Abstract
Introduction
Dose (number of repetitions) has been suggested as a key element in the effectiveness of rehabilitation exercises to promote motor recovery of the hemiparetic upper limb. However, rehabilitation exercises tend to be monotonous and require significant motivation to continue, making it difficult to increase the exercise dose. To address this issue, gamification technology has been implemented in exercises to promote self-engagement for people with hemiparesis in continuing monotonous repetitive movements. This study aimed to investigate how subjective perspectives, specifically enjoyability, motivation to continue, and expectancy of effectiveness, change through continuous daily exercise using a developed gamified exercise system.
Materials and Method
Ten people with stroke suffering upper limb dysfunction underwent daily gamified exercise for seven days. The gamified exercise consisted of an electromyography (EMG)-controlled operating system that enabled users to play virtual games using repetitive finger movements. The participants performed conventional self-exercise on the same day as the control exercise, and rated their subjective perspectives on both exercises on a numerical rating scale on each exercise day.
Results
Ratings for enjoyability and motivation to continue consistently showed significantly higher scores for the gamified exercise than for conventional self-exercise on all exercise days. A similar trend was observed in the ratings for the expectancy of effectiveness. No changes over time were found in any of the ratings throughout the exercise period.
Conclusions
Exercise using the developed EMG-controlled gamified system may have the potential to maintain motivation and enjoyment in people with stroke to continue monotonous repetitive finger movements.
KEY MESSAGES
Although dose (number of repetitions) has been suggested as a key element in the effectiveness of rehabilitation exercises to promote motor recovery of the hemiparetic upper limb, rehabilitation exercises tend to be monotonous and require significant motivation to continue.
Gamification technology has been implemented in exercises to promote self-engagement for people with hemiparesis in continuing monotonous repetitive movements.
Exercises using the developed EMG-controlled gamified system may have the potential to maintain motivation and enjoyment in people with stroke to continue monotonous repetitive finger movements.
Introduction
Stroke is a leading cause of disability with a global prevalence of approximately 1% [Citation1]. Among the various dysfunctions in people with stroke, upper limb dysfunction is observed in at least 70% of them [Citation2]. Upper limb dysfunction is known to have a significant impact on daily life. It reduces the level of independence in activities of daily living, diminishes the likelihood of returning to work, and impairs engagement in hobbies, and has detrimental effects on mental health and quality of life [Citation3,Citation4].
Intensive and repetitive exercise in people with stroke has been reported to be an important factor in improving upper limb motor function [Citation5,Citation6] for strengthening the musculoskeletal system, and promoting neuroplasticity [Citation7,Citation8] and motor learning [Citation9]. Indeed, previous studies have suggested that higher dose of exercise, such as increased duration [Citation10,Citation11] and number of repetitions [Citation12], results in better rehabilitation outcomes. Among the several approaches attempting to improve hemiparetic upper limb function in people with stroke, task-specific training, constraint-induced movement therapy, and robotic therapy have been recommended as effective exercises with high levels of evidence [Citation13]. Importantly, despite some differences between studies, the effectiveness of all these exercise approaches has been demonstrated in clinical trials in which targeted exercises were provided continuously for at least a few weeks (e.g. 2–12 weeks) [Citation14–22]. This suggests that the sustainability of exercise over a longer period may be an important element influencing the outcome of upper limb motor function.
However, continuous engagement of affected limbs during exercise is difficult. In particular, in the case of self-exercise, which is often provided to obtain a dose of exercise but requires voluntary engagement by the individual, only approximately 30% of people with stroke were able to continue as instructed [Citation23,Citation24]. One possible reason for this may be that self-exercise with the expectation of functional improvement often involves monotonous, repetitive movements that lack enjoyment [Citation23,Citation24].
Recently, gamification has been widely implemented in rehabilitation exercises as a tool to entertain people with stroke and motivate them to continue exercising [Citation25–27]. Motivation is defined in the field of psychology as ‘a force that initiates, sustains, directs, activates, or drives purpose-oriented behaviour’ [Citation28] and is thought to influence the outcomes of rehabilitation exercises [Citation25,Citation26]. Contrary to extrinsic motivation, which is driven by rewards, consequences, or external pressures, intrinsic motivation, which is driven by the satisfaction inherent in an activity [Citation29], is known to contribute to the persistence of activities, and is therefore important for the self-driven continuation of rehabilitation [Citation30]. Gamified rehabilitation exercises have been reported to increase intrinsic motivation by increasing enjoyment and promoting voluntary engagement in exercise [Citation31,Citation32].
