Abstract
Background: Essential hypertension, a prevalent cardiovascular condition, poses a significant health burden worldwide. Based on the latest American clinical guidelines, half of adults in the United States have hypertension. Of these, only about a half are treated and about a quarter are adequately controlled for hypertension. Given its impact on morbidity and mortality, ensuring effective management of high blood pressure is crucial to reduce associated risks and improve patient outcomes.Objective: This review aims to provide a comprehensive and up-to-date summary of the latest cardiology guidelines and evidence-based research on essential hypertension, with a focus on guiding outpatient clinical practice.Methods: The review evaluates both non-pharmacological approaches and pharmacological interventions to offer clinicians practical insights. Notably, it emphasizes the importance of individualized treatment plans tailored to patients’ specific risk profiles and comorbidities.Results: By consolidating the latest advancements in hypertension management, this review provides clinicians with an up-to-date reference, offering a nuanced understanding of treatment goals and strategies.Conclusion: Through the incorporation of evidence-based recommendations, healthcare practitioners can optimize patient care, mitigate potential complications, and improve overall outcomes in essential hypertension.
Introduction
Hypertension is one of the most common chronic medical conditions characterized by a persistent elevation in arterial blood pressure (BP) [Citation1], which contributes to the development of stroke [Citation2,Citation3], myocardial infarction [Citation4,Citation5], heart failure [Citation6], and renal failure [Citation7]. In the United States, hypertension accounts for more cardiovascular disease (CVD)-related deaths than any other modifiable risk factor and comes in second to cigarette smoking as a preventable cause of death for any reason [Citation8]. According to the latest diagnostic criteria as defined by the 2017 ACC/AHA guidelines [Citation1], nearly half of American adults have hypertension [Citation9]. Although approximately 80% of these adults are recommended prescription BP medication and lifestyle changes, 25% are adequately controlled for hypertension [Citation9]. The prevalence of hypertension increases with age and is higher in men (50.4%) than women (44.3%), and in Africans Americans (56.2%) than any other ethnic group [Citation9]. Additionally, there is a marked discrepancy between the high-income Western world and the world at large in terms of the diagnosis, treatment, and adequate control of hypertension [Citation10]. This review aims to summarize the latest cardiology guidelines and evidence-based research to provide an overview of the current treatment goals and strategies for essential hypertension, and to guide outpatient clinical practice. The literature search was conducted using PubMed and Google Scholar with a focus on articles with high quality of evidence and low risk of bias. Relevant clinical guidelines, randomized controlled trials, systematic reviews or meta-analyses pertaining to essential hypertension were identified through title screening or snowballing references of included articles.
Etiology
Hypertension can be divided into two major categories: essential (or primary) hypertension and secondary hypertension [Citation1]. Essential hypertension, which does not have a single identifiable cause, represents the vast majority of cases (85–95%) [Citation1,Citation11]. Risk factors for essential hypertension include genetics, obesity, diabetes, smoking, high alcohol consumption, high sodium intake, lack of exercise, and stress [Citation1]. In contrast, secondary hypertension is caused by an identifiable underlying condition which can be divided into four categories: renal, endocrine, vascular, or other [Citation12]. Among these, renal causes include renal parenchymal disease or renovascular disease; endocrine causes are adrenal-dependent, thyroid-dependent, or pituitary dependent; vascular causes include coarctation of the aorta; and other causes include obstructive sleep apnea, drug-induced, and pregnancy. Since the prevalence of secondary hypertension is relatively low (5–15%), routine evaluations in the absence of compelling clinical findings are time-consuming and not cost-effective [Citation13].
Diagnostic criteria
In 2017, the American College of Cardiology in conjunction with the American Heart Association (ACC/AHA) released an updated document for the prevention, detection, evaluation, and management of high BP in adults [Citation1]. Of note, this document lowered the diagnostic threshold of hypertension from the preceding 2003 Joint National Committee definition of 140/90 mmHg [Citation14] to 130/80 mmHg (). This threshold was set irrespective of age and co-morbid illness status. Based on this new definition, the prevalence of hypertension increased by approximately 14% in the American population with an additional 4.2 million patients requiring antihypertensive treatment and 7.9 million patients requiring intensification of treatment [Citation15]. In parallel, a year later, the European Society of Cardiology and European Society of Hypertension (ESC/ESH) updated and released their clinical practice guidelines, however, retaining the previous 140/90 mmHg diagnostic threshold () [Citation13]. The discrepancy regarding the diagnostic threshold between these two guidelines carried significant implications with respect to treatment initiation and therapeutic goals across populations. While the new American guidelines were seen as pragmatic, aiming to reduce hypertension-related disease burden by early risk detection and intervention, their European counterpart were more conservative with focus on the individual patient rather than epidemiological concerns [Citation16]. New studies are required to elucidate which strategy has had a bigger impact on the reduction of cardiovascular morbidity and mortality. The STEP trial investigated the ideal systolic BP target in older patients to reduce cardiovascular risk [Citation17]. It found that treatment with a systolic BP target of 110 to <130 mmHg resulted in lower incidence of cardiovascular events compared to standard treatment with a target of 130 to <150 mmHg. However, since this trial was conducted in population of Chinese hypertensive patients aged 60–80, its generalizability to a younger population is unclear.
Table 1. Classification of hypertension based on office blood pressure (BP) measurement.
Despite differences in the definition of hypertension, there is a lot of congruity between the ACC/AHA and ESC/ESH guidelines. Both guidelines, in addition to the 2020 Hypertension Canada guidelines [Citation18], place a strong emphasis on measurement accuracy and are consistent with the approach of BP measurement. The outlined methodology is similar to that used in the ACCORD [Citation19] and SPRINT [Citation20] trials: the patient must be seated in a quiet area for five minutes before a measurement is taken, their feet must be flat on the ground and their back firmly supported, and the appropriate size of cuff must be used. Furthermore, all guidelines stress the importance of repeated readings and out-of-office monitoring in conjunction with automated office BP measurement [Citation1,Citation13,Citation18,Citation21,Citation22]. These are used to rule out masked and white coat hypertension which lead to the under- or over- diagnosis of hypertension, respectively [Citation21].
