Abstract
Background
Ulcerative colitis (UC) is a disease characterized by chronic relapsing-remitting inflammatory disorders and is associated with environmental changes.
Aim
To explore the disease patterns of Chinese UC patients and to determine controllable related environmental factors.
Methods
This multicentre cross-sectional study was performed using a questionnaire survey. Data on clinical characteristics and environmental factors were collected. Patients with a disease course ≥5 years were defined as the long course group, and those with a disease course < 5 years were defined as the short course group.
Results
A total of 588 effective questionnaires were collected. The proportion of the chronic continuous pattern was the highest among patients with a long disease course (46.8%), and in patients with a short disease course, the proportion of the active to remission pattern was the highest (53.3%). In patients with a long disease course, a higher proportion of patients with adequate sleep was found in the active to remission pattern than in the chronic intermittent (72.1% vs. 43.3%, p = 0.008) and chronic continuous (72.1% vs. 52.4%, p = 0.016) patterns. In patients with a short disease course, the frequency of shellfish and shrimp was higher in the chronic continuous pattern group than in the active to remission pattern group (P = 0.001 and 0.017 respectively).
Conclusions
For early diagnosis patients, dietary guidance should be actively carried out. With the prolongation of the disease course, attention should be given to the sleep quality of patients.
KEY MESSAGES
1.UC exhibits various disease patterns, which may be associated with differences in patient prognosis and treatment response.
2.Environmental factors, especially sleep and dietary factors, correlated strongly with disease patterns, which varied in different disease courses.
3.Early diagnosis patients should receive active dietary guidance, while patients with a prolonged disease course require attention to their sleep quality and appropriate drug interventions when necessary.
Introduction
Ulcerative colitis (UC) is a disease of incompletely understood aetiology and is characterized by chronic relapsing-remitting inflammatory disorders of the entire colon and rectum [Citation1]. Although novel treatment strategies have been introduced to improve long-term disease outcomes, researchers have found that the natural course of UC differs among patients. A better understanding of the natural disease course of UC is crucial to improve patient management, assess the effectiveness of treatments, and provide predictors for prognosis. In 2006, Henriksen et al. first proposed the concept of the UC disease pattern and divided UC patients into four disease patterns based on patient self-evaluation [Citation2]. Then, Danish researchers classified inflammatory bowel disease (IBD) patients into three disease patterns, known as aggressive, moderate, and indolent [Citation3]. In a 2016 Italian cohort, patients were divided into three patterns: disease onset and subsequent mild or no activity, relapsing behaviour, and chronically active disease [Citation4]. A retrospective cohort of children with UC in Denmark in 2020 divided patients into five disease modes: active to remission, remission to active, moderate-severe chronically active, chronic intermittent, and quiescent [Citation5].
The course of disease in UC patients has often been described in terms of the degree of progression of many objective parameters in recent decades, such as the inflammatory process, the need for surgery, the number of relapses, and mortality, but these variables provide little information about patients’ daily habits, such as dietary habits and sleep quality, and how they affect the disease pattern.
Environmental factors such as diet, smoking, sleep, and psychological health are associated with the onset and course of IBD [Citation6–9]. Accumulating evidence has shown that gut dysbiosis combined with an aberrant immune response caused by environmental changes in genetically predisposed individuals is one of the pathogeneses of IBD [Citation10]. As controllable factors, if the relationship between environmental factors and disease patterns is found, it will be of great significance for the prevention and management of chronic IBD. Therefore, this study aimed to explore the characteristics of the different natural courses of UC patients. In addition, IBD is an emerging digestive system disease in China. The genetic, environmental, and other influencing factors in the Chinese population are not the same as those in other populations. Thus, studies on the environmental factors of IBD in the Chinese population are of great significance for understanding the overall profile of IBD worldwide.
Methods
Design and study population
This cross-sectional study included individuals with UC recruited from multiple inflammatory bowel disease centres (including The Second Hospital of Hebei Medical University, The Affiliated Hospital of Qingdao University, Qilu Hospital of Shandong University, Renji Hospital of Shanghai Jiaotong University School of Medicine, and The Affiliated Hospital of Shanxi Medical University) in China. A survey invitation was distributed electronically to patients. Data were collected from June 22, 2022, to January 22, 2023. The inclusion criteria were a confirmed diagnosis of UC based on clinical, endoscopic, and histological examinations according to the criteria of Chinese consensus and the guidelines of ECCO [Citation11,Citation12]. The exclusion criteria were patients who refused to sign the informed consent form and those with a clinical course of less than 6 months.