To increase motivation for continuous exercise, we developed a gamified exercise system that allows people with stroke to train motor functions in the distal part of the upper limb in a fun and motivating manner [Citation33]. Our previous work showed that through a single bout of training, the developed system has relatively good usability, satisfaction, enjoyability, motivation to continue, and expectancy of effectiveness, which leads to the assumption that the system can motivate people with stroke to train continuously. However, it remains unclear how these subjective perspectives on the developed gamified exercise system change over time, which is a critical question that needs to be addressed when expecting daily clinical use.
This study aimed to investigate how subjective perspectives, specifically enjoyability, motivation to continue, and expectancy of effectiveness, change through continuous daily exercise using the developed gamified exercise system. We hypothesized that these subjective perspectives on the gamified exercise would be more positive than conventional self-exercises without significant decline even with continuous use of the system.
Materials and methods
Participants
Ten individuals with subacute stroke (3 women, mean age 72.6 ± 12.8 years) were recruited from a rehabilitation ward in Fujita Health University Hospital between April and July 2021. All participants provided written informed consent before participating in the study. The study protocol was approved by the Ethics Review Committee of Fujita Health University (approval no. HM19-231). The experiment was conducted in accordance with the Declaration of Helsinki of 1964, as revised in 2013.
The inclusion criteria were as follows: (1) diagnosis of ischemic or haemorrhagic stroke; (2) subacute phase (7 days to 6 months after stroke onset) [Citation34]; (3) cognitive ability to follow instructions and understand the rules of the task; (4) ability to sit independently; and (5) demonstrating no less than 1 A (minimal voluntary movement or mass flexion) on the Stroke Impairment Assessment Set for finger function (SIAS-FF) [Citation35,Citation36].
Study design
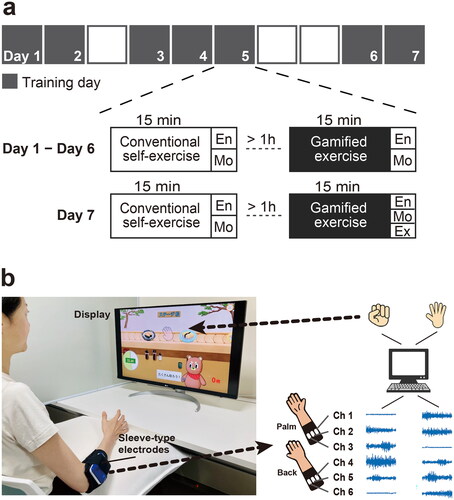
All participants performed the gamified exercise and conventional self-exercise involving finger movements for 15 min per day, repeated for seven non-consecutive days. Each day, both exercises were performed in a fixed order (first, conventional self-exercise), separated by at least one hour between them (). The specific fixed order was chosen because a researcher, who is also an occupational therapist (OT) clinician, had to do clinical work during the day; therefore, the gamified exercise had to be provided after that, between 5 pm and 6 pm.
Figure 1. Experimental protocol and developed electromyography-controlled gamified system. (a) Example of protocol. A participant performed gamified exercise and conventional self-exercise for 15 min per day and repeated for non-consecutive seven days. Each day, both exercises were performed in a fixed order, separated by at least one hour between them. A participant was asked to rate the exercises regarding enjoyability (En), motivation to continue (Mo), and expectancy of effectiveness (Ex) based on numerical rating scales. Expectancy of effectiveness was evaluated only on the last day. (b) An overview of gamified exercise for training hand-finger movements. This is an example of the game contents at stage 3. Muscle activities were measured from the forearm using a custom-made elastic sleeve with 6 pairs of wireless surface electromyography electrodes. When muscle activity patterns matched the reference and the level of activity exceeded a certain level, characters’ movements were generated.
To evaluate the subjective perspectives on each exercise and track their time-course changes, we asked the participants to rate the gamified exercise and conventional self-exercise on a numerical rating scale (NRS) regarding enjoyability, motivation to continue, and expectancy of effectiveness. All participants also received the usual rehabilitation training, including 60 min of occupational, physical, and speech-language therapies (if needed) every day.
