Abstract
Introduction: Adverse effects of opioids are common among older individuals, and undertreatment as well as overuse can be an issue. Epidemiological data on opioid use in older individuals are available, but scarce in hospitalized patients.Aims: The aim of this study is to examine the one-day prevalence of opioid use among older inpatients and identify the factors associated with both opioid use and dosage.Materials and methods: One-day cross-sectional study with data collected from geriatric units across 14 Belgian hospitals. The primary focus of the study is to assess the prevalence of opioid use and dosage, along with identifying associated factors. To achieve this, a multiple binary logistic regression model was fitted for opioid use, and a multiple linear regression model for opioid dose.Results: Opioids were used in 24.4% of 784 patients, of which 57.9% was treated with tramadol, 13.2% with oxycodone or morphine and 28.9% with transdermal buprenorphine or fentanyl. The odds for opioid use were 4.2 times higher in patients in orthogeriatric units compared to other patients (OR=4.2, 95% CI=2.50-7.05). The prevalence of opioid use was 34% higher in patients without dementia compared to patients with dementia (OR=0.66, 95% CI=0.46-0.95). The overall mean daily dosage was 14.07mg subcutaneous morphine equivalent. After adjustment for age, gender and dementia, dosage was only associated with type of opioid: the estimated mean opioid dose was 70% lower with tramadol (mean ratio=0,30,95% CI=0,23-0,39) and 67% lower with oxycodone and morphine (mean ratio=0,33, 95% CI=0,22-0,48) compared to transdermal buprenorphine and transdermal fentanyl.Conclusions: One in four patients received opioid treatment. It is not clear whether this reflects under- or overtreatment, but these results can serve as a benchmark for geriatric units to guide future pain management practices. The utilization of transdermal fentanyl and buprenorphine, resulting in higher doses of morphine equivalent, poses significant risks for side effects.
Introduction
Pain is highly prevalent among older adults. In community-dwelling older individuals, approximately 25 to 50% report pain on a frequent basis, while the prevalence of pain increases to about 45 to 80% in institutionalized older adults [Citation1]. Localization and patterns of pain tend to change with advancing age. Pain in older age, acute as well as chronic pain, is often caused by locomotor problems, such as osteo-arthritis, leading to a higher prevalence of pain localized in lower back and legs, particularly the joints [Citation2]. The high prevalence of pain in older adults has a high impact on quality of life and activities of daily living [Citation3]. In line with younger populations, the use of opioids is on the rise among older adults [Citation4–8]. Furthermore, overuse of opioids is widespread in some countries, such as the US but also in Germany, Belgium, Austria, Spain and the Netherlands, and is becoming an urgent public health problem, at least partly due to excessive prescription of opioids [Citation9–13]. In Belgium, opioid prescription rates raised from 2012 to 2019. Since 2019, there is a trend towards a decrease in prescription rates, but this trend needs to be confirmed in the years to follow, partly to evaluate the influence of the Covid pandemic, as well as the influence of a higher awareness among prescribers [Citation14].
Despite opioids being widely known as potent pain relievers and being put forward to manage pain in older adults in a stepwise approach [Citation15,Citation16] and despite increasing use of opioids [Citation12,Citation13], pain in older individuals is still often undertreated for several reasons. These reasons include the misconception that pain is part of aging, concerns about potential side effects in older patients or the influence of cognitive impairment on recognizing and expressing pain [Citation17–19]. Undertreating pain, often attributed to suboptimal prescribing and administration of pain relievers, can lead to frequent falls, reduced mobility and loss of independence, delirium, more frequent hospitalizations and extended hospital stays [Citation20–22]. By treating pain during hospital stay and applying a proper pain management, falls, loss of mobility, loss of independence and delirium can be avoided to some extent, and therefore length of hospital stay can be reduced and rehabilitation in case of acute and chronic pain can be promoted. Use of opioids can indeed have a positive effect on physical rehabilitation and quality of life [Citation23–26], as well as on the use of healthcare facilities [Citation27].
However, because older individuals are more susceptible to adverse effects of opioids, such as sedation, delirium, respiratory depression, decline in liver function, neurotoxicity with seizures, constipation, nausea and vomitus, risk of falling and urinary retention [Citation28–30], and because polypharmacy is a real problem in older patients, use of opioids can cause a higher risk of complications, in turn leading to higher hospital admission rates or longer hospital stay. Given these considerations, it is crucial to investigate the prevalence and characteristics of opioid use in older adults, identify the factors associated with opioid use and dosage, and understand the prescribing practices of treating geriatricians. Such information serves as a vital starting point to improve pain management in older adults within geriatric units.
Until now and to the best of our knowledge, the epidemiological patterns of opioid use in older individuals have primarily been described in community-dwelling older patients, in nursing homes and in emergency departments, with limited information available for hospitalized patients [Citation10, Citation31–33]. However, considering the potential risk of pain undertreatment in older patients on one hand, and the risks of side-effects and opioid overuse on the other, it becomes essential to map the prevalence of opioid use in older in-patients.
This multicentric study aimed to determine the one-day prevalence of opioid use in geriatric units, asses the mean opioid dose received by patients within a 24-h period, and identify the factors associated with both the use and dosage of opioids administered in these units.