Out-of-office BP measurements include home BP monitoring (HBPM) which is now very commonly used among hypertensive patients [Citation22]. HBPM has been shown to improve patient motivation for self-care as well as to help patients increase their adherence to antihypertensive medications [Citation23]. Patients are encouraged to take at least two readings, one minute apart in the morning before taking their medications and in the evening before supper. BP measurements are averaged every 2 to 4 weeks to assess the effects of antihypertensive treatment [Citation1].
Out-of-office BP measurements also include ambulatory BP monitoring (ABPM) [Citation22]. Typically, the BP monitor inflates once every half an hour during the day and once an hour when the patient is asleep, allowing for detection of changes in circadian BP [Citation24]. This is a valuable tool for measuring BP variation both day and night in patients with chronic kidney disease who exhibit altered diurnal variation in BP [Citation25,Citation26]. Furthermore, higher 24-h and nighttime BP readings have been associated with greater risks of deaths and composite cardiovascular outcomes, including nonfatal coronary events, heart failure, or stroke [Citation27]. ABPM, therefore, is superior to office-based BP measurement in predicting chronic kidney disease progression and cardiovascular risk [Citation26,Citation28,Citation29]. The identification of sleep disorders is also important as patients with obstructive sleep apnea have increased sympathetic activity and frequently resistant hypertension. A meta-analysis of 1904 participants with obstructive sleep apnea and hypertension found that the use of continuous positive airway pressure significantly reduced 24-h systolic BP (−5.01 mmHg) and 24-h diastolic BP (−3.30 mmHg) [Citation30].
Treatment goals
Due to differences in the definition of hypertension, guidelines consequently differ in BP targets (). The ACC/AHA guidelines set a universal BP goal of <130/80 mmHg, arguing that the ‘one size fits all’ strategy simplifies decisions regarding therapy [Citation1]. In contrast, the ESC/ESH guidelines generally recommend an initial target of <140/90 mmHg and close to 130/80 mmHg, with lower targets individualized on the basis of treatment tolerance and adherence [Citation13]. Similarly, the 2020 Hypertension Canada and the 2021 World Health Organization (WHO) guidelines both recommend a target BP goal of <140/90 mmHg in all patients without comorbidities, with lower thresholds in patients with a high risk of CVD, diabetes mellitus, and chronic kidney disease [Citation18,Citation31].
Table 2. Blood pressure targets in hypertensive patients according to clinical conditions.
Treatment strategies
Non-pharmacological management
Lifestyle modification is seen as the cornerstone for essential hypertension prevention and treatment. High BP is often related to unhealthy dietary habits, lack of physical activity, and/or high alcohol intake. Thus, weight loss in adults who are overweight/obese, adherence to a healthy diet, reduction in sodium intake, enhancement in potassium intake, increased physical activity, moderation in alcohol consumption, and smoking cessation are all prescribed prior to initiation of antihypertensive medication in stage I patients [Citation1,Citation13,Citation18]. Effective lifestyle changes may be sufficient to delay or prevent the need for pharmacological therapy or augment its effects when used in conjunction [Citation13].
Weight loss
There is a continuous almost-linear relationship between body mass index (BMI) and hypertension, with no evidence of a threshold [Citation32,Citation33]. Risk estimates from the Framingham Heart Study suggest that approximately 65–80% of essential hypertension can be ascribed to excess weight gain, particularly increased visceral adiposity [Citation34]. Studies have shown that a loss of 6–8% in body weight can reduce systolic BP and diastolic BP by more than 5 and 4 mmHg, respectively [Citation35], and weight loss of 10 kg may reduce systolic BP by 5–20 mmHg [Citation36]. It is recommended that weight loss strategies employ a multidisciplinary approach which combines dietary education, regular physical activity, and behavioral intervention [Citation37,Citation38].
Physical activity
The antihypertensive effects of exercise training on resting and ambulatory BP have been consistently shown in many trials over the past two decades [Citation39–41]. A meta-analysis of 5223 participants showed that moderate to high intensity aerobic exercise (<210 min/week) reduced resting systolic and diastolic BP by 8.3 and 5.2 mmHg, respectively, in adults with hypertension [Citation39]. Another meta-analysis showed that regardless of intensity, frequency, and duration, aerobic exercise reduced around 4 and 3 mmHg of 24-h ambulatory systolic and diastolic BP, respectively [Citation41]. Dynamic resistance training should be considered as an important supplement to aerobic training as it elicits both antihypertensive and neuromuscular benefits, such as increases in strength, power, and muscle mass [Citation42]. A meta-analysis showed that moderate-intensity dynamic resistance training (65–75% of 1 repetition max, ∼3 days/week) reduced resting systolic and diastolic BP by approximately 6 and 5 mmHg, respectively, in adults with hypertension [Citation40]. A network analysis comparing exercise treatments with antihypertensive medications found no detectable differences in the systolic BP-lowering effects of diuretics, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, calcium channel blockers and beta-blockers when compared to endurance or dynamic resistance training in hypertensive participants [Citation43].
As such, current hypertension guidelines all recommend moderate-intensity aerobic training complimented with resistance training, with variations in frequency intensity, and duration of sessions, for hypertensive adults () [Citation1,Citation13,Citation18]. There is no difference in the magnitude of BP reduction between moderate-intensity continuous training (MICT) and high-intensity interval training (HIIT) [Citation44]. HIIT, however, improves vascular function and general cardiorespiratory fitness to a greater extent that MICT, and can be considered as an alternative approach to the traditional recommendation of MICT to hypertensive patients [Citation44].
Table 3. Professional recommendations of physical activity for adults with hypertension.
Diet
The most established diets for hypertensive patients include the Dietary Approaches to Stop Hypertension (DASH) diet and the Mediterranean diet due to their robust BP-lowering effects [Citation45,Citation46]. In a meta-analysis of dietary pattern interventions, the DASH diet showed significant effects on systolic (−7.6 mmHg) and diastolic (−4.2 mmHg) BP, with higher reductions seen in patients with higher baseline BP [Citation47]. Similarly, the Mediterranean diet has shown beneficial effects on systolic (−3.0 mmHg) and diastolic (−2.0 mmHg) BP, in addition to reductions in body weight (−1.8 kg) and BMI (−0.6 kg/m2) [Citation48]. These two dietary patterns are rich in fruits, vegetables, legumes, nuts and seeds, moderate in fish, seafood, poultry and dairy, and low in red or processed meats and sweets [Citation49].