Gamified exercise
Gamified exercise was performed using the custom-made electromyography (EMG)-controlled gamified exercise system [Citation33] (). In brief, this system implements a computer game in which the user controls the actions of the character developed using computer graphics. The actions of the character are controlled by the activity of the forearm muscles exerted during specific finger movements. The surface EMG signals were captured by a custom-made elastic sleeve made of interknitted fabric comprising polyurethane elastic yarns and nylon yarns integrated with 6 pairs of wireless surface EMG electrodes (size: 1 cm × 1 cm, SMK Co., Ltd, Tokyo, Japan). The elastic sleeve used in the present study was a modified model from the original one with 12 pairs of electrodes [Citation33]. The number of electrode pairs was reduced by half, and the length of the sleeve was shortened to make it easier to apply. We confirmed that the accuracy of motion estimation was comparable when using the current sleeve. The location of the EMG electrodes did not necessarily have to correspond to the belly of specific muscles but were roughly positioned so that three pairs were over the flexors (e.g. flexor digitorum superficialis and profundus muscles) and the other three were over the extensors (e.g. extensor digitorum muscle) of the forearm. Surface EMG signals from the forearm muscles were analyzed for patterns based on a mathematical model (see details in [Citation33]) to estimate the types of finger movements attempted while playing the game. Prior to starting the exercise, EMG signals were recorded during specific finger movements, which were then used as references to detect the movements performed and the level of muscle activity. The actions of a character with computer sound appeared when muscle activity patterns matched the reference and the level of activity exceeded a certain level (see details in [Citation33]).
The game comprises four stages, each of which requires the user to repeat different sets of movements. The finger movements required at each stage were designed to accommodate a wide range of hemiparesis severity, as modified from the previous version [Citation33] ().
Table 1. The finger movements required in each stage.
In stages 1 and 2, users were required to repeatedly attempt a set of movements, including whole-finger flexion (stage 1) and extension (stage 2), followed by relaxation. Stage 3 required a set of whole-finger flexion movements followed by extension. If a participant was unable to exert reproducible EMG patterns and thus could not play even at stage 1, the participant played stage 0, where any EMG signal could make the character act as in stage 1.
When muscle activity patterns matched the reference and the level of activity exceeded a certain level, characters’ movements (i.e. making, serving, grabbing, or eating sushi) were generated with the computer sound and sushi plates were stacked as a positive visual feedback. The number of successful performances was fed back to the participants as a score on each trial (60 s of play), and the highest score on a trial and the total score for each day were recorded. In addition, each individual’s highest score on a trial between exercise days was recorded and updated. At all times prior to the session, the participants were instructed once to attempt to exceed this individual’s highest score. For entertainment purposes, the graphics of the characters and objects changed when the total score or number of trials reached a certain value.
Experimental protocol
Gamified exercise
The participants sat in a wheelchair or chair with a backrest in front of the display. The affected arm was placed on a cushion to support the forearm and to provide a comfortable limb position. All participants were briefed on how to operate the game before the exercise and allowed to experience stage 1 or stage 0 once for practice depending on their level of motor function. As for the exercise, all participants started with stage 1 and proceeded to the next stage until they reached the highest playable stage, where they were able to control the actions of a character at least once in the first 10 s. The play time for each stage was set to 60 s (one trial). After completing the highest playable stage, they played again from stage 1 and repeated the cycle until the end of the exercise duration of 15 min (one session). The researcher was present at every session to ensure that the exercise was carried out appropriately, but not to provide special encouragement.
Conventional self-exercise
The participants performed individualized self-exercises involving finger movements on the affected side while seated in a wheelchair or chair. Individualized tasks were selected by an assigned OT according to the level of motor function. The participants who could grasp objects performed tasks such as corn transport, peg transport, and grasping multiple otedama bags (small bags filled with beans). The participants who could not grasp objects due to severe hand-finger dysfunction performed active-assistive hand-finger exercises, such as repetition of finger flexion and relaxation, with assistive technology of functional electrical stimulation to the flexor muscles in the forearm. The conventional self-exercises did not include any cognitive training or passive training such as stretching. The duration of the exercise was 15 min per session, similar to the gamified exercise. The assigned OT prepared the task but was not present during the exercise. The same exercise task was repeated for each participant throughout the seven exercise days.
Clinical motor assessments
SIAS-FF, Fugl-Meyer Assessment for the upper extremity (FMA-UE) [Citation37], and Action Research Arm Test (ARAT) [Citation38] were used as clinical motor assessments to specify participants’ characteristics. These scores were assessed every two weeks by an OT in charge, and the scores obtained on the day closest to the first exercise day were used.
Subjective assessments
Subjective perspectives on enjoyability, motivation to continue, and expectancy of effectiveness were assessed using a NRS (Figure S1). The NRS is an 11-point Likert scale ranging from 0 to 10 with higher values indicating positive responses. Enjoyability and motivation to continue were assessed after completion of each exercise each day, and the expectancy of effectiveness was evaluated once after all the 7-day exercises were completed (). The examiner stayed back and was blinded to the scale during the participants scoring to prevent them from making additional considerations.