Materials and methods
Design and study population
Patients hospitalized in the geriatric units of 14 Belgian hospitals were included on a fixed day in June 2022. Data were collected on that specific day. The only inclusion criterion was being admitted to the geriatric unit. No exclusion criteria were applied. The data collection process was conducted by the treating geriatrician.
All 21 hospitals with geriatric departments in two provinces (East-Flanders and West-Flanders) were contacted and asked to cooperate with the study. Out of 21 hospitals, 14 agreed to cooperate.
In Belgian hospitals, geriatric units are commonly categorized into two types: acute geriatric units, where patients with typical geriatric syndromes receive care, and orthogeriatric units, where geriatric patients with, often acute, orthopaedic problems, such as hip fractures, are hospitalized, regardless of whether they are undergoing surgical treatment or not. However, some hospitals or departments do not make this distinction and operate mixed geriatric units [Citation34,Citation35]. Patients hospitalised on a geriatric ward are 70 years or older.
The study was approved by the central ethics committee (Ghent University Hospital, Belgian Registration number BC-11504) and all local ethics committees. Informed consent was waived due to the noninterventional nature of the study and because strict anonymity during data processing was guaranteed. Study data were collected and managed using REDCap electronic data capture tools hosted at Ghent University [Citation36,Citation37].
Variables
Socio-demographic data, including age category and gender, were recorded for each participant. Diagnosis of dementia was made by the treating senior geriatrician. Administration of pain medication was documented, including the type of pain medication (acetaminophen, anticonvulsant drugs, tricyclic antidepressants, non-steroidal anti-inflammatory drugs (NSAIDs) and opioids). All of those medications were registered only if they were utilized specifically for pain management purposes.
For patients who received any opioids, the following data were collected as it applied to the day of inclusion: type of opioid, total dose administered over a 24-h period (converted to a subcutaneous (SC) morphine equivalent using a conversion table [Citation38]), whether opioids were started during hospitalization or were already taken before admission to the hospital, and the indication for opioid treatment. Patients who were on an as-needed-scheme for opioid administration, but did not receive any opioids during the 24-h registration period, were considered as not being treated with opioids. The classification of opioid types was divided into 3 groups, based on the WHO analgesic ladder [Citation39] and the route of administration: tramadol (step 2 (weak) opioid, orally administered), oxycodone and morphine (step 3 (strong) opioids, orally or subcutaneously administered) and buprenorphine and fentanyl (step 3 (strong) opioids, transdermal administered).
Statistics
A multiple binary logistic regression model was fitted for opioid use, including hospital, gender, dementia status and department type as fixed effects. The (conditional) odds ratios with 95% Wald confidence interval and p-value are reported. Analyses were performed in SPSS version 28 (Statistical Package for the Social Sciences – Windows). All hypothesis testing was performed two-sided at the 5% significance level.
A multiple linear regression model was fitted for opioid dose (log-transformed), including hospital, department type, gender, dementia status, type of opioid, and indication for opioid use in the fixed effects part. Results were back-transformed to the original scale by taking the exponential. Geometric mean ratios with 95% CI and p-value are reported.
Results
A total of 784 patients, divided over 14 hospitals, were included. Participating geriatricians included all patients hospitalized on their wards, no patients were excluded. Patient characteristics are shown in .
Table 1. Patient characteristics and univariate analysis for opioid use and opioid dosage.
Prevalence of opioids and co-medications
On the day of registration, 191 patients (24.4%) were treated with opioids, 486 (62.0%) with paracetamol, 27 (3.4%) with anticonvulsants, 11 (1.4%) with antidepressants and 7 (0.9%) with NSAIDs.
Eighty-eight percent of patients treated with opioids, also received acetaminophen as pain treatment. Vice versa, 34.6% of patients treated with acetaminophen were also treated with opioids. Combination of opioids with other pain treatment was less prevalent: of all patients receiving opioids, 2.1% received NSAID’s, 7.9% anticonvulsants and 2.1% antidepressants.
Tramadol, oxycodone, morphine, buprenorphine, fentanyl and piritramide were the opioid molecules used. Tramadol was used in 57.9% (110 patients) of patients treated with opioids, oxycodone and morphine in 13.2% (25 patients) and buprenorphine and fentanyl in 28.9% (55 patients). Only 1 patient was treated with piritramide (excluded from further statistical analysis).
A high percentage of patients being treated with tramadol or oxycodone were initiated with opioids during their hospital stay (77.0% resp 54.3%), while more patients on fentanyl or buprenorphine were already on that medication before admission to the geriatric unit (77.3% resp 81.8%) (p < 0.001).
Indications for the use of opioids were divided into acute and chronic pathologies. Acute pathologies include acute locomotor problems (fracture as well as non-fracture), acute visceral problems and post-operative episode (until 72 h post-operative). Chronic pathologies include chronic locomotor problems, malignancy and neuropathic pain. A total of 105 (60.3%) patients treated with opioids had an acute pathology (locomotor fracture: 76 (39.8%); locomotor non-fracture: 23 (12.0%); visceral 4 (2.1%); post-operative 3 (1.6%)), compared to 69 (39.7%) patients with a chronic pathology (locomotor 50 (26.2%); malignancy 9 (4.7%); neuropathic 9 (4.7%)). Missing indications (10 patients, 5.2%) and terminal phase/palliative sedation (7 patients, 3.7%) were excluded from further statistical evaluation.