Data collection and definitions
The data on clinical characteristics and environmental factors were collected via the questionnaire, such as demographic characteristics (gender, age, ethnicity, education level, income level, and place of residence), lifestyle factors (drinking and smoking), clinical characteristics (disease location, extraintestinal manifestations, comorbidities, and medical history), dietary habits (vegetables, fruits, meat, seafood, eggs, milk, yogurt, coffee, processed food, etc.), and sleep factors (sleep duration, sleep quality). The evaluation of diet mainly included the frequency of consumption of certain foods, which was divided into four categories: everyday, sometimes (at least once a week), occasionally (at least once a month), and rarely. The sleep evaluation mainly included sleep duration and sleep quality. The sleep duration was divided into 2 groups: ≥ 7 h and <7 h [Citation13]. The sleep quality was divided into three categories: poor, average, and good. The evaluation of sleep quality is based on the patient’s self-evaluation. If the patient feels refreshed and without any sense of fatigue, it is defined as good sleep quality. Conversely, if the patient wakes up feeling tired, drowsy, or experiencing decreased work efficiency, it is classified as poor sleep quality. Sleep quality is considered average if it falls between these two categories. The criteria of a valid questionnaire were that all questions had been answered and no items were missing.
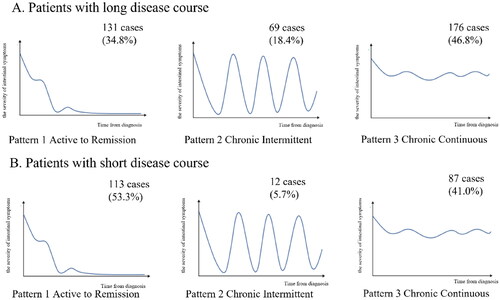
The disease patterns were categorized based on previous studies [Citation2–5,Citation14] as follows:
Pattern 1 (active to remission): patients experienced a decline in the severity of symptoms during the follow-up.
Pattern 2 (chronic intermittent): relapsing behaviour, with remission time >3 months.
Pattern 3 (chronic continuous): no remission lasting more than 3 months.
Because of the differences in the perception of disease patterns and environmental factors among patients with different disease courses, to better compare the characteristics of disease patterns and environmental factors between short and long disease courses, patients in this study were divided into two groups using 5 years as the boundary. A long disease course was defined as a disease course ≥5 years, while a short disease course was defined as a disease course <5 years.
Statistical analysis
Continuous variables are shown as the mean ± standard deviation (x ̅±s) or median (interquartile range, IQR) depending on whether they satisfied a normal distribution. Univariate analysis was performed by Student’s t test or the Mann–Whitney U-test. Categorical variables are expressed as frequencies (%), and univariate analysis was performed by the chi-square test (or Fisher’s exact test). Significance tests were two-tailed, while results with P values of < 0.05 were deemed significant. A multivariable logistic regression model was used to analyse the association between related environmental factors and the disease activity patterns. Variables with a P value <0.1 were included in the multivariate regression model. SPSS 26.0 (IBM Corporation, Chicago, IL, USA) was used for data analysis.
Results
1. Questionnaire collection and disease patterns
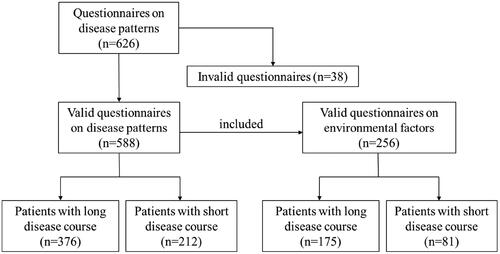
A total of 626 questionnaires on disease patterns were distributed in this study, of which 588 were effective and 38 were invalid (with incomplete data). In addition, 256 cases of effective environmental factor questionnaires were collected from patients with valid disease patterns. The median disease course of a long disease course was 8 years, and the median disease course of a short disease course was 2 years. The disease pattern questionnaire revealed 376 cases (63.9%) in patients with a long disease course. In the short disease course group, 212 cases (36.1%) were revealed by the disease pattern questionnaire (). The distribution of patients over the three disease patterns is presented in . In summary, among patients with a long disease course, 131 cases (34.8%) were pattern 1, 69 cases (18.4%) were pattern 2, and 176 cases (46.8%) were pattern 3 (). In patients with a short disease course, 113 cases (53.3%) were pattern 1, 12 cases (5.7%) were pattern 2, and 87 cases (41.0%) were pattern 3 (). In the comparison of drugs among the three patterns, we found that the proportions of glucocorticoids, immunosuppressants, and biologics used in pattern 2 and pattern 3 were higher than in pattern 1, suggesting that the natural course of patients in pattern 1 was less aggressive than in pattern 2 or pattern 3 (Supplementary Table S1). Among the 588 patients, there were 318 males (54.1%), with a male-to-female ratio of 1.18:1. And the median age at diagnosis was 37.0 years.