Statistical analysis
To compare subjective ratings and patterns of time-course changes in enjoyability and motivation to continue between the gamified exercise and conventional self-exercise, a two-way analysis of variance (ANOVA) was applied with factors of exercise (two levels) and time (seven levels: days 1–7). All ANOVAs were tested for the assumption of homogeneity of variance with Mauchly’s test of sphericity. For those tests in which this assumption was violated, the Greenhouse-Geisser correction statistic was reported. A paired t-test was used to compare the ratings for the expectancy of effectiveness between the exercises. Statistical significance was set at p < 0.05. Effect sizes were reported in partial eta squared value for ANOVA and Cohen’s d value for t-test. All tests were performed using SPSS version 26 (IBM Inc., Armonk, New York, USA).
Results
Participants’ characteristics are presented in . The participants had a wide range of motor impairments in finger function (1B–4 on SIAS-FF) and upper extremity function (10–65 in FMA-UE). They also showed a wide range of motor activity capacities (3–57 in ARAT). The non-consecutive 7-day exercise lasted for 8–12 days, and the interval between exercise days was between 0 and 2 days among the participants, depending on the experimenter’s work shift. No participants dropped out of the study. There were no adverse events during the study.
Table 2. Participants’ characteristics.
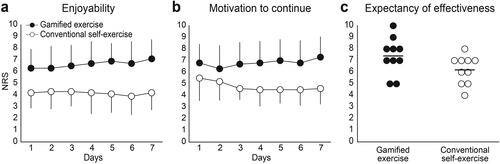
Regarding subjective assessments, the NRS for enjoyability showed higher scores for the gamified exercise than for conventional self-exercise (two-way ANOVA: main effect of exercise: F1,9 = 16.8, p = 0.003, ηp2 = 0.65) (). The scores did not change over the seven exercise days for either exercise (main effect of days: F6,54 = 0.4, p = 0.85, ηp2 = 0.05; interaction: F1,9 = 1.4, p = 0.20, ηp2 = 0.14). A similar trend was observed for motivation to continue, where the NRS for the gamified exercise was higher than that for conventional self-exercise (two-way ANOVA: main effect of exercise: F1,9 = 25.1, p = 0.001, ηp2 = 0.74) (). Notable time-course changes were not found for either exercise (main effect of time: F6,54 = 0.7, p = 0.68, ηp2 = 0.07; interaction: F2.5, 22.6 = 2.6, p = 0.08, ηp2 = 0.23). Regarding the expectancy of effectiveness, there were no statistically significant differences between the exercises (t9 = 2.3, p = 0.051, d = 1.01), although the gamified exercise showed relatively higher scores than conventional self-exercise (). To qualitatively investigate differences in subjective perspectives on the gamified exercise between subgroups with different playable stage or motor function, we subdivided participants into those who were able to play stage 2 (n = 3) or stage 3 (n = 7), or those who had more severe (1 C or less on SIAS-FF, n = 5) or milder (3 or greater on SIAS-FF, n = 5) finger motor function. As a result, participants who were able to play stage 3 tended to score higher on the gamified exercise than those who were able to play stage 2, while no such trend was observed on the conventional self-exercise. A similar trend was observed for finger motor function (Figure S2).
Figure 2. Numerical rating scale (NRS) scores for a) enjoyability, b) motivation to continue, c) expectancy of effectiveness for the gamified exercise, and conventional self-exercise. Mean scores for enjoyability and motivation to continue are presented using black dots for the gamified exercise and white dots for conventional self-exercise. Error bars indicate standard deviation. Individual scores for expectancy of effectiveness are shown in each dot, and mean scores are shown in horizontal lines.
Discussion
The present study investigated the feasibility of the developed EMG-controlled gamified exercise system designed to repeatedly train hand-finger movements by comparing daily changes in subjective perspectives on the developed exercise with those on conventional self-exercise. The results showed that the perspectives on enjoyability and motivation to continue were consistently significantly more positive than those on conventional self-exercise throughout the 7-day exercises, with the scores of both exercises maintained without clear decreases. These results indicate that the developed gamified system may have the potential to maintain motivation and enjoyment for people with stroke to continue monotonous repetitive hand-finger movements.