Tramadol (78.9% in acute vs 21.1% in chronic pathology) and oxycodone (61.3% in acute vs 38.7% in chronic pathology) were more used in acute indications, while buprenorphine (9.1% in acute vs 90.9% in chronic pathology) and fentanyl (10.8% in acute vs 89.2% in chronic pathology) were more frequently used in patients with chronic pathology (p < 0.001).
shows the univariate analysis for opioid use. Prevalence of opioid use varied from 12.2% to 47.7% between hospitals (p = 0.039) ().
shows the multivariable logistic regression model on opioid use. If in the univariate analysis for opioid use, p was <0.5, the variable was used in the multivariable logistic regression. Age category was used as a forced variable. The estimated odds for opioid use are 4.2 times higher in patients in orthogeriatric units compared to other patients, adjusted for hospital, gender, age category and dementia status (OR = 4.2, 95% CI = 2.50-7.05, p < 0.0001). The estimated odds for opioid use are 34% lower in patients with dementia compared to patients without dementia, given that we compare patients from the same hospital, gender, age category and department type (OR = 0.66, 95% CI = 0.46-0.95, p = 0.024). An interaction term between hospital and department for opioid use was not statistically significant and thus left out of the final model as presented in .
Table 2. Multivariable logistic regression for opioid use.
Dosage and associated factors
The overall mean daily dosage was 14.07 mg SC morphine equivalent.
Differences in mean opioid dose for different hospitals and departments are shown in Supplementary eFigures 1-4.
shows the linear regression model on opioid dose. We found no indication of an association of gender, hospital, age category or dementia with mean opioid dose. The estimated geometric mean opioid dose (in SC morphine equivalent) is 70% lower with step 2 oral drugs compared to step 3 transdermal drugs (mean ratio = 0,30, 95% CI = 0,23-0,39, p < 0,001), adjusted for hospital, department type, gender, dementia status and indication. The estimated geometric mean opioid dose (in SC morphine equivalent) is 67% lower with step 3 oral/subcutaneous drugs compared to step 3 transdermal drugs (mean ratio = 0,33, 95% CI = 0,22-0,48, p < 0,001), given that we compare patients from the same hospital, department type, gender, dementia status and indication. The association of opioid indication with mean opioid dose was no longer found, when type of opioid was taken into account. An interaction term between hospital and department for opioid dose was not statistically significant, and thus removed from the final model as presented in Table 3.
Table 3. Multivariable linear regression for opioid dosage.
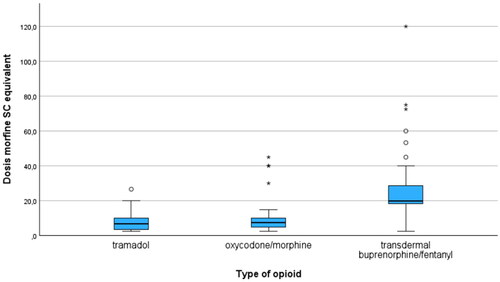
Differences in mean dose (SC morphine equivalent) for type of opioid are shown in . In the group patients treated with step 2 oral drugs, mean daily dose SC morphine equivalent was 7.85 mg (range 2.5-120; SD 4.37), while it was 12.13 mg (range 2.5-45; SD 12.45) in the group treated with step 3 oral/subcutaneous drugs. In the transdermal group, where the mean daily dose was 27.33 mg (range 10-120; SD 19.44), mean daily dose SC morphine equivalent for buprenorphine was 30.84 mg (range 19-53.3; SD 9.66) and 24.55 mg for fentanyl (range 10-120; SD 21.17).
Discussion
In this multicentric study on the use of opioids in patients hospitalized on a geriatric ward, nearly one out of four patients received opioid treatment on the day of registration. The utilization of opioids was found to be associated with the specific department where patients were hospitalized, with a higher frequency of opioid use observed in orthogeriatric departments. Additionally, opioid use was linked to the dementia status of patients, with a higher frequency of opioid use noted in patients without cognitive decline. However, the dosage of opioids administered was only associated with the type of opioid used. Patients in orthogeriatric departments were more likely to be treated with opioids, likely reflecting the specific pathology in such departments, such as fractures, but there was no difference in mean doses administered. Tramadol and oxycodone were more commonly used in patients with an acute pathology, with tramadol being significantly more prevalent than oxycodone, while transdermal buprenorphine and fentanyl were more commonly used in patients with chronic conditions. Patients treated with buprenorphine or fentanyl patches received a significantly higher dose of SC morphine equivalent. Patients with dementia were less likely to receive opioid treatment compared to those without a dementia diagnosis, but there was no statistical difference in the doses used. Once treatment with opioids started, doses used were comparable. Additionally, a majority of patients treated with opioids were also treated with acetaminophen as pain treatment. Only a few patients received NSAIDs.
The high prevalence of opioid use observed in our study aligns with previous results in in-patients [Citation32] and in emergency departments in the US [Citation20]. However, when compared to other investigations, the prevalence of opioid use in our study appears to be higher than some [Citation40], and comparable to findings from studies on community-dwelling older individuals [Citation31, Citation41–43]. On the contrary, certain studies reported lower percentages of opioid use [Citation8, Citation44–47], similar to the observations made in nursing home and assisted living facilities residents in Finland [Citation7, Citation48]. The reasons for these variations in opioid use prevalence across different studies remain unclear, and it is challenging to determine whether the observed prevalence in our study or in others reflects undertreatment or overtreatment of opioids. More comprehensive research and analysis are required to elucidate the factors contributing to these differences and to gain a deeper understanding of the appropriate use of opioids in older patient populations.