2. The differences and related environmental factors between disease patterns in patients with a long disease course
2.1. Pattern 1 (active to remission) versus pattern 2 (chronic intermittent)
There were no significant differences between the two patterns in general demographic data, disease location, extraintestinal manifestations, complications, or drugs (Supplementary Table S2). However, in a univariate comparison of related environmental factors, a higher proportion of patients with adequate sleep was found in pattern 1 than in pattern 2 (72.1% vs. 43.3%, p = 0.008). The frequency of yogurt consumption in pattern 1 was higher than that in pattern 2 (U = 656.0, p = 0.021), and there were no statistically significant differences among the remaining variables ().
Table 1. Environmental factors in disease pattern 1 and pattern 2.
2.2. Pattern 1 (active to remission) versus pattern 3 (chronic continuous)
The results of the univariate comparisons suggest that the proportion of biologics in pattern 3 was higher than that in pattern 1 (39.8% vs. 26.7%, p = 0.017). In terms of specific biologics, the proportion of vedolizumab in patients with pattern 3 was higher than that in patients with pattern 1 (29.0% vs. 14.5%, p = 0.003). And the proportion of cholelithiasis was higher in pattern 3 than in pattern 1 (7.4% vs. 2.3%, p = 0.047) (Supplementary Table S3). Furthermore, a higher proportion of pattern 1 patients had adequate sleep than pattern 3 patients (72.1% vs. 52.4%, p = 0.016), and the proportion of patients with less than 7 h sleep duration was higher in pattern 3 than in pattern 1 (82.1% vs. 47.5%, p < 0.001) (). Sleep factors were further analysed by the multivariable logistic regression model. And our results showed that patients with less than 7 h sleep duration were prone to be pattern 3(OR 4.285, 95%CI 1.899–9.669, p < 0.001) (Supplementary Table S4).
2.3. Pattern 2 (chronic intermittent) versus pattern 3 (chronic continuous)
According to the univariate comparisons, the proportion of male patients with pattern 2 was higher than that of pattern 3 (53.6% vs. 39.8, p = 0.049) (Supplementary Table S5). Additionally, the frequency of yogurt consumption in pattern 2 was lower than that in pattern 3 (U = 964.5, p = 0.045) ().
3. The differences and related environmental factors between disease patterns in patients with a short disease course
3.1. Pattern 1 (active to remission) versus pattern 2 (chronic intermittent)
The proportion of patients using glucocorticoids (66.7% vs. 29.2%, p = 0.024) and composite sophora (41.7% vs. 13.3%, p = 0.024) in pattern 2 was higher than that in pattern 1. Although there was no significant difference in other variables, we found that the age of onset and diagnosis in patients with pattern 1 was smaller than that in patients with pattern 2 (Supplementary Table S2). However, no significant difference was found in the comparison of environmental factors between the two patterns ().
3.2. Pattern 1 (active to remission) versus pattern 3 (chronic continuous)
Our findings revealed that the proportion of patients using glucocorticoids in pattern 3 was higher than that in pattern 1 (42.5% vs. 29.2%, p = 0.050) (Supplementary Table S2). In terms of environmental factors, we observed that the frequency of shellfish and shrimp consumption was higher in pattern 3 than in pattern 1 (p = 0.001 and 0.017, respectively). Additionally, although not statistically significant, the frequency of vegetable and salted product consumption in pattern 3 was higher than that in pattern 1 (p = 0.075 and 0.058, respectively) (). Furthermore, the results of the multivariate analysis indicate that the frequency vegetable consumption was an independent risk factor in pattern 3 (OR 5.397, 95%CI 1.130-25.775, p = 0.035).