Independently of dietary pattern, many trials have shown significant BP-lowering effects of sodium restriction [Citation50,Citation51]. The global usual intake of sodium is anywhere between 3.5–5.5 g per day, corresponding to 9–12 g of salt per day [Citation13]. The Canadian and European guidelines both recommend limiting sodium intake to 2 g (or 5 g of salt) per day for individuals with hypertension [Citation13,Citation18]. American guidelines note an optimal goal of less than 1.5 g per day but advise to aim for at least a 1 mg daily reduction in most adults [Citation1]. Most hypertension guidelines acknowledge the benefit of increased potassium intake on BP with only the 2017 AHA/ACC guidelines providing a recommended dose of 3500–5000 mg per day [Citation1].
Alcohol
Due to its detrimental effect on cardiovascular health, most guidelines, including the International Society of Hypertension (ISH), ESC/ESH, ACC/AHA and Hypertension Canada, suggest that no alcohol is the safe threshold for alcohol consumption [Citation1,Citation13,Citation18,Citation52]. The 2020 Hypertension Canada guidelines recommend that both healthy and hypertensive adults should abstain from or limit alcohol consumption to less than 2 drinks a day (the equivalent of 17.2 mL of ethanol or approximately 44 mL of 40% spirits, 355 mL of 5% beer, or 148 mL of 12% wine) [Citation18]. The 2017 AHA/ACC guidelines advise on moderation to two daily drinks for men and one daily drink from women [Citation1]. Similarly, the 2018 ESC/ESH guidelines recommend limiting alcohol consumption to 14 weekly units for men and 8 weekly units for women (1 unit is equal to 125 mL of wine or 250 mL of beer) [Citation13]. Additionally, European guidelines encourage alcohol-free days during the week and avoidance of binge drinking [Citation13].
Smoking
Cigarette smoking has adverse effects on BP and is a major risk factor for CVD [Citation53]. Therefore, it is important to establish a patient’s history regarding tobacco use, update their status on a regular basis, and advise on smoking cessation to hypertensive smokers. The 2017 Canadian and 2018 European guidelines both recommend the use of pharmacological measures (e.g. varenicline, bupropion, nicotine replacement therapy) in combination with behavioral intervention for smoking cessation [Citation1,Citation13]. The 2020 ISH guidelines advise on referral to smoking cessation programs [Citation52].
Electronic nicotine delivery devices, or e-cigarettes, are increasingly gaining popularity, especially among youth and former cigarette smokers. Given their novelty, knowledge on the long-term health consequences of e-cigarettes remains limited. Several small studies assessing the effects of e-cigarettes on cardiovascular risk factors report significant acute increases in both systolic and diastolic BP, similar to those observed with the use of traditional cigarettes [Citation54–56]. A 2020 umbrella review of 183 studies corroborates the adverse effect of e-cigarettes on BP management but highlights that the detrimental impact of e-cigarettes is of lesser magnitude than that of traditional cigarettes [Citation57]. Another cross-sectional study, assessing the association of smoking and e-cigarette use to self-reported diagnosed hypertension, shows that current vaping (aOR= 1.31) and current smoking (aOR = 1.27) are both associated with similar odds of hypertension [Citation58]. Altogether, these findings raise concerns about the harms of chronic e-cigarette use and necessitate further investigation into their long-term cardiovascular impact.
Pharmacological management
The BP thresholds for initiation of antihypertensive therapy vary across guidelines, and are contingent on the grade of hypertension, risk of cardiovascular disease, and presence of comorbidities (). There are five major classes of first-line antihypertensive medications: thiazide/thiazide-like diuretics, angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARB), calcium channel blockers (CCB) and beta-blockers [Citation1]. Although beta-blockers are maintained as first line agents in Canadian guidelines [Citation18], most guidelines restrict them to compelling indications, such as angina, post myocardial infarction, arrythmia, heart failure with reduced ejection fraction, or as an alternative to an ACE inhibitor or ARB in women of child-bearing potential [Citation1,Citation13].
Table 4. Blood pressure thresholds for initiation of antihypertensive therapy.
Single-pill combinations are highly favored since they have been shown to improve patient adherence and increase the rate of BP control by targeting complimentary mechanisms of first-line antihypertensive agents [Citation59,Citation60]. The TRIUMPH and QUARTET trials showed that initiating treatment with a fixed low-dose triple or quadruple antihypertensive combination achieved and maintained greater BP reduction compared to usual care or standard dose monotherapy [Citation61,Citation62]. Recommended single-pill combinations are those in which an ACE inhibitor or ARB is combined with a CCB or a thiazide/thiazide-like diuretic. Typically, triple therapy is initiated when patients do not achieve adequate BP control with a dual combination of antihypertensive agents and require treatment escalation. An ACE inhibitor or ARB combined with a CCB and a thiazide/thiazide-like diuretic is recommended (). If BP remains above-goal with the concurrent use of three first-line agents at maximally tolerated doses, a diagnosis of resistant hypertension may be made [Citation63]. In such cases, further investigation is required to assess treatment adherence, optimal dosing of antihypertensives, and treatment resistance due to secondary causes.
Figure 1. General treatment algorithm for patients with high blood pressure and no compelling indications.
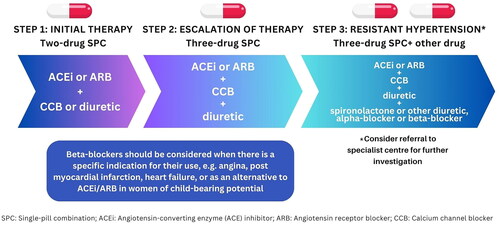
Other antihypertensive medications, including alpha-blockers or mineralocorticoid receptor agonists, are no longer recommended for the routine treatment of hypertension, and are primarily reserved as add-on therapy in cases of resistant hypertension where all other treatment options have failed [Citation13]. Spironolactone has been shown to be the most effective add-on agent in this patient population and is preferred by all guidelines as a fourth-line agent [Citation1,Citation13,Citation18,Citation64].