Although a few studies have investigated the subjective perspective on daily gamified exercises, most were conducted in healthy volunteers, and the perspective of people with stroke remains unclear. van der Kooij et al. (2019) reported a comparison between gamified exercises and usual training in healthy elderly and young adults, with the former showing higher enjoyability and motivation scores, which did not decline after multiple sessions (three sessions per day for a total of nine sessions in three days) [Citation31]. The present study demonstrated similar results in people with stroke who suffer motor impairments. People with stroke are easily fatigued [Citation39,Citation40], and movements require extensive effort because of motor paresis. In addition, their motivation for rehabilitation is negatively affected by various factors, including physical and cognitive disabilities [Citation41], indicating that it is difficult for them to maintain motivation for rehabilitation exercises. In fact, it has been reported that people with stroke have the lowest participation in activities as exercise and the highest percentage of inactivity among people with stroke, musculoskeletal disease, vascular/heart diseases, degenerative disease, neurological disease, diabetes, and respiratory disease [Citation42]. Nevertheless, the present study suggests that our gamified system can motivate people with stroke to continue exercising to a greater extent than conventional self-exercise. To generalize our results, further studies are needed in people with other health conditions such as spinal cord injury who also have lower levels of physical activity.
In addition, it is noteworthy that the participants performed the gamified exercise with a high frequency and long duration per session. In the previous study, each of the three days of exercise was performed sporadically, with at least one week between each day, and one session lasted for only 4 min [Citation31]. In contrast, in the present study, the minimum interval between exercise days (seven non-consecutive days within 8–12 days) was 0 and 2 days at maximum, with one session per day lasting for 15 min. It is known that, when training for upper limb motor paresis, increasing dose as much as possible is essential for promoting motor recovery [Citation5,Citation6], indicating that training with higher frequency and longer duration may work positively. Therefore, the fact that similar results (no clear decline in subjective perspective scores) were obtained with a relatively higher frequency and longer duration compared to the previous study indicates a potential contribution of our gamified exercise in maintaining repetitive training aimed at upper limb motor recovery in people with stroke.
Although the present study examined changes in subjective perspectives over a 7-day period, it remains unclear how they change when the exercise continues over a longer period. Most recovery from post-stroke motor paresis occurs in the first three months after onset, followed by a slow, long-lasting recovery that can be expected with continuous exercise [Citation43]. Since this implies that long-term rehabilitation is important for people with stroke, further examination is needed to explore how subjective perspectives change after more than seven days of exercise.
Although gamified exercise can generally increase enjoyment and motivation for exercise, users may lose interest at some point if the same content continues. By contrast, our system was devised based on essential factors in stroke rehabilitation to maintain motivation, such as goal setting, task difficulty control, and feedback [Citation44]. First, the system implements settings for recording scores per attempt and increasing the type of graphics for each goal achieved, which can encourage users to achieve and surpass previous scores. Second, this system can control the level of difficulty by selecting stages according to the severity of motor impairment, and can be adjusted to suit a wide range of severities of motor impairment [Citation33]. Furthermore, this system indirectly provides feedback on the amount of muscle activity (i.e. whether the muscle activity is sufficient) as actions of graphics and the success rate of those movements as a total score. Controlling the difficulty level and providing appropriate feedback have been reported to be effective in promoting intrinsic motivation (motivation driven by enjoyment and satisfaction inherent in an activity) in gamified exercises [Citation29,Citation45,Citation46]. For these reasons, the present exercise system is expected to maintain participants’ motivation even with long-term use. However, sub-analysis revealed that the perspectives on the gamified exercise may be influenced by the level of hand-finger motor function and the level of playable stage, in such a way that those who had better motor function or those who were able to play the higher stages tended to have more positive perspectives on the gamified system. These results suggest that there is still room for improvement in the system so that people with more severe hemiparesis can have more positive perspectives to the same extent as people with milder hemiparesis.
Interestingly, the present study revealed that the conventional self-exercise did not lead to a significant decrease in enjoyment and motivation with continued exercise, similar to the gamified exercise, even with generally lower scores. Furthermore, the expectancy of effectiveness was not significantly different between the two exercises. Since the conventional self-exercises were determined by therapists who understood the participants’ impairments well, expectations of their effectiveness may have been relatively high. Another possibility is that this may be simply due to the small sample size. The expectancy of effectiveness increases motivation to exercise in the elderly [Citation47]. Therefore, the relatively positive expectancy of effectiveness may have contributed to the sustained motivation for the conventional self-exercise training. The result that there was no significant difference in expectancy of effectiveness of the gamified exercise compared to conventional self-exercise also indicates that our gamified system may induce sufficient expectations as the participants have on personalized self-exercise provided by therapists.