Table 2. Environmental factors in disease pattern 1 and pattern 3.
3.3. Pattern 2 (chronic intermittent) versus pattern 3 (chronic continuous)
Our results indicated that patients in pattern 3 exhibited higher monthly income (p = 0.009) and frequency of vegetable consumption (p = 0.038) compared to those in pattern 2. However, there were no significant differences observed in the remaining variables (, Supplementary Table 3).
Table 3. Environmental factors in disease pattern 2 and pattern 3.
Discussion
To the best of our knowledge, this is the first study conducted in China to investigate the disease pattern. One highlight of this study is the comprehensive analysis of environmental factors and disease patterns, which provides new theoretical support for understanding the characteristics of different disease patterns and exploring potentially modifiable environmental factors. In terms of distinguishing the three disease patterns, we found that patients in pattern 1 maintained remission even with weaker treatment strategies, suggesting a less aggressive natural course compared to pattern 2 or 3. Intriguingly, when comparing environmental factors across different patterns, sleep duration and sleep quality showed greater significance in patients with long disease courses, while dietary factors were more significant in patients with a short disease course. Among patients with a long disease course, there was a higher proportion of individuals with short sleep duration in the aggressive pattern. In addition, in comparing patients with a short disease course, it was observed that patients with aggressive patterns are more likely to have unsuitable dietary habits.
The concept of disease patterns was initially introduced in 2006 by Norwegian scholars, who showed that the remission type had the highest proportion (59%) [Citation2]. A retrospective Italian study in 2016 defined three disease patterns, namely, disease onset and subsequent mild or no activity, relapsing behaviour (more than one flare per year, with remission time >3 months), and chronically active disease, defined as no remission lasting more than 3 months [Citation4]. The study further analysed patients by age at onset and suggested that the highest proportion of patients with relapsing behaviour (45%) was <40 years of age, while the highest proportion of patients with subsequent mild or no activity (more than 55%) had a disease onset age of ≥40 years [Citation4]. The highest proportion of patients had the quiescent pattern (33%). With the increased understanding of the disease pattern of UC, UC patients have been divided into various disease patterns combined with the characteristics of patients. Researchers have discussed the disease patterns of UC in different populations, such as children and elderly onset UC patients. However, it is of great significance to comprehensively understand the disease patterns of UC by exploring the disease patterns of different categories and populations. Our study combined previous studies to define the natural course of the patients into 3 patterns: active to remission, chronic intermittent, and chronic continuous. Due to conducting a preliminary questionnaire evaluation in a small number of patients using the Norwegian four-category model before the official questionnaire release, patients generally reported more severe symptoms at disease onset. Therefore, based on patients’ feedback, we ultimately decided to exclude the disease mode of remission to activity when the formal questionnaire was released. Additionally, we also discussed disease patterns in terms of the duration of the disease. In contrast to the Norwegian study, the highest proportion of patients with a long disease course was chronic continuous in this study, while the highest proportion of patients with a short disease course was active to remission (53.3%). The difference in the proportion of disease patterns may be due to the diverse methods of categorization. Our study also found that the use of biologics was lower in patients with active to remission patterns than in patients with a chronic continuous pattern for the long disease course, suggesting that patients with active to remission patterns may have less aggressive natural course characteristics than patients with a chronic continuous pattern and can maintain remission without escalating therapy. Therefore, for patients with aggressive patterns, early intervention and timely escalation of therapies may have a positive impact on the prognosis of patients. For patients with a long disease course and stable disease patterns, unnecessary drugs should be avoided to reduce toxic side effects.