It is important to note that when treating hypertensive patients, therapy needs to be individualized according to their comorbidities, contraindications, and tolerances. Common comorbidities include diabetes mellitus, coronary artery disease, heart failure, atrial fibrillation, and chronic kidney disease [Citation65]. In these cases, the choice of first-line antihypertensive agents and their doses vary.
Diuretics
Thiazide and thiazide-like diuretics decrease intravascular volume by promoting natriuresis and diuresis via blockage of the sodium-chloride channel in the distal convoluted tubule [Citation66]. They are widely used for pharmacological treatment due to their robust effectiveness in reducing BP, favorable safety profile, and low cost. There are number of different thiazides that are available for the treatment of hypertension (). Current guidelines state that long-acting (thiazide-like) diuretics are preferred over shorter-acting (thiazide) diuretics based on their greater effectiveness in reducing the risk of cardiovascular events (12%, p = 0.049) and heart failure (21%, p = 0.023) [Citation67]. Additionally, in contrast to thiazide diuretics, thiazide-like diuretics have also been shown to reduce coronary events and all-cause mortality [Citation67]. A recent trial of 13,523 patients, however, showed no difference in major cardiovascular outcome events or non–cancer-related deaths after treatment with chlorthalidone or hydrochlorothiazide, bringing the superiority of thiazide-like diuretics into question [Citation68]. Nonetheless, these results were met with controversy due to the design and inclusion criteria of the trial. Some critiques include the confounding effect of hypokalemia which may have influenced cardiosvascular outcomes as well as the lower-than-usual doses of chlorthalidone used in the trial compared to other major trials establishing the benefits of chlorthalidone therapy [Citation69].
Table 5. Summary of first-line antihypertensive agents.
Adverse effects of thiazides include the following: hypokalemia, hyponatremia, metabolic alkalosis, hypercalcemia, hyperglycemia, hyperuricemia, hyperlipidemia, and sulfonamide allergy [Citation66]. These effects stem from the ionic imbalance caused by initial sodium loss in the distal convoluted tubule. Thiazide may also increase the risk of developing acute pancreatitis [Citation70]. As such, clinicians should closely monitor for electrolyte abnormalities and symptoms of acute pancreatitis. A recent network meta-analysis involving 58,807 participants showed that thiazides combined with potassium-sparing diuretics increased BP-lowering efficacy compared with thiazides alone, while minimizing hypokalemia and hyperglycemia [Citation71]. These findings suggest that the combination of thiazide and potassium-sparing diuretics should be considered more frequently in the management of hypertension.
Current guidelines state that thiazides and thiazide-like agents are less effective antihypertensive agents in patients with a reduced glomerular filtration rate (estimated glomerular filtration rate <45 mL/min) and become ineffective when the estimated glomerular filtration rate is <30 mL/min [Citation1,Citation13,Citation72]. In these cases, the guidelines recommend loop diuretics such as furosemide or torsemide over thiazides for their antihypertensive effects () [Citation1]. However, the CLICK trial as well as a recent meta-analysis suggest that thiazide and thiazide-like diuretics maintain their effectiveness in lowering BP in patients with advanced chronic kidney disease [Citation72,Citation73]. Loop diuretics function by inhibiting the reabsorption of sodium and chloride at the apical membrane of the thick ascending limb of the loop of Henle [Citation74]. Like thiazides, adverse effects for loop diuretics occur from electrolyte imbalance secondary to the diuresis effects which should be closely monitored.
Renin-angiotensin-aldosterone system inhibitors
ACE inhibitors and ARBs both act on the renin-angiotensin-aldosterone system (RAAS) which mediates BP through the regulation of vascular tone, and sodium and fluid homeostasis [Citation75]. Although ACE inhibitors and ARBs do not differ in effectiveness, ARBs have shown to present a better safety profile [Citation76]. There are a large number of ACE inhibitors that are available for the treatment of hypertension () [Citation77]. Clinicians should be aware about potential side effects associated with ACE inhibitors include angioedema, hyperkalemia, elevated blood urea nitrogen, creatinine increase, dizziness, and syncope. Monitoring for these effects may be advisable, particularly in older patients or after dosage adjustments [Citation78–80]. Importantly, a dry cough may also develop in approximately 10% of patients treated with ACE inhibitors, half of which will ultimately have to discontinue use [Citation81,Citation82]. This is the most common adverse effect of ACE inhibitors which may occur months or even a year after the institution of therapy. The cough will usually resolve within a few days after withdrawal of treatment [Citation82].
In contrast, ARBs are generally well tolerated and are associated with significantly lower treatment discontinuation rates for adverse events than those of all other antihypertensive therapies [Citation83]. As such, they are suggested as an alternative to patients who cannot tolerate ACE inhibitor therapy due to an induced cough or angioneurotic edema (). Of note, ACE inhibitors and ARBs should not be used in combination [Citation84]. Furthermore, both ACE inhibitors and ARBs should be used with caution in patients with abnormal renal function, aortic valve stenosis, hypovolemia, or bilateral renal artery stenosis. They are contraindicated in pregnancy, and in patients with hyperkalemia (potassium >5.5 mmol/L). Recent guidelines also recommend discontinuation of RAAS inhibitors in women who are considering pregnancy, and caution in women of child-bearing potential who are not using reliable contraception.
There is conflicting evidence on the efficacy of ACE inhibitors and ARBs in Black hypertensive patients [Citation85,Citation86]. Several proposed pathophysiologic mechanisms for decreased efficacy have been advanced, including ethnic differences in metabolism and renin angiotensin aldosterone system, lack of genetic variants in cytochrome P450 2C9 which metabolizes some ARBS, and naturally lower renin levels due to baseline sodium retention [Citation85]. There is also a higher prevalence of ACE inhibitor-induced angioedema in people of black African origin [Citation85]. As such, an ARB is preferred over an ACE inhibitor for antihypertensive treatment in these patients. An ACE inhibitor or ARB may also be less effective in older hypertensive patients due to their decreased plasma renin levels [Citation87]. Therefore, measuring renin levels prior to pharmacotherapy initiation may help individualize treatments in both Black and elderly populations.