Literature suggests that tramadol can have a positive effect on moderate chronic osteoarthritis pain and may be well-tolerated [Citation49]. However, data on older patients with acute or chronic pain are scarce. In a previous study on patients 70 years and above, both tramadol and oxycodone were found to have similar effects on pain in a short-term treatment schedule for acute moderate to severe locomotor pain. Nevertheless, patients treated with tramadol reported a higher frequency of nausea and a trend towards more frequent occurrences of delirium and fall accidents compared to those on oxycodone [Citation17]. The controversy surrounding the use of tramadol in older individuals is related to its potential to cause delirium, primarily due to the existence of metabolites with anticholinergic effects [Citation50]. Despite this concern, tramadol remains widely prescribed as a weak opioid [Citation26], as confirmed in our study. In contrast, oxycodone is considered one of the preferred evidence-based choices for opioid therapy in older adults [Citation51].
The high percentage of buprenorphine and fentanyl patch usage in our study is noteworthy. Comparable results are found in the Pain and Opioids IN treatment study on patients 65 years and older with chronic non-cancer pain [Citation52], in a study on the entire Danish population in nursing home residents with dementia [Citation31] and in home dwelling patients in Finland (particularly in those suffering from dementia) [Citation44]. The high morphine equivalent in the transdermal group (buprenorphine and fentanyl) was not driven by fentanyl, as shown by the fact that the mean daily dose of SC morphine equivalent was not higher in patients treated with fentanyl compared to those treated with buprenorphine. The high usage of fentanyl and buprenorphine patches, potentially resulting in a higher dose of SC morphine equivalent, is not without any risks, while one could wonder if those patients would have received a lower dose (SC morphine equivalent) if they would have been treated with orally administered opioids. Although the safety profiles of transdermal opioids are not necessarily worse than those of orally administered opioids [Citation52], the higher dosage may lead to a heightened frequency of side effects [Citation31, Citation53].
The finding that patients with dementia were less likely to be treated with opioids raises concerns about the potential undertreatment of pain in this group, not in terms of dosage, but in the frequency of opioid treatment. This observation is consistent with earlier findings that pain is often considered to be undertreated in older patients with dementia [Citation31, Citation43, Citation45, Citation54–59], likely due to challenges in assessing pain and concerns about potential side effects, such as sedation and cognitive decline [Citation23]. However, recent studies have presented conflicting results [Citation46, Citation60]. Depending on the setting, opioids were either used more frequently (home dwelling) or less frequently (nursing homes) in patients with dementia [Citation31]. Other studies show either a higher frequency of opioid use in individuals with dementia [Citation43, Citation60] or conversely, a lower frequency [Citation32, Citation44, Citation61]. It is important to note that most studies focus on the prevalence of opioid use, while data regarding differences in dosage between patients with or without dementia, remain limited.
Our results show a variation in opioid use between different hospitals. This reflects possibly an interhospital difference in policy on opioid use [Citation62]. The lack of guidelines on treatment of acute and chronic pain in older patients can lead to differences in prescribing behavior between geriatricians and between hospitals [Citation63,Citation64]. Interhospital collaboration and benchmarking could help to set up guidelines on use of opioids in geriatric patients [Citation65]. In this way, differences in prevalence of opioid use between hospitals could diminish. However, there was no significant difference in opioid dose between hospitals. Once opioids were started, doses were comparable between hospitals.
The use of acetaminophen as an add-on treatment in our study appears to be either lower [Citation33] or higher [Citation40] compared to previous studies. The use of acetaminophen in older individuals with pain, especially chronic locomotor pain, is debatable. While acetaminophen has a better safety profile compared to opioids and can be effective in managing mild acute or chronic pain [Citation66–69], its efficacy in treating moderate to severe chronic pain is lower as it being considered first-line therapy. Furthermore, there is limited data available on the use of acetaminophen in patients aged 75 years or older [Citation70,Citation71]. The low frequency of NSAID use in our study is consistent with the generally accepted approach to avoid the use of NSAIDs in a geriatric population due to their potential adverse effects. One could argue that there is rather limited evidence on the effect of opioids on pain in older individuals, while adverse effects of opioids are frequent. However, the American Geriatric Society has stated that long-term opioid use has a lower risk of life-threatening side effects compared to long-term and daily use of high doses of non-selective NSAIDs [Citation15].