In this study, the comparison of environmental factors revealed that sleep factors differed more significantly in patients with long disease courses. The proportion of longer sleep duration (≥7 h) in pattern 1 (active to remission) was higher than that in the other two patterns. Several studies have suggested that IBD is closely related to sleep disorders, and the activity of IBD disease leads to insomnia, which also aggravates IBD [Citation8,Citation15]. Previously, we showed that, in line with other population-based studies, the proportion of poor sleep quality before onset in UC was higher than that in the healthy control group (15.1% vs. 5.4%) [Citation9]. Moreover, poor sleep was more common in patients with clinically active disease and was positively correlated with the levels of serum IL-6, IL-17, and IL-23 [Citation16]. A study of 166 patients with IBD in Italy showed that 67.5% of the patients suffered from sleep disturbances, and sleep quality was not directly associated with an active or inactive IBD state or with ongoing treatment, but it was mostly related to the patient’s mood state. Furthermore, a positive correlation was reported between both anxiety and depression scores and PSQI (Pittsburgh Sleep Quality Index) scores (Spearman correlation: r = 0.31 and r = 0.38, respectively) in this study [Citation17]. A study of sleep quality in patients with IBD and peripheral arthritis (PA) suggested that the sleep quality of patients with IBD was poorer than that of the control group and that that of patients with IBD and PA was even worse [Citation18]. A recent study on IBD and fatigue suggested that the overall fatigue prevalence in UC patients was 36%, and sleep disturbances were one of the risk factors for fatigue [Citation19]. Shuai Yuan et al. found that when comparing sleep duration ≤5 with 7 h/day, the HR of UC was 1.29 (95% CI, 1.07–1.56), which revealed a positive association between short sleep duration and UC risk [Citation13]. Our results also found that the proportion of sleep deprivation was higher in patients with a longer aggressive natural course, suggesting that sleep duration was associated with the severity of the natural disease course. Considering that the influence of sleep factors on UC patients may have a cumulative effect over time, in addition to the treatment of the primary disease, patients’ sleep and mood should also be properly managed, which is conducive to improving the long-term prognosis and quality of life of patients.
Dietary factors differed significantly between the three disease patterns in short-course patients, whereas they were not observed in long-course patients. Our results showed that patients with pattern 3 had a higher intake of vegetables than those with pattern 1, and patients with pattern 3 hade a higher intake of shellfish and shrimp than those with pattern 2. Although a meta-analysis suggested that vegetable intake was negatively associated with UC incidence [Citation20], vegetables should be reduced to alleviate the intestinal burden for active UC patients based on the Chinese consensus and our results [Citation21]. Shellfish and shrimp are high-quality sources of protein and fat; however, higher seafood consumption was associated with a higher UC risk [Citation22]. Although there is no literature suggesting that the intake of seafood is related to disease relapse in UC, current studies have shown that shellfish and shrimp are common allergenic foods that cause a high proportion of allergic reactions and are more likely to be complicated with digestive tract symptoms [Citation23,Citation24]. Moreover, UC patients frequently experience varying degrees of IgG-mediated food intolerance. Foods such as eggs, shrimp, crab, and others are commonly associated with these intolerances. It has been discovered that partial relief of UC symptoms can be achieved by eliminating IgG antibody-reactive foods from the diet [Citation25]. Additionally, diet plays a significant role in the composition of the gut microbiota. A randomized controlled study suggested that a Mediterranean diet pattern can alleviate intestinal symptoms in patients with quiescent UC and reshape the gut microbiota [Citation26]. Furthermore, the gut microbiota composition of UC patients who maintain long-term remission tends to resemble that of healthy individuals [Citation27]. Therefore, dietary education for UC patients should be emphasized in the early stage to reduce the influence of dietary factors on the course of the disease.
In terms of other related factors, this study found that among patients with short disease courses, the monthly income level of patients with pattern 3 was higher than that of patients with pattern 1 or pattern 2. Further analysis found that the proportion of patients with pattern 3 who had high work intensity and pressure, such as programmers and businessmen, was higher. Psychological stress is related to IBD disease activity [Citation28,Citation29], and most patients show a decrease in work efficiency [Citation30]. Therefore, psychological counselling should be emphasized in UC patients, and appropriate psychological intervention should be given, if necessary, to relieve anxiety, depression, and other emotions of patients and reduce psychological pressure, which may have a positive impact on the alleviation of the disease [Citation31].
The strengths of the present study were the focus on environmental factors and disease patterns, and patients with different patterns were divided into two groups by disease course. As a multicentre study, this study reduced the population bias of a single centre. In contrast, the limitations of this study are that PSQI and anxiety and depression scores were not further evaluated in the sleep study, and the exploration of sleep-influencing factors was not sufficiently in-depth. Future studies are needed to explore the differences and influencing factors of sleep factors in different disease patterns, and prospective studies are needed to evaluate whether there are changes in the disease patterns of patients after sleep intervention.