Calcium channel blockers
CCBs function by blocking the inward movement of calcium binding to voltage-gated calcium channels in the heart and vascular smooth muscle [Citation88]. They can be divided into two major classes based on their primary physiologic effects: dihydropyridines and non-dihydropyridines [Citation89]. Dihydropyridine CCBs are vascular selective and have antihypertensive properties, predominately affecting peripheral vasodilatation. Contrarily, non-dihydropyridine CCBs are myocardial selective and have antiarrhythmic properties. Both classes of CCBs are used for the treatment of hypertension () with similar effectiveness as other major drug classes on BP, major cardiovascular events, and mortality outcomes [Citation90,Citation91]. Additionally, CCBs are superior to other drugs for the prevention of stroke, but inferior for the prevention of heart failure [Citation90,Citation91].
In general, long-acting dihydropyridines (e.g. amlodipine) are preferred over intermediate-acting (e.g. nicardipine) or short-acting (e.g. nifedipine) dihydropyridines [Citation1,Citation18] to provide greater cardiovascular protection [Citation92] and to simplify dosing to once-daily. Common adverse events in dihydropyridines are related to their vasodilation effects, including dizziness, facial flushing, headaches, and peripheral edema [Citation93]. Dihydropyridines are contraindicated in patients with severe stenotic heart valve defects and hypertrophic obstructive cardiomyopathy.
Non-dihydropyridines, which include verapamil and diltiazem, may cause constipation, worsening cardiac output, and bradycardia [Citation94]. As such, they are contraindicated in patients with heart failure with reduced ejection fraction, second or third-degree AV blockade, sick sinus syndrome, and Wolff-Parkinson-White syndrome. Furthermore, non-dihydropyridines should not be combined with beta-blockers because they can enhance the negative inotropic, chronotropic, and dromotropic effects of beta-blockers [Citation95].
Beta-blockers
Beta-blockers are a class of medication which mediate cardiac activity, control various aspects of metabolic activity and induce smooth muscle relaxation [Citation96]. Due to their effect, they lead to low cardiac output, bradycardia, higher total peripheral resistance, reduced renal blood flow and glomerular filtration rate, and low plasma renin activity. As such, beta-blockers are not recommended as first-line therapy for hypertension in high-risk patients or patients over the age of 60 unless they have comorbid diseases which necessitate beta-blocker need, such as, heart failure or ischemic heart disease [Citation97,Citation98].
Most beta-blockers are dosed at least twice per day while long-acting beta-blockers, such as metoprolol succinate, typically include once-daily dosing (). Labetalol is often used intravenously in patients with hypertensive crises but can also be used as an oral medication. It is not typically used as a first-line therapy for hypertension except in pregnant women [Citation1,Citation13,Citation18].
All beta-blockers, especially in patients with cardiac risk factors, carry a risk of atrioventricular conduction disorders [Citation96]. As such, clinicians should measure the heart rate of patients using these medications at each visit. Patients may also be encouraged to monitor and record their heart rates at home using BP monitors or wearable devices as needed.
Since beta receptors are found all over the body and induce a broad range of physiologic effects, their blockade may lead to many adverse effects. Some commonly reported adverse effects include fatigue, dizziness, nausea, and constipation [Citation96]. Furthermore, beta-blockers should be used with caution in patients with asthma or chronic obstructive pulmonary disorder.
Pregnancy and lactation
Pregnant and lactating patients require special attention, however evidence for this population is lacking and non-contemporaneous () [Citation99,Citation100]. First-line oral medications that are commonly used in pregnancy include labetalol, methyldopa, and long-acting nifedipine [Citation18,Citation99,Citation100]. Other oral beta-blockers, such as acebutolol, metoprolol, pindolol and propranolol, are also considered safe [Citation18]. Second-line agents that can be used in pregnancy include clonidine, hydralazine, and thiazide diuretics. ACE inhibitors and ARBs should be avoided as they are associated with an increased risk of fetal malformations, particularly fetal renal damage [Citation101,Citation102]. For lactating women, and up to 6 weeks postpartum, labetalol, methyldopa, long-acting nifedipine, enalapril, and captopril are commonly used [Citation18]. However, some guidelines suggest avoiding methyldopa due to its increased risk of postpartum depression [Citation13,Citation52,Citation103].
Table 6. Summary of antihypertensive agents used in pregnancy.
Novel therapies
Drug therapies
Novel therapies targeting specific pathways involved in BP regulation are now being investigated for the management of hypertension. Zilebesiran is a small-interfering RNA which inhibits hepatic angiotensinogen synthesis, subsequently lowering BP [Citation104]. A phase I study demonstrated that a single subcutaneous dose of zilebesiran reduced systolic BP by 10 mmHg and diastolic BP by 5 mmHg in 8 weeks [Citation105]. Neprilysin inhibitors, in combination with angiotensin receptor blockers, enhance natriuretic peptide levels, promoting vasodilation and diuresis. In a meta-analysis of 6028 participants, the dual agent sacubitril/valsartan was more effective (−4.62 mmHg systolic BP, −2.13 mmHg diastolic BP) than an ARB in BP reduction among hypertensive patients [Citation106]. Aldosterone synthase inhibitors and endothelin receptor agonists have shown promising results in phase II and phase III trials of BP reduction in patients with resistant hypertension [Citation107,Citation108]. Glucagon-like peptide-1 receptor agonists, previously used for glycemic control in patients with diabetes, are now being investigated for their weight loss effects which consequentially lead to BP reduction [Citation109–112]. In a meta-analysis of 4,567 patients, semaglutide induced a body weight loss of −10.09%, and reduced systolic BP by −5.10 mmHg compared to placebo [Citation113]. SGLT-2 inhibitors, also designed for diabetes, have shown significant BP reduction. A meta-analysis of 9,913 participants showed a systolic BP reduction of −5.06 mmHg and a diastolic BP reduction of −2.39 mmHg compared to placebo [Citation114].