This study can have practice and research implications. The prevalence of opioid use in patients hospitalized on geriatric units is high, but comparable to other studies. Nevertheless, while pain assessment is lacking, there is no certainty that patients are undertreated or overtreated, but these results can be used as a benchmark for geriatric units to guide future pain management practices. The significant usage of fentanyl and buprenorphine patches in our study, possibly leading to a higher dose of SC morphine equivalent, raises concerns about the potential for increased side effects. Thorough reconsideration of treatment schedules for geriatric patients is warranted to mitigate risks. Polypharmacy among older patients is common and can lead to higher rates of adverse effects and drug-drug interactions [Citation40]. Therefore, an evidence-based schedule for pain treatment in older adults is essential. While the 3-step guideline for pain treatment by the World Health Organization (WHO) was originally developed for cancer pain [Citation39], and while the guideline on treatment of pain in older adults by the American Geriatrics Society in 2009 [Citation15] focused on chronic pain, international guidelines [Citation64] on treatment of acute pain in older adults are missing. While non-pharmacological treatment and acetaminophen are often insufficient in treating moderate to severe pain, and NSAIDs have limited use because of the risk of, potentially life-threatening, side effects (gastro-intestinal, renal, cardiac), opioids are often needed as pain relievers. Further research is needed to evaluate the effect and safety of add-on acetaminophen, the use of NSAIDs in short treatment schedules for acute pain, and the comparison between step 2 (weak) and step 3 (strong) opioids in order to draw up guidelines on pain treatment for older adults with frailty, co-morbidities and polypharmacy. When necessary, selecting the correct opioid based on indication, pain duration, and patient characteristics is vital, taking into account the risk of side effects and drug-drug interactions. Therefore, tackling polypharmacy remains a key element in pharmacological treatment for older adults. If opioids are initiated, starting low and going slow remains important, as well as choosing the correct dosing scheme (as-needed or not) and the form of administration (oral, transdermal) and release (slow-release vs immediate release). Once a treatment is started, one must constantly consider if treatment is still needed and useful, and if deprescription is an option. Therefore, adequate monitoring and teaching on side effects is a necessity.
The strengths of our study lie in the inclusion of a large number of patients from multiple hospitals. The use of real-life data, without exclusion criteria, has minimized selection bias. Equally, there is little risk of bias in the selection of hospitals given the high response rate of 66% and the fact that all Belgian hospitals are subject to the same legislative framework concerning staffing and operating standards, making this sample representative for Belgian geriatric units. As one of the first large-scale, multicentric studies on the use of opioids in geriatric in-patients, it contributes significantly to the understanding of opioid usage in this specific setting. The calculating of the mean dosage received by patients (converted to a SC morphine equivalent) adds depth to the study, going beyond merely assessing the prevalence of opioid prescription. This approach allows for a comprehensive examination of differences in dosages and provides valuable insights into opioid administration patterns in geriatric units.
An important limitation of our study is the lack of pain assessment. Thence, we were not able to differentiate between patients with slight, moderate or severe pain. Furthermore, as the focus of the study was on opioid use rather than pain assessment, the analysis began with patients on opioids, potentially leading to the exclusion of patients who might have had pain but were not receiving any treatment. For future research in this area, incorporating pain assessment could enhance the accuracy and comprehensiveness of the findings. This would allow for a more comprehensive analysis of pain management and opioid utilization patterns, ensuring that patients’ pain needs are properly addressed, regardless of their current opioid use status.
Another limitation of our study is the lack of data concerning comorbidity and frailty. However, the fact that most acute indications for opioid treatment were locomotor, and that numbers of patients with acute visceral or post-operative (non-orthopaedic) indications were low, and that most patients with chronic pain also had a locomotor pathology (low numbers of patients with malignancy or neuropathic pain) shows that most patients were treated with opioids because of locomotor problems, where comorbidity and frailty had possibly less influence. However, further research should also focus on patients with important comorbidities and frailty.
Conclusions
In our study involving 14 Belgian hospitals, opioids were used in one out of four patients hospitalized on geriatric units. Tramadol and, to a lesser extent, oxycodone were predominantly used for acute pain, while transdermal buprenorphine and fentanyl were more used in chronic pain. Two significant factors associated with opioid use were dementia status and the department where patients were hospitalized, while the type of opioid administered was associated with the dosage patients received.
Those insights into the prevalence and patterns of opioid use in geriatric in-patients, as well as the factors associated with opioid usage and dosage can help guide clinical decision-making and improve pain management practices in this vulnerable patient population.
Ethics approval statement
The study was approved by the central ethics committee (Ghent University Hospital, Belgian Registration number BC-11504) as well as all local ethics committees.
Patient consent statement
Informed consent was waived due to the noninterventional nature of the study and the assurance of strict anonymity during data processing.
Author contributions
Janssens WH was responsible for study concept and design, data acquisition, data analysis and interpretation, as well as writing the manuscript.
Van Den Noortgate NJ and Piers RD made significant contributions to the study concept and design, as well as analysis and interpretation of data.
Mouton V, Desmet P, Van Puyvelde K, Steen E, Maere C, Van Mulders K, De Raes E, Dekoninck J, Kympers C, Werbrouck B and Delaere J played substantial roles in the acquisition of data.
All authors (Janssens WH, Van Den Noortgate NJ, Mouton V, Desmet P, Van Puyvelde K, Steen E, Maere C, Van Mulders K, De Raes E, Dekoninck J, Kympers C, Werbrouck B, Delaere J and Piers RD) critically revised the article and provided final approval for its publication.
Supplemental Material
Download MS Word (155.7 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Full trial protocol and all data are accessible on request to the corresponding author.
Additional information
Funding
References
- Shega JW, Hougham GW, Stocking CB, et al. Management of noncancer pain in community-dwelling persons with dementia. J Am Geriatr Soc. 2006;54(12):1–10. doi: 10.1111/j.1532-5415.2006.00986.x.
- Pautex S, Michon A, Guedira M, et al. Pain in severe dementia: self-assessment or observational scales? J Am Geriatr Soc. 2006;54(7):1040–1045. doi: 10.1111/j.1532-5415.2006.00766.x.