Conclusion
In conclusion, we found that environmental factors vary among patients with different patterns and disease courses. In patients with a long disease course, short sleep duration was more common in the aggressive pattern, while among patients with a short disease course, unsuitable dietary habits were more common in the aggressive pattern. Therefore, for early diagnosis patients, dietary guidance should be actively carried out. With the prolongation of the disease course, attention should be given to the mental health of patients, and appropriate drug interventions should be given when necessary.
Author contributions
Mingyue Guo, Xueli Ding, Yanbo yu, Linglin Tian, Jun Shen, Weiyang Zheng, and Huijun Shu performed the data collection, statistical analyses and wrote the manuscript. Mingyue Guo, Gechong Ruan, Xiaoyin Bai, Lingjuan Jiang, and Hong Yang participated in designing this study and revising the draft. Hong Yang and Xiaolan Zhang supervised this study and revised the draft. All authors approved the final submitted version.
Ethical approval and consent to participate
This study was approved by the Ethics Committee of Peking Union Medical College Hospital (No: ZS-3563D), and informed consent was obtained from all participants.
Supplemental Material
Download MS Word (40.1 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data used during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Ordás I, Eckmann L, Talamini M, et al. Ulcerative colitis. Lancet. 2012;380(9853):1–14. doi: 10.1016/S0140-6736(12)60150-0.
- Henriksen M, Jahnsen J, Lygren I, et al. Ulcerative colitis and clinical course: results of a 5-year population-based follow-up study (the IBSEN study). Inflamm Bowel Dis. 2006;12(7):543–550. doi: 10.1097/01.MIB.0000225339.91484.fc.
- Jess T, Riis L, Vind I, et al. Changes in clinical characteristics, course, and prognosis of inflammatory bowel disease during the last 5 decades: a population-based study from Copenhagen, Denmark. Inflamm Bowel Dis. 2007;13(4):481–489. doi: 10.1002/ibd.20036.
- Fries W, Viola A, Manetti N, et al. Disease patterns in late-onset ulcerative colitis: results from the IG-IBD "AGED study. Dig Liver Dis. 2017;49(1):17–23. doi: 10.1016/j.dld.2016.09.006.
- Aloi M, Bramuzzo M, Norsa L, et al. Disease activity patterns in the first 5 years after diagnosis in children with ulcerative colitis: a population-based study. J Crohns Colitis. 2021;15(3):367–374. doi: 10.1093/ecco-jcc/jjaa203.
- Singh N, Bernstein CN. Environmental risk factors for inflammatory bowel disease. United European Gastroenterol J. 2022;10(10):1047–1053. doi: 10.1002/ueg2.12319.
- Ananthakrishnan AN, Kaplan GG, Bernstein CN, et al. Lifestyle, behaviour, and environmental modification for the management of patients with inflammatory bowel diseases: an international organization for study of inflammatory bowel diseases consensus. Lancet Gastroenterol Hepatol. 2022;7(7):666–678. doi: 10.1016/S2468-1253(22)00021-8.
- Rozich JJ, Holmer A, Singh S. Effect of lifestyle factors on outcomes in patients with inflammatory bowel diseases. Am J Gastroenterol. 2020;115(6):832–840. doi: 10.14309/ajg.0000000000000608.
- Wu M, Chen X, Zhang H, et al. Evaluation of the effect of sleep on inflammatory bowel disease patients based on propensity score matching. Natl Med J China. 2020;100(20):1551–1556.
- Pickard JM, Zeng MY, Caruso R, et al. Gut microbiota: role in pathogen colonization, immune responses, and inflammatory disease. Immunol Rev. 2017;279(1):70–89. doi: 10.1111/imr.12567.
- Inflammatory Bowel Disease Group, Chinese Society of Gastroenterology, Chinese Medical Association. Chinese consensus on diagnosis and treatment in inflammatory bowel disease (2018, Beijing). J Dig Dis. 2021;22(6):298–317. doi: 10.1111/1751-2980.12994.
- Magro F, Gionchetti P, Eliakim R, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis. 2017;11(6):649–670. doi: 10.1093/ecco-jcc/jjx008.
- Yuan S, Sun Y, Tan X, et al. Sleep duration and daytime napping in relation to incident inflammatory bowel disease: a prospective cohort study. Aliment Pharmacol Ther. 2023; 57(5):475–485. doi: 10.1111/apt.17285.
- Wewer MD, Langholz E, Munkholm P, et al. Disease activity patterns of inflammatory bowel disease-a anish nationwide cohort study 1995-2018. J Crohns Colitis. 2023;17(3):329–337. doi: 10.1093/ecco-jcc/jjac140.