Although the results of the present feasibility study are promising, several limitations must be addressed in future studies. As discussed above, this study was conducted over a period of seven days. It remains unclear how these perspectives change when the exercise is performed over a longer period. In addition, the level of difficulty could not be fully matched between the exercises, which could lead to different subjective perspectives of the participants regarding the exercises. Although the playable stage of the gamified exercise and the task for conventional self-exercise were chosen based on the individual’s motor function, matching the level of difficulty of both exercises can be challenging. The order of intervention for the two conditions was fixed, the gamified exercise was done later, which may have influenced the results. For example, the subjective perspectives may have been influenced by the fatigue that the participants may have felt while performing the gamified exercise. We used the NRS to assess subjective perspectives on enjoyability, motivation to continue, and expectancy of effectiveness because the NRS has been widely used for similar purposes in several previous studies [Citation31,Citation48]. However, its use for these purposes has not been validated and thus further test would be required for validation. In the present study, the number of movements during the 15-min intervention could not be compared between both conditions because that during conventional self-exercise was not recorded. Future investigation of the impact of the gamified system on the number of movements during exercise would be an interesting topic to address. Finally, this study could not verify whether repetitive exercises using this system prevented decline or improved motor function. Future studies should examine the effects of the gamified exercise on motor recovery.
Conclusion
The present study investigated the feasibility of the developed EMG-controlled gamified exercise system for repetitive hand-finger movement training in people with stroke. The developed gamified system showed consistently positive perspectives on enjoyability and motivation to continue throughout the 7-day exercises as compared to the conventional self-exercise. Exercises using the developed gamified system may have the potential to maintain motivation and enjoyment in people with stroke to continue monotonous repetitive hand-finger movements.
Authors’ contributions
KI, SU, AY, and YO contributed to conception and design of the study. KI collected data and performed the statistical analysis. KI, SU, and AY wrote the first draft of the manuscript. KU, ST, and YO revised the manuscript critically for intellectual content. All authors approved the submitted version of the manuscript.
Supplemental Material
Download MS Word (2.5 MB)Acknowledgements
We would like to thank Mr. Kosuke Otsubo, a software engineer, and Ms. Yui Minemura and Mr. Shinji Kato, computer graphic designers at SPEED Inc., as well as Mr. Chang Man Kim, an engineer at SMK Corporation, for their invaluable contributions to the development of game elements in our gamified system.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding authors, upon reasonable request.
Additional information
Funding
References
- GBD 2016 Stroke Collaborators. Global, regional, and national burden of stroke, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019;18(5):1–9. doi: 10.1016/S1474-4422(19)30034-1.
- Broeks JG, Lankhorst GJ, Rumping K, et al. The long-term outcome of arm function after stroke: results of a follow-up study. Disabil Rehabil. 1999;21(8):357–364. doi: 10.1080/096382899297459.
- Chen CM, Tsai CC, Chung CY, et al. Potential predictors for health-related quality of life in stroke patients undergoing inpatient rehabilitation. Health Qual Life Outcomes. 2015;13(1):118. doi: 10.1186/s12955-015-0314-5.
- Lieshout ECC, van de Port IG, Dijkhuizen RM, et al. Does upper limb strength play a prominent role in health-related quality of life in stroke patients discharged from inpatient rehabilitation? Top Stroke Rehabil. 2020;27(7):525–533. doi: 10.1080/10749357.2020.1738662.
- Arya KN, Pandian S, Verma R, et al. Movement therapy induced neural reorganization and motor recovery in stroke: a review. J Bodyw Mov Ther. 2011;15(4):528–537. doi: 10.1016/j.jbmt.2011.01.023.
- Takeuchi N, Izumi S. Rehabilitation with poststroke motor recovery: a review with a focus on neural plasticity. Stroke Res Treat. 2013;2013:128641. doi: 10.1155/2013/128641.
- Nudo RJ, Milliken GW, Jenkins WM, et al. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci. 1996;16(2):785–807. doi: 10.1523/JNEUROSCI.16-02-00785.1996.
- Nudo RJ, Wise BM, SiFuentes F, et al. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science. 1996;272(5269):1791–1794. doi: 10.1126/science.272.5269.1791.
- Aflalo TN, Graziano MS. Possible origins of the complex topographic organization of motor cortex: reduction of a multidimensional space onto a two-dimensional array. J Neurosci. 2006;26(23):6288–6297. doi: 10.1523/jneurosci.0768-06.2006.
- Peurala SH, Kantanen MP, Sjögren T, et al. Effectiveness of constraint-induced movement therapy on activity and participation after stroke: a systematic review and meta-analysis of randomized controlled trials. Clin Rehabil. 2012;26(3):209–223. doi: 10.1177/0269215511420306.