- Thomas E, Peat G, Harris L, et al. The prevalence of pain and pain interference in a general population of older adults: cross-sectional findings from the North staffordshire osteoarthritis project (NorStOP). Pain. 2004;110(1-2):361–368. doi: 10.1016/j.pain.2004.04.017.
- Kaye AD, Baluch A, Scott JT. Pain management in the elderly population: a review. Ochsner J. 2010;10(3):179–187.
- Li C, Santaella-Tenorio J, Mauro PM, et al. Past-year use of prescription opioids and/or benzodiazepines among adults in the United States: estimating medical and nonmedical use in 2015-2016. Drug Alcohol Depend. 2019;204:107458. doi: 10.1016/j.drugalcdep.2019.04.029.
- Elliot AM, Smith BH, Penny KI, et al. The epidemiology of chronic pain in the community. Lancet. 1999;354(9186):1248–1252. doi: 10.1016/s0140-6736(99)03057-3.
- Pitkala KH, Juola A-L, Hosia H, et al. Eight-year trends in the use of opioids, other analgesics, and psychotropic medications among institutionalized older people in Finland. J Am Med Dir Assoc. 2015;16(11):973–978. doi: 10.1016/j.jamda.2015.06.009.
- Steinman MA, Komaiko KDR, Fung KZ, et al. Use of opioids and other analgesics by older adults in the United States, 1999-2010. Pain Med. 2015;16(2):319–327. doi: 10.1111/pme.12613.
- Skolnick P. The opioid epidemic: crisis and solutions. Annu Rev Pharmacol Toxicol. 2018;58(1):143–159. doi: 10.1146/annurev-pharmtox-010617-052534.
- West NA, Severtson SG, Green JL, et al. Trends in abuse and misuse of prescription opioids among older adults. Drug Alcohol Depend. 2015;149:117–121. doi: 10.1016/j.drugalcdep.2015.01.027.
- Organisation for economic co-operation, development. OECD – Opioids. https://www.oecd.org/health/health-systems/opioids.htm.
- Volkow D, Blanco C. The changing opioid crisis: development, challenges and opportunities. Mol Psychiatry. 2021;26(1):218–233. doi: 10.1038/s41380-020-0661-4.
- Ekstein Y, Jans D, Pieters L, et al. Hoe zorgvuldig worden voorschriften voor opioïden opgesteld? Een analyse van 1.000 voorschriften uitgevoerd in Belgische openbare apotheken. Tijdschr Geneesk. 2022;79:709–720. doi: 10.47671/TVG.78.22.100.
- RAPPORT-NL-Opiaces_2022.pdf (gezondbelgie.be). Flash VIG-news: misbruik van opioïde pijnstillers - voor een rationeel gebruik van opioïden | FAGG;2022.
- American Geriatric Society Panel. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc. 2009;57:1331–1346. doi: 10.1111/j.1532-5415.2009.02376.x.
- Dowd LA, Cross AJ, Liau SJ, et al. Pain in the frail or elderly patient: does tapentadol have a role? Drugs Aging. 2015;40(5):449–459. doi: 10.1007/s40266-015-0268-7.
- Janssens WH, Verhoestraete P, Piers RD, et al. Short-term opioid treatment of acute locomotor pain in older adults: comparison of effectiveness and safety between tramadol and oxycodone: a randomized trial. Br J Pain.
- Achterberg WP, Gambassi G, Finne-Soveri H, et al. Pain in European long-term care facilities: cross-national study in Finland, Italy and The Netherlands. Pain. 2010;148(1):70–74. doi: 10.1016/j.pain.2009.10.008.
- Nygaard HA, Jarland M. Are nursing home patients with dementia diagnosis at increased risk for inadequate pain treatment? Int J Geriatr Psychiatry. 2005;20(8):730–737. doi: 10.1002/gps.1350.
- Marra EM, Mazer-Amirshahi M, Mullins P, et al. Opioid administration and prescribing in older adults in U.S. emergency departments (2005-2015). West J Emerg Med. 2018;19(4):678–688. doi: 10.5811/westjem.2018.5.37853.
- Sawyer P, Bodner EV, Ritchie CS, et al. Pain and pain medication use in community-dwelling older adults. Am J Geriatr Pharmacother. 2006;4(4):316–324. doi: 10.1016/j.amjopharm.2006.12.005.
- Leveille SG, Jones RN, Kiely DK, et al. Chronic musculoskeletal pain and the occurrence of falls in an older population. JAMA. 2009;302(20):2214–2221. doi: 10.1001/jama.2009.1738.
- Papaleontiou M, Henderson CR, Jr, Turner BJ, et al. Outcomes associated with opioid use in the treatment of chronic noncancer pain in older adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2010;58(7):1353–1369. doi: 10.1111/j.1532-5415.2010.02920.x.
- Galicia-Castillo M. Opioids for persistent pain in older adults. Cleve Clin J Med. 2016;83(6):443–451. doi: 10.3949/ccjm.83a.15023.
- Avouac J, Gossec L, Dougados M. Efficacy and safety of opioids for osteoarthritis: a meta-analysis of randomized controlled trials. Osteoarthritis Cartilage. 2007;15(8):957–965. doi: 10.1016/j.joca.2007.02.006.
- Furlan AD, Sandoval JA, Mailis-Gagnon A, et al. Opioids for chronic noncancer pain: a meta-analysis of effectiveness and side effects. CMAJ. 2006;174(11):1589–1594. doi: 10.1503/cmaj.051528.