- Parekh PJ, Oldfield Iv EC, Challapallisri V, et al. Sleep disorders and inflammatory disease activity: chicken or the egg? Am J Gastroenterol. 2015;110(4):484–488. doi: 10.1038/ajg.2014.247.
- Sobolewska-Włodarczyk A, Włodarczyk M, Talar M, et al. The association of the quality of sleep with proinflammatory cytokine profile in inflammatory bowel disease patients. Pharmacol Rep. 2021;73(6):1660–1669. doi: 10.1007/s43440-021-00333-0.
- Marinelli C, Savarino EV, Marsilio I, et al. Sleep disturbance in inflammatory bowel disease: prevalence and risk factors – a cross-sectional study. Sci Rep. 2020;10(1):507. doi: 10.1038/s41598-020-57460-6.
- Zhang Y, Pi B, Xu X, et al. Sleep characteristics and influencing factors of sleep quality in patients with inflammatory bowel disease-Peripheral arthritis. Front Med (Lausanne). 2019;6:190. doi: 10.3389/fmed.2019.00190.
- D’Silva A, Fox DE, Nasser Y, et al. Prevalence and risk factors for fatigue in adults with inflammatory bowel disease: a systematic review with Meta-Analysis. Clin Gastroenterol Hepatol. 2022;20(5):995–1009.e7. doi: 10.1016/j.cgh.2021.06.034.
- Hou JK, Abraham B, El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol. 2011;106(4):563–573. doi: 10.1038/ajg.2011.44.
- Li S, Shi H, Yang H. Chinese recommendations on diet in inflammatory bowel disease. Chin J Digest Med Imageol. (Electronic Edition)2021;11(3):97–105.
- Campmans-Kuijpers MJE, Dijkstra G. Food and food groups in inflammatory bowel disease (IBD): the design of the Groningen anti-inflammatory diet (GrAID). Nutrients. 2021;13(4):1067. doi: 10.3390/nu13041067.
- Tasson L, Canova C, Vettorato MG, et al. Influence of diet on the course of inflammatory bowel disease. Dig Dis Sci. 2017; 62(8):2087–2094. doi: 10.1007/s10620-017-4620-0.
- Patel BY, Volcheck GW. Food allergy: common causes, diagnosis, and treatment. Mayo Clin Proc. 2015;90(10):1411–1419. doi: 10.1016/j.mayocp.2015.07.012.
- Jian L, Anqi H, Gang L, et al. Food exclusion based on IgG antibodies alleviates symptoms in ulcerative colitis: a prospective study. Inflamm Bowel Dis. 2018;24(9):1918–1925. doi: 10.1093/ibd/izy110.
- Haskey N, Estaki M, Ye J, et al. A mediterranean diet pattern improves intestinal inflammation concomitant with reshaping of the bacteriome in ulcerative colitis: a randomised controlled trial. J Crohns Colitis. 2023; 17(10):1569–1578. doi: 10.1093/ecco-jcc/jjad073.
- Herrera-deGuise C, Varela E, Sarrabayrouse G, et al. Gut microbiota composition in long-remission ulcerative colitis is close to a healthy gut microbiota. Inflamm Bowel Dis. 2023;29(9):1362–1369. doi: 10.1093/ibd/izad058.
- Bisgaard TH, Allin KH, Keefer L, et al. Depression and anxiety in inflammatory bowel disease: epidemiology, mechanisms and treatment. Nat Rev Gastroenterol Hepatol. 2022;19(11):717–726. doi: 10.1038/s41575-022-00634-6.
- Black J, Sweeney L, Yuan Y, et al. Systematic review: the role of psychological stress in inflammatory bowel disease. Aliment Pharmacol Ther. 2022;56(8):1235–1249. doi: 10.1111/apt.17202.
- Sciberras M, Karmiris K, Nascimento C, et al. Mental health, work presenteeism, and exercise in inflammatory bowel disease. J Crohns Colitis. 2022;16(8):1197–1201. doi: 10.1093/ecco-jcc/jjac037.
- Wynne B, McHugh L, Gao W, et al. Acceptance and commitment therapy reduces psychological stress in patients with inflammatory bowel diseases. Gastroenterology. 2019; 156(4):935–945.e1. doi: 10.1053/j.gastro.2018.11.030.