Interventional therapies
Recent advancements have introduced novel approaches to effectively manage hypertension beyond conventional pharmacological therapy. Clinicians should be aware of these options, particularly in cases of resistant hypertension. Renal denervation is a minimally invasive catheter-based procedure which disrupts the sympathetic nerves surrounding the renal arteries to achieve sustained BP reductions. Renal denervation has shown reductions of around 5 mmHg in systolic BP and 2 mmHg in diastolic BP [Citation115]. Furthermore, recent 9-year follow up data shows robust reduction in both office and ambulatory systolic and diastolic BP in patients with resistant hypertension [Citation116]. Baroreceptor activation therapy is a surgical technique which electrically stimulates the carotid baroreceptors to reduce sympathetic nerve activity and, subsequently, BP through an implantable device. It has also shown significant reductions of systolic BP in patients with resistant hypertension [Citation117].
Monitoring and follow-up
Routine tests for all hypertensive patients include hemoglobin and hematocrit, fasting blood glucose and/or HBA1c, lipid profile, serum sodium and potassium, serum creatinine with estimated glomerular filtration rate, serum uric acid, liver enzymes, urinalysis, and 12-lead electrocardiogram [Citation18]. Regular monitoring and follow-ups are crucial when treating hypertension to assess response to treatment, detect any adverse events or complications, and adjust the treatment plan as required. Monthly office follow-ups are recommended after initiation or change in antihypertensive medication until patients reach their target BP [Citation1,Citation13,Citation18]. For patients with controlled BP, follow-ups are recommended every 3–6 months [Citation1,Citation13,Citation18,Citation118]. In addition, patients who have difficulty remembering to take their medication and patients with diabetes may benefit from daily or frequent HBPM [Citation18,Citation119,Citation120].
Telemedicine has shown significant potential in the management of essential hypertension by enhancing patient engagement and treatment adherence in the outpatient setting [Citation121–123]. Telemedicine enables patients to connect with clinicians remotely for regular check-ins which not only saves time and resources but also improves access to care, especially for individuals in rural or underserved areas [Citation121]. Telemedicine can be particularly useful for patients who require close monitoring, as it allows clinicians to assess progress without requiring in-person visits.
Conclusion
In conclusion, hypertension is globally the leading cause of cardiovascular disease and premature death [Citation124]. Although the classifications and definitions of hypertension vary across guidelines, there is a shared goal of utilizing evidence-based research to provide effective strategies to prevent and manage hypertension. Treatment goals and strategies must be individualized to a patient’s lifestyle, comorbidities, and preferences to minimize potential harm and increase the likelihood of long-term compliance. Lifestyle modifications are recommended before initiation of pharmacological therapy in low-moderate risk patients, and alongside pharmacological therapy in higher risk patients. Monotherapy with first-line antihypertensive agents, including diuretics, RAAS inhibitors, CCBs, and beta-blockers, is often inadequate for most hypertensive patients. As such, single-pill combinations are recommended to approve the speed, efficiency, and consistency of initial BP reduction, and long-term BP control. Although great strides have been made in North America and Europe in terms of the identification of patients with hypertension and their treatment, there is still a long way to go. Adequate identification and treatment of hypertension will substantially decrease morbidity and mortality in this population.
Ethical approval
Not applicable
Consent to participate
Not applicable
Authors’ contributions
A.M. conducted the research and drafted the manuscript. T.Z. provided edits to the manuscript. M.E. designed and oversaw the project by providing comments and edits to the manuscript.
Disclosure statement
The authors have no conflicts of interest to disclose.
Code availability
Not applicable
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Additional information
Funding
References
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2018;138(17):1–17. Oct 23 doi: 10.1161/CIR.0000000000000597.
- Feigin VL, Norrving B, Mensah GA. Global burden of stroke. Circ Res. 2017;120(3):439–448. doi: 10.1161/CIRCRESAHA.116.308413.
- O’Donnell MJ, Xavier D, Liu L, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet. 2010;376(9735):112–123. doi: 10.1016/S0140-6736(10)60834-3.
- Carrick D, Haig C, Maznyczka AM, et al. Hypertension, microvascular pathology, and prognosis after an acute myocardial infarction. Hypertension. 2018;72(3):720–730. doi: 10.1161/HYPERTENSIONAHA.117.10786.
- Psaty BM, Furberg CD, Kuller LH, et al. Association between blood pressure level and the risk of myocardial infarction, stroke, and total mortality: the cardiovascular health study. Arch Intern Med. 2001;161(9):1183–1192. doi: 10.1001/archinte.161.9.1183.
- Levy D, Larson MG, Vasan RS, et al. The progression from hypertension to congestive heart failure. JAMA. 1996;275(20):1557–1562. doi: 10.1001/jama.1996.03530440037034.
- Bidani AK, Griffin KA. Pathophysiology of hypertensive renal damage: implications for therapy. Hypertension. 2004;44(5):595–601. Nov doi: 10.1161/01.HYP.0000145180.38707.84.
- Danaei G, Ding EL, Mozaffarian D, et al. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLOS Med. 2009;6(4):e1000058. doi: 10.1371/journal.pmed.1000058.
- Data from: National Center for Health Statistics, Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey (NHANES). 2015–2018.
- Zhou B, Carrillo-Larco RM, Danaei G, et al. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. 2021;398(10304):957–980. doi: 10.1016/S0140-6736(21)01330-1.
- Rossi GP, Bisogni V, Rossitto G, et al. Practice recommendations for diagnosis and treatment of the most common forms of secondary hypertension. High Blood Press Cardiovasc Prev. 2020;27(6):547–560. Dec doi: 10.1007/s40292-020-00415-9.
- Taler, SJ. Secondary causes of hypertension. Prim Care Clin Office Pract. 2008;35(3):489–500. doi: 10.1016/j.pop.2008.06.001.
- Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European society of cardiology and the European society of hypertension: the task force for the management of arterial hypertension of the European society of cardiology and the European society of hypertension. J Hypertens. 2018;36(10):1953–2041. doi: 10.1097/HJH.0000000000001940.
- Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA.2003;289(19):2560–2572. doi: 10.1001/jama.289.19.2560.
- Garies S, Hao S, McBrien K, et al. Prevalence of hypertension, treatment, and blood pressure targets in Canada associated with the 2017 American college of cardiology and American heart association blood pressure guidelines. JAMA Netw Open. 2019;2(3):e190406. doi: 10.1001/jamanetworkopen.2019.0406.
- de la Sierra A. New American and european hypertension guidelines, reconciling the differences. Cardiol Ther. 2019;8(2):157–166. doi: 10.1007/s40119-019-0144-3.
- Zhang W, Zhang S, Deng Y, et al. Trial of intensive blood-pressure control in older patients with hypertension. N Engl J Med. 2021;385(14):1268–1279. doi: 10.1056/NEJMoa2111437.
- Rabi DM, McBrien KA, Sapir-Pichhadze R, et al. Hypertension Canada’s 2020 comprehensive guidelines for the prevention, diagnosis, risk assessment, and treatment of hypertension in adults and children. Can J Cardiol. 2020;36(5):596–624. doi: 10.1016/j.cjca.2020.02.086.
- Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1575–1585. doi: 10.1056/NEJMoa1001286.
- Wright JT, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103–2116. doi: 10.1056/NEJMoa1511939.
- Drawz PE, Ix JH. BP measurement in clinical practice: time to SPRINT to guideline-recommended protocols. J Am Soc Nephrol. 2018;29(2):383–388. Feb doi: 10.1681/ASN.2017070753.
- Stergiou GS, Palatini P, Parati G, et al. 2021 European society of hypertension practice guidelines for office and out-of-office blood pressure measurement. J Hypertens. 2021;39(7):1293–1302. doi: 10.1097/HJH.0000000000002843.
- McManus RJ, Mant J, Franssen M, et al. Efficacy of self-monitored blood pressure, with or without telemonitoring, for titration of antihypertensive medication (TASMINH4): an unmasked randomised controlled trial. Lancet. 2018;391(10124):949–959. doi: 10.1016/S0140-6736(18)30309-X.
- Velasquez MT, Beddhu S, Nobakht E, et al. Ambulatory blood pressure in chronic kidney disease: ready for prime time? Kidney Int Rep. 2016;1(2):94–104. doi: 10.1016/j.ekir.2016.05.001.
- Kim CS, Choi HS, Bae EH, et al. Optimal blood pressure target and measurement in patients with chronic kidney disease. Korean J Intern Med. 2019;34(6):1181–1187.v doi: 10.3904/kjim.2019.164.
- Kanno A, Kikuya M, Asayama K, et al. Night-time blood pressure is associated with the development of chronic kidney disease in a general population: the ohasama study. J Hypertens. 2013;31(12):2410–2417. doi: 10.1097/HJH.0b013e328364dd0f.
- Yang WY, Melgarejo JD, Thijs L, et al. Association of office and ambulatory blood pressure with mortality and cardiovascular outcomes. JAMA. 2019;322(5):409–420. doi: 10.1001/jama.2019.9811.
- Parati G, Ochoa JE, Salvi P, et al. Prognostic value of blood pressure variability and average blood pressure levels in patients with hypertension and diabetes. Diabetes Care. 2013;36 Suppl 2(Suppl 2):S312–S24. doi: 10.2337/dcS13-2043.
- Pickering TG, Shimbo D, Haas D. Ambulatory blood-pressure monitoring. N Engl J Med. 2006;354(22):2368–2374. doi: 10.1056/NEJMra060433.
- Shang W, Zhang Y, Liu L, et al. Benefits of continuous positive airway pressure on blood pressure in patients with hypertension and obstructive sleep apnea: a meta-analysis. Hypertens Res. 2022;45(11):1802–1813. doi: 10.1038/s41440-022-00954-9.
- Campbell NRC, Paccot Burnens M, Whelton PK, et al. 2021 World health organization guideline on pharmacological treatment of hypertension: policy implications for the region of the Americas. Lancet Reg Health Am. 2022;9: none. doi: 10.1016/j.lana.2022.100219.
- Hall JE. The kidney, hypertension, and obesity. Hypertension. 2003;41(3 Pt 2):625–633. doi: 10.1161/01.HYP.0000052314.95497.78.
- Jones DW, Kim JS, Andrew ME, et al. Body mass index and blood pressure in Korean men and women: the Korean National blood pressure survey. J Hypertens. 1994;12(12):1433–1437. doi: 10.1097/00004872-199412000-00018.
- Garrison RJ, Kannel WB, Stokes J, 3rd, et al. Incidence and precursors of hypertension in young adults: the Framingham offspring study. Prev Med. 1987;16(2):235–251. doi: 10.1016/0091-7435(87)90087-9.
- Weiss EP, Albert SG, Reeds DN, et al. Effects of matched weight loss from calorie restriction, exercise, or both on cardiovascular disease risk factors: a randomized intervention trial. Am J Clin Nutr. 2016;104(3):576–586. doi: 10.3945/ajcn.116.131391.
- Garvey WT, Mechanick JI, Brett EM, et al. American association of clinical endocrinologists and American college of endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016;22 Suppl 3:1–203. Jul doi: 10.4158/EP161365.GL.
- Jebb SA, Ahern AL, Olson AD, et al. Primary care referral to a commercial provider for weight loss treatment versus standard care: a randomised controlled trial. Lancet. 2011;378(9801):1485–1492. doi: 10.1016/S0140-6736(11)61344-5.
- Piepoli MF, Hoes AW, Agewall S, et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice: the sixth joint task force of the European society of cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of 10 societies and by invited experts)developed with the special contribution of the European association for cardiovascular prevention & rehabilitation (EACPR). Eur Heart J. 2016;37(29):2315–2381. doi: 10.1093/eurheartj/ehw106.
- Cornelissen VA, Smart NA. Exercise training for blood pressure: a systematic review and meta-analysis. J Am Heart Assoc. 2013;2(1):e004473. doi: 10.1161/JAHA.112.004473.