- American Geriatrics Society Panel on Persistent Pain in Older Persons. The management of persistent pain in older persons. J Am Geriatr Soc. 2002;50:205–224. doi: 10.1046/j.1532-5415.50.6s.1.x.
- Pergolizzi J, Böger RH, Budd K, et al. Opioids and the management of chronic severe pain in the elderly: consensus statement of an International Expert Panel with focus on the six clinically most often used World Health Organization Step III opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone). Pain Pract. 2008;8(4):287–313. doi: 10.1111/j.1533-2500.2008.00204.
- Wongrakpanich S, Wongrakpanich A, Melhado K, et al. A comprehensive review of non-steroidal anti-inflammatory drug use in The elderly. Aging Dis. 2018;9(1):143–150. doi: 10.14336/AD.2017.0306.
- Labianca R, Sarzi-Puttini P, Zuccaro SM, et al. Adverse effects associated with non-opioid and opioid treatment in patients with chronic pain. Clin Drug Investig. 2012;32 Suppl 1:53–63. doi: 10.2165/11630080-000000000-00000.
- Jensen-Dahm C, Gasse C, Astrup A, et al. Frequent use of opioids in patients with dementia and nursing home residents: a study of the entire elderly population of Denmark. Alzheimers Dement. 2015;11(6):691–699. doi: 10.1016/j.jalz.2014.06.013.
- Maiti S, Sinvani L, Pisano M, et al. Opiate prescribing in hospitalized older adults: patterns and outcomes. J Am Geriatr Soc. 2018;66(1):70–75. doi: 10.1111/jgs.15127.
- Murnion BP, Gnjidic D, Hilmer SN. Prescription and administration of opioids to hospital in-patients, and barriers to effective use. Pain Med. 2010;11(1):58–66. doi: 10.1111/j.1526-4637.2009.00747.x.
- Kappenschneider T, Grifka J. Orthogeriatrics: a bridge between orthopaedics and geriatrics. Orthopade. 2022;51(2):79–80. doi: 10.1007/s00132-021-04202-9.
- Falashi P, Marsh D. Orthogeriatrics: the management of older patients with fragility fractures (Internet). 2nd ed. Cham (CH): Springer; 2021.
- Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) – A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010.
- Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208.
- Pijn bij patiënten met kanker - Richtlijnen Palliatieve zorg (palliaweb.nl). Available from: https://palliaweb.nl/richtlijnen-palliatieve-zorg/richtlijn/pijn-bij-patiënten-met-kanker
- Ventafridda V, Saita L, Ripamonti C, et al. WHO guidelines for the use of analgesics in cancer pain. Int J Tissue React. 1985;7(1):93–96.
- Deng LX, Patel K, Miaskowski C, et al. Prevalence and characteristics of hospitalized older adults with moderate to severe pain. J Am Geriatr Soc. 2018;66(9):1744–1751. doi: 10.1111/jgs.15459.
- Williams R, Bosnic N, Duncan AW, et al. Prevalence of opioid dispensing and concurrent gastrointestinal medications in an elderly population from Ontario, Canada. J Opioid Manag. 2008;4(4):193–200. doi: 10.5055/jom.2008.0025.
- Barry LC, Gill TM, Kerns RD, et al. Identification of pain-reduction strategies used by community-dwelling older persons. J Gerontol A Biol Sci Med Sci. 2005;60(12):1569–1575. doi: 10.1093/gerona/60.12.1569.
- Cotton BP, Lohman MC, Brooks JM, et al. Prevalence of and factors related to prescription opioids, benzodiazepines, and hypnotics among medicare home health recipients. Home Healthc Now. 2017;35(6):304–313. doi: 10.1097/NHH.0000000000000553.
- Mörttinen-Vallius H, Hartikainen S, Seinelä L, et al. The prevalence of and exact indications for daily opioid use among aged home care clients with and without dementia. Aging Clin Exp Res. 2021;33(5):1239–1247. doi: 10.1007/s40520-020-01627-8.
- Hamina A, Taipale H, Tanskanen A, et al. Differences in analgesic use in community-dwelling persons with and without Alzheimer’s disease. Eur J Pain. 2017;21(4):658–667. doi: 10.1002/ejp.969.
- Haasum Y, Fastbom J, Fratiglioni L, et al. Pain treatment in elderly persons with and without dementia: a population-based study of institutionalized and home dwelling elderly. Drugs Aging. 2011;28(4):283–293. doi: 10.2165/11587040-000000000-00000.
- Jeffery MM, Hooten WM, Henk HJ, et al. Trends in opioid use in commercially insured and medicare advantage populations in 2007-16: retrospective cohort study. BMJ. 2018;362:k2833. doi: 10.1136/bmj.k2833.
- Roitto H, Kautiainen H, Aalto UL, et al. Fourteen-year trends in the use of psychotropic medications, opioids, and other sedatives among institutionalized older people in helsinki, Finland. J Am Med Dir Assoc. 2019;20(3):305–311. doi: 10.1016/j.jamda.2018.12.022.
- Vorsanger G, Xiang J, Jordan D, et al. Post hoc analysis of a randomized, double-blind, placebo-controlled efficacy and tolerability study of tramadol extended release for the treatment of osteoarthritis pain in geriatric patients. Clin Ther. 2007;29 Suppl(11):2520–2535. doi: 10.1016/j.clinthera.2007.12.009.