- Sterr A, Elbert T, Berthold I, et al. Longer versus shorter daily constraint-induced movement therapy of chronic hemiparesis: an exploratory study. Arch Phys Med Rehabil. 2002;83(10):1374–1377. doi: 10.1053/apmr.2002.35108.
- Han CE, Arbib MA, Schweighofer N. Stroke rehabilitation reaches a threshold. PLOS Comput Biol. 2008;4(8):e1000133. doi: 10.1371/journal.pcbi.1000133.
- Winstein CJ, Stein J, Arena R, et al. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2016;47(6):e98–e169. doi: 10.1161/STR.0000000000000098.
- Hsieh YW, Wu CY, Liao WW, et al. Effects of treatment intensity in upper limb robot-assisted therapy for chronic stroke: a pilot randomized controlled trial. Neurorehabil Neural Repair. 2011;25(6):503–511. doi: 10.1177/1545968310394871.
- Klamroth-Marganska V, Blanco J, Campen K, et al. Three-dimensional, task-specific robot therapy of the arm after stroke: a multicentre, parallel-group randomised trial. Lancet Neurol. 2014;13(2):159–166. doi: 10.1016/S1474-4422(13)70305-3.
- Lo AC, Guarino PD, Richards LG, et al. Robot-assisted therapy for long-term upper-limb impairment after stroke. N Engl J Med. 2010;362(19):1772–1783. doi: 10.1056/NEJMoa0911341.
- Smania N, Gandolfi M, Paolucci S, et al. Reduced-intensity modified constraint-induced movement therapy versus conventional therapy for upper extremity rehabilitation after stroke: a multicenter trial. Neurorehabil Neural Repair. 2012;26(9):1035–1045. doi: 10.1177/1545968312446003.
- Wang Q, Zhao JL, Zhu QX, et al. Comparison of conventional therapy, intensive therapy and modified constraint-induced movement therapy to improve upper extremity function after stroke. J Rehabil Med. 2011;43(7):619–625. doi: 10.2340/16501977-0819.
- Winstein CJ, Wolf SL, Dromerick AW, et al. Effect of a task-oriented rehabilitation program on upper extremity recovery following motor stroke: the ICARE randomized clinical trial. JAMA. 2016;315(6):571–581. doi: 10.1001/jama.2016.0276.
- Wolf SL, Thompson PA, Winstein CJ, et al. The EXCITE stroke trial: comparing early and delayed constraint-induced movement therapy. Stroke. 2010;41(10):2309–2315. doi: 10.1161/STROKEAHA.110.588723.
- Wolf SL, Winstein CJ, Miller JP, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA. 2006;296(17):2095–2104. doi: 10.1001/jama.296.17.2095.
- Wu CY, Chen YA, Lin KC, et al. Constraint-induced therapy with trunk restraint for improving functional outcomes and trunk-arm control after stroke: a randomized controlled trial. Phys Ther. 2012;92(4):483–492. doi: 10.2522/ptj.20110213.
- Hung YX, Huang PC, Chen KT, et al. What do stroke patients look for in game-based rehabilitation: a survey study. Medicine. 2016;95(11):e3032. doi: 10.1097/MD.0000000000003032.
- Shaughnessy M, Resnick BM, Macko RF. Testing a model of post-stroke exercise behavior. Rehabil Nurs. 2006;31(1):15–21. doi: 10.1002/j.2048-7940.2006.tb00005.x.
- Karamians R, Proffitt R, Kline D, et al. Effectiveness of virtual reality- and gaming-based interventions for upper extremity rehabilitation poststroke: a meta-analysis. Arch Phys Med Rehabil. 2020;101(5):885–896. doi: 10.1016/j.apmr.2019.10.195.
- Lohse KR, Hilderman CG, Cheung KL, et al. Virtual reality therapy for adults post-stroke: a systematic review and meta-analysis exploring virtual environments and commercial games in therapy. PLOS One. 2014;9(3):e93318. doi: 10.1371/journal.pone.0093318.
- Swanson LR, Whittinghill DM. Intrinsic or extrinsic? Using videogames to motivate stroke survivors: a systematic review. Games Health J. 2015;4(3):253–258. doi: 10.1089/g4h.2014.0074.
- Colman AM. A dictionary of psychology. Oxford (UK): Oxford University Press; 2009. doi: 10.1093/acref/9780199534067.001.0001
- Ryan RM, Deci EL. Intrinsic and extrinsic motivations: classic definitions and new directions. Contemp Educ Psychol. 2000;25(1):54–67. doi: 10.1006/ceps.1999.1020.