- MacDonald HV, Johnson BT, Huedo-Medina TB, et al. Dynamic resistance training as stand-alone antihypertensive lifestyle therapy: a meta-analysis. J Am Heart Assoc. 2016;5(10):e003231. doi: 10.1161/JAHA.116.003231.
- Sosner P, Guiraud T, Gremeaux V, et al. The ambulatory hypotensive effect of aerobic training: a reappraisal through a meta-analysis of selected moderators. Scand J Med Sci Sports. 2017;27(3):327–341. Mar doi: 10.1111/sms.12661.
- Williams MA, Haskell WL, Ades PA, et al. Resistance exercise in individuals with and without cardiovascular disease: 2007 update: a scientific statement from the American heart association council on clinical cardiology and council on nutrition, physical activity, and metabolism. Circulation. 2007;116(5):572–584. doi: 10.1161/CIRCULATIONAHA.107.185214.
- Naci H, Salcher-Konrad M, Dias S, et al. How does exercise treatment compare with antihypertensive medications? A network meta-analysis of 391 randomised controlled trials assessing exercise and medication effects on systolic blood pressure. Br J Sports Med. 2019;53(14):859–869. doi: 10.1136/bjsports-2018-099921.
- Costa EC, Hay JL, Kehler DS, et al. Effects of high-intensity interval training versus moderate-intensity continuous training on blood pressure in adults with pre- to established hypertension: a systematic review and meta-analysis of randomized trials. Sports Med. 2018;48(9):2127–2142. Sep doi: 10.1007/s40279-018-0944-y.
- Psaltopoulou T, Naska A, Orfanos P, et al. Olive oil, the mediterranean diet, and arterial blood pressure: the greek European prospective investigation into cancer and nutrition (EPIC) study. Am J Clin Nutr. 2004;80(4):1012–1018. doi: 10.1093/ajcn/80.4.1012.
- Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the dietary approaches to stop hypertension (DASH) diet. DASH-sodium collaborative research group. N Engl J Med. 2001;344(1):3–10. doi: 10.1056/NEJM200101043440101.
- Gay HC, Rao SG, Vaccarino V, et al. Effects of different dietary interventions on blood pressure: systematic review and meta-analysis of randomized controlled trials. Hypertension. 2016;67(4):733–739. doi: 10.1161/HYPERTENSIONAHA.115.06853.
- Esposito K, Kastorini CM, Panagiotakos DB, et al. Mediterranean diet and weight loss: meta-analysis of randomized controlled trials. Metab Syndr Relat Disord. 2011;9(1):1–12. doi: 10.1089/met.2010.0031.
- Hall ME, Cohen JB, Ard JD, et al. Weight-Loss strategies for prevention and treatment of hypertension: a scientific statement from the American heart association. Hypertension. 2021;78(5):e38–e50. doi: 10.1161/HYP.0000000000000202.
- Graudal N, Hubeck-Graudal T, Jurgens G, et al. Dose-response relation between dietary sodium and blood pressure: a meta-regression analysis of 133 randomized controlled trials. Am J Clin Nutr. 2019;109(5):1273–1278. doi: 10.1093/ajcn/nqy384.
- Huang L, Trieu K, Yoshimura S, et al. Effect of dose and duration of reduction in dietary sodium on blood pressure levels: systematic review and meta-analysis of randomised trials. BMJ. 2020;368:m315. doi: 10.1136/bmj.m315.
- Unger T, Borghi C, Charchar F, et al. 2020 International society of hypertension global hypertension practice guidelines. Hypertension. 2020;75(6):1334–1357. doi: 10.1161/HYPERTENSIONAHA.120.15026.
- Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380(9859):2224–2260. doi: 10.1016/S0140-6736(12)61766-8.
- Lundbäck M, Antoniewicz L, Brynedal A, et al. Acute effects of active e-cigarette inhalation on arterial stiffness. Eur Respir J. 2017;50(61) OA1979. doi: 10.1183/1393003.congress-2017.
- Tattersall MC, Hughey CM, Piasecki TM, et al. Abstract 11674: acute effects of nicotine-containing product challenges on cardiovascular and autonomic function among electronic cigarette vapers, combustible cigarette smokers, and controls: the CLUES study. Circulation. 2022;146:A11674. doi: 10.1161/circ.146.suppl_1.11674Circulation.
- Vlachopoulos C, Ioakeimidis N, Abdelrasoul M, et al. Electronic cigarette smoking increases aortic stiffness and blood pressure in young smokers. J Am Coll Cardiol. 2016;67(23):2802–2803. doi: 10.1016/j.jacc.2016.03.569.
- Peruzzi M, Biondi-Zoccai G, Carnevale R, et al. Vaping cardiovascular health risks: an updated umbrella review. Curr Emerg Hosp Med Rep. 2020;8(3):103–109. doi: 10.1007/s40138-020-00219-0.
- Miller CR, Shi H, Li D, et al. Cross-Sectional associations of smoking and E-cigarette use with self-Reported diagnosed hypertension: findings from wave 3 of the population assessment of tobacco and health study. Toxics. 2021;9(3):52. doi: 10.3390/toxics9030052.
- Parati G, Kjeldsen S, Coca A, et al. Adherence to single-pill versus free-equivalent combination therapy in hypertension: a systematic review and meta-analysis. Hypertension. 2021;77(2):692–705. doi: 10.1161/HYPERTENSIONAHA.120.15781.
- Volpe M, Gallo G, Tocci G. New approach to blood pressure control: triple combination pill. Trends Cardiovasc Med. 2020;30(2):72–77. doi: 10.1016/j.tcm.2019.03.002.
- Chow CK, Atkins ER, Hillis GS, et al. Initial treatment with a single pill containing quadruple combination of quarter doses of blood pressure medicines versus standard dose monotherapy in patients with hypertension (QUARTET): a phase 3, randomised, double-blind, active-controlled trial. Lancet. 2021;398(10305):1043–1052. doi: 10.1016/S0140-6736(21)01922-X.
- Webster R, Salam A, de Silva HA, et al. Fixed low-dose triple combination antihypertensive medication vs usual care for blood pressure control in patients with mild to moderate hypertension in