- Chau DL, Walker V, Pai L, et al. Opiates and elderly: use and side effects. Clin Interv Aging. 2008;3(2):273–278. doi: 10.2147/cia.s1847.
- Prostran M, Vujović KS, Vučković S, et al. Pharmacotherapy of pain in the older population: the place of opioids. Front Aging Neurosci. 2016;8:144. doi: 10.3389/fnagi.2016.00144.
- Campbell G, Nielsen S, Bruno R, et al. The pain and opioids IN treatment study: characteristics of a cohort using opioids to manage chronic non-cancer pain. Pain. 2015;156(2):231–242. doi: 10.1097/01.j.pain.0000460303.63948.8e.
- Dosa DM, Dore DD, Mor V, et al. Frequency of long-acting opioid analgesic initiation in opioid-naïve nursing home residents. J Pain Symptom Manage. 2009;38(4):515–521. doi: 10.1016/j.jpainsymman.2008.11.008.
- McLachlan AJ, Bath S, Naganathan V, et al. Clinical pharmacology of analgesic medicines in older people: impact of frailty and cognitive impairment. Br J Clin Pharmacol. 2011;71(3):351–364. doi: 10.1111/j.1365-2125.2010.03847.x.
- Scherder E, Oosterman J, Swaab D, et al. Recent developments in pain in dementia. BMJ. 2005;330(7489):461–464. doi: 10.1136/bmj.330.7489.461.
- Hamina A, Taipale H, Tanskanen A, et al. Long-term use of opioids for nonmalignant pain among community-dwelling persons with and without alzheimer disease in Finland: a nationwide register-based study. Pain. 2017;158(2):252–260. doi: 10.1097/j.pain.0000000000000752.
- Maxwell CJ, Dalby DM, Slater M, et al. The prevalence and management of current daily pain among older home care clients. Pain. 2008;138(1):208–216. doi: 10.1016/j.pain.2008.04.007.
- Reynolds KS, Hanson LC, DeVellis RF, et al. Disparities in pain management between cognitively intact and cognitively impaired nursing home residents. J Pain Symp Manage. 2008;35(4):388–396. doi: 10.1016/j.jpainsymman.2008.01.001.
- de Souto Barreto P, Lapeyre-Mestre M, Vellas B, et al. Potential underuse of analgesics for recognized pain in nursing home residents with dementia: a cross-sectional study. Pain. 2013;154(11):2427–2431. doi: 10.1016/j.pain.2013.07.017.
- Lövheim H, Karlsson S, Gustafson Y. The use of Central nervous system drugs and analgesics among very old people with and without dementia. Pharmacoepidemiol Drug Saf. 2008;17(9):912–918. doi: 10.1002/pds.1600.
- Bell SJ, Laitinen M, Lavikainen P, et al. Use of strong opioids among community-dwelling persons with and without alzheimer’s disease in Finland. Pain. 2011;152(3):543–547. doi: 10.1016/j.pain.2010.11.003.
- Janssens WH, Van Den Noortgate NJ, Piers RD. Pharmacological treatment in the dying geriatric patient: describing use and dosage of opioids in the acute geriatric wards and palliative care units of three hospitals. Eur Geriatr Med. 2021;12(3):545–550. doi: 10.1007/s41999-021-00496-2.
- Ferreira-González I, Carrillo X, Martín V, ACDC research group, et al. Interhospital variability in drug prescription after acute coronary syndrome: insights from the ACDC study. Rev Esp Cardiol (Engl Ed). 2016;69(2):117–124. doi: 10.1016/j.rec.2015.04.018.
- Schofield P, Dunham M, Martin D, et al. Evidence-based clinical practice guidelines on the management of pain in older people – a summary report. Br J Pain. 2022;16(1):6–13. doi: 10.1177/2049463720976155.
- Del Giorno R, Ottini A, Greco A, et al. Peer-pressure and overuse: the effect of a multimodal approach on variation in benzodiazepine prescroptions in a network of public hospitals. Int J Clin Pract. 2020;74(3):e13448. doi: 10.1111/ijcp.13448.
- Hoel RW, Giddings Connolly RM, Takahashi PY. Polypharmacy management in older patients. Mayo Clin Proc. 2021;96(1):242–256. doi: 10.1016/j.mayocp.2020.06.012.
- Makris UE, Abrams RC, Gurland B, et al. Management of persistent pain in the older patient: a clinical review. JAMA. 2014;312(8):825–836. doi: 10.1001/jama.2014.9405.
- American Geriatrics Society. Beers criteria update panel. American geriatrics society updated criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60:616–631. doi: 10.1111/j.1532-5415.2012.03923.x.
- Abdulla A, Adams N, Bone M, et al. Guidance on the management of pain in older people. Age Ageing. 2013;42 Suppl 1(2):i1–57. doi: 10.1093/ageing/afs200.
- Reid MC, Shengelia R, Parker SSJ. Pharmacological management of osteoarthritis-related pain in older adults. Am J Nurs. 2012;112(3 Suppl 1):S38–S43. doi: 10.1097/01.NAJ.0000412650.02926.e3.
- Seed SM, Dunican KKC, Lynch AM. Osteoarthritis: a review of treatment options. Geriatrics. 2009;64(10):20–29.