernaehrungsempfehlungen/10-regeln/.
- Physicians Committee for Responsible Medicine. Portals – factsheets [Internet]. 2023 [cited 2023 Jul 21]. https://pcrm.widencollective.com/portals/gr0kpkol/factsheets.
- Physicians Association for Nutrition. 2023 [cited 2023 Jul 21]. https://tinyurl.com/mrykfr66.
- QS. Wenns ums Fleisch geht. 2023 [cited 2023 Jul 21]. https://tinyurl.com/ycyznhkm.
- Storz MA, Lombardo M, Rizzo G, et al. Bowel health in U.S. Shift workers: insights from a cross-sectional study (NHANES). Int J Environ Res Public Health. 2022;19(6):3334. doi: 10.3390/ijerph19063334.
- Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32(9):920–924. doi: 10.3109/00365529709011203.
- Eypasch E, Williams JI, Wood-Dauphinee S, et al. Gastrointestinal quality of life index: development, validation and application of a new instrument. Br J Surg. 1995;82(2):216–222. doi: 10.1002/bjs.1800820229.
- Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum. 1993;36(1):77–97. doi: 10.1007/BF02050307.
- Blake MR, Raker JM, Whelan K. Validity and reliability of the bristol stool form scale in healthy adults and patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2016;44(7):693–703. doi: 10.1111/apt.13746.
- Vork L, Wilms E, Penders J, et al. Stool consistency: looking Beyond the bristol stool form scale. J Neurogastroenterol Motil. 2019;25(4):625–625. doi: 10.5056/jnm19086.
- Jaruvongvanich V, Patcharatrakul T, Gonlachanvit S. Prediction of delayed colonic transit using bristol stool form and stool frequency in Eastern constipated patients: a difference From the west. J Neurogastroenterol Motil. 2017;23(4):561–568. doi: 10.5056/jnm17022.
- Wilson PB. Associations between physical activity and constipation in adult americans: results from the national health and nutrition examination survey. Neurogastroenterol Motil. 2020;32(5):e13789. doi: 10.1111/nmo.13789.
- Nevler A. The epidemiology of anal incontinence and symptom severity scoring. Gastroenterol Rep (Oxf). 2014;2(2):79–84. doi: 10.1093/gastro/gou005.
- Alexy U, Fischer M, Weder S, et al. Nutrient intake and status of german children and adolescents consuming vegetarian, vegan or omnivore diets: results of the VeChi youth study. Nutrients. 2021;13(5):1707. doi: 10.3390/nu13051707.
- Hoebel J, Müters S, Kuntz B, et al. Messung des subjektiven sozialen status in der gesundheitsforschung mit einer deutschen version der MacArthur scale. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2015;58(7):749–757. doi: 10.1007/s00103-015-2166-x.
- Giatti L, Camelo L do V, Rodrigues Jf de C, et al. Reliability of the MacArthur scale of subjective social status - Brazilian longitudinal study of adult health (ELSA-Brasil). BMC Public Health. 2012;12(1):1096. doi: 10.1186/1471-2458-12-1096.
- Lee PH, Macfarlane DJ, Lam T, et al. Validity of the international physical activity questionnaire short form (IPAQ-SF): A systematic review. Int J Behav Nutr Phys Act. 2011;8(1):115. doi: 10.1186/1479-5868-8-115.
- Silva-Batista C, Urso RP, Lima Silva AE, et al. Associations Between fitness tests and the international physical activity questionnaire—short form in healthy men. J Strength Cond Res. 2013;27(12):3481–3487. doi: 10.1519/JSC.0b013e31828f1efa.
- Kristensen NB, Madsen ML, Hansen TH, et al. Intake of macro- and micronutrients in danish vegans. Nutr J. 2015;14(1):115. doi: 10.1186/s12937-015-0103-3.
- Jones D, Molitor D, Reif J. What do workplace wellness programs do? Evidence from the Illinois workplace wellness study. Q J Econ. 2019;134(4):1747–1791. doi: 10.1093/qje/qjz023.
- Deutsche Gesellschaft für Ernährung (DGE); Österreichische Gesellschaft für Ernährung (ÖGE); Schweizerische Gesellschaft für Ernährung (SGE). D-A-CH-Referenzwerte für die nährstoffzufuhr. Bonn, Germany: Neuer Umschau Buchverlag; 2018
- Ito M, Yoshimoto J, Maeda T, et al. Effects of high-fiber food product consumption and personal health record use on body mass index and bowel movement. J Funct Foods. 2023;102:105443. doi: 10.1016/j.jff.2023.105443.
- Home. Dietary Guidelines for Americans [Internet]. 2023 [cited 2023 Aug 8]. https://www.dietaryguidelines.gov/.
- Arnaud MJ. Mild dehydration: a risk factor of constipation? Eur J Clin Nutr. 2003;57(Suppl 2):S88–S95. doi: 10.1038/sj.ejcn.1601907.
- Klauser AG, Beck A, Schindlbeck NE, et al. Low fluid intake lowers stool output in healthy male volunteers. Z Gastroenterol. 1990;28(11):606–609.
- Markland AD, Palsson O, Goode PS, et al. Association of low dietary intake of fiber and liquids with constipation: evidence from the national health and nutrition examination survey. Am J Gastroenterol. 2013;108(5):796–803. doi: 10.1038/ajg.2013.73.
- Tantawy SA, Kamel DM, Abdelbasset WK, et al. Effects of a proposed physical activity and diet control to manage constipation in middle-aged obese women. Diabetes Metab Syndr Obes. 2017;10:513–519. doi: 10.2147/DMSO.S140250.
- Clarys P, Deliens T, Huybrechts I, et al. Comparison of nutritional quality of the vegan, vegetarian, semi-vegetarian, pesco-vegetarian and omnivorous diet. Nutrients. 2014;6(3):1318–1332. doi: 10.3390/nu6031318.
- Alcorta A, Porta A, Tárrega A, et al. Foods for plant-based diets: challenges and innovations. Foods. 2021;10(2):293. doi: 10.3390/foods10020293.
- Henn K, Goddyn H, Olsen SB, et al. Identifying behavioral and attitudinal barriers and drivers to promote consumption of pulses: a quantitative survey across five european countries. Food Qual Preference. 2022;98:104455. doi: 10.1016/j.foodqual.2021.104455.
- Farapti F, Buanasita A, Atmaka DR, et al. Potassium intake is associated with nutritional quality and actual diet cost: a study at formulating a low sodium high potassium (LSHP) healthy diet. J Nutr Sci. 2022;11:e11. doi: 10.1017/jns.2021.104.
- Carlsen MH, Lillegaard IT, Karlsen A, et al. Evaluation of energy and dietary intake estimates from a food frequency questionnaire using independent energy expenditure measurement and weighed food records. Nutr J. 2010;9(1):37. doi: 10.1186/1475-2891-9-37.
- Vandeputte D, Falony G, Vieira-Silva S, et al. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut. 2016;65(1):57–62. doi: 10.1136/gutjnl-2015-309618.
- National Heart, Lung, and Blood Institute. Assessing your weight and health risk [Internet]. 2023 [cited 2023 Dec 25]. https://www.nhlbi.nih.gov/health/educational/lose_wt/risk.htm.