- Kelley SA, Brownell CA, Campbell SB. Mastery motivation and self-evaluative affect in toddlers: longitudinal relations with maternal behavior. Child Dev. 2000;71(4):1061–1071. doi: 10.1111/1467-8624.00209.
- van der Kooij K, van Dijsseldonk R, van Veen M, et al. Gamification as a sustainable source of enjoyment during balance and gait exercises. Front Psychol. 2019;10:294. doi: 10.3389/fpsyg.2019.00294.
- Vansteenkiste M, Lens W, Deci EL. Intrinsic versus extrinsic goal contents in self-determination theory: another look at the quality of academic motivation. Educ Psychol. 2006;41(1):19–31. doi: 10.1207/s15326985ep4101_4.
- Ito K, Uehara S, Yuasa A, et al. Electromyography-controlled gamified exercise system for the distal upper extremity: a usability assessment in subacute post-stroke patients. Disabil Rehabil Assist Technol. 2021;18(6):883–888. doi: 10.1080/17483107.2021.1936663.
- Bernhardt J, Hayward KS, Kwakkel G, et al. Agreed definitions and a shared vision for new standards in stroke recovery research: the stroke recovery and rehabilitation roundtable taskforce. Int J Stroke. 2017;12(5):444–450. doi: 10.1177/1747493017711816.
- Chino N, Sonoda S, Domen K, et al. Stroke impairment assessment set (SIAS). In: Chino N, Melvin JL, editors. Functional evaluation of stroke patients. Tokyo: Springer Japan; 1996:19–31.
- Domen K, Sonoda S, Chino N, et al. Evaluation of motor function in stroke patients using the stroke impairment assessment set (SIAS). Functional evaluation of stroke patients. Tokyo: Springer-Verlag; 1996. p. 33–44. doi: 10.1007/978-4-431-68461-9_4.
- Fugl-Meyer AR, Jaasko L, Leyman I, et al. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. JRM. 1975;7(1):13–31. doi: 10.2340/1650197771331.
- Lyle RC. A performance test for assessment of upper limb function in physical rehabilitation treatment and research. Int J Rehabil Res. 1981;4(4):483–492. doi: 10.1097/00004356-198112000-00001.
- Acciarresi M, Bogousslavsky J, Paciaroni M. Post-stroke fatigue: epidemiology, clinical characteristics and treatment. Eur Neurol. 2014;72(5-6):255–261. doi: 10.1159/s000363763.
- Ponchel A, Bombois S, Bordet R, et al. Factors associated with poststroke fatigue: a systematic review. Stroke Res Treat. 2015;2015:347920. doi: 10.1155/2015/347920.
- Yoshida T, Otaka Y, Osu R, et al. Motivation for rehabilitation in patients with subacute stroke: a qualitative study. Front Rehabil Sci. 2021;2:664758. doi: 10.3389/fresc.2021.664758.
- Ashe MC, Miller WC, Eng JJ, et al. Older adults, chronic disease and leisure-time physical activity. Gerontology. 2009;55(1):64–72. doi: 10.1159/000141518.
- Hatem SM, Saussez G, Della Faille M, et al. Rehabilitation of motor function after stroke: a multiple systematic review focused on techniques to stimulate upper extremity recovery. Front Hum Neurosci. 2016;10:442. doi: 10.3389/fnhum.2016.00442.
- Oyake K, Suzuki M, Otaka Y, et al. Motivational strategies for stroke rehabilitation: a delphi study. Arch Phys Med Rehabil. 2020;101(11):1929–1936. doi: 10.1016/j.apmr.2020.06.007.
- Ryan RM, Rigby CS, Przybylski A. The motivational pull of video games: a self-determination theory approach. Motiv Emot. 2006;30(4):344–360. doi: 10.1007/s11031-006-9051-8.
- Przybylski AK, Rigby CS, Ryan RM. A motivational model of video game engagement. Rev Gen Psychol. 2010;14(2):154–166. doi: 10.1037/a0019440.
- Subramanian S, Dahl Y, Skjæret Maroni N, et al. Assessing motivational differences between young and older adults when playing an exergame. Games Health J. 2020;9(1):24–30. doi: 10.1089/g4h.2019.0082.
- Ozaki K, Kondo I, Hirano S, et al. Training with a balance exercise assist robot is more effective than conventional training for frail older adults. Geriatr Gerontol Int. 2017;17(11):1982–1990. doi: 10.1111/ggi.13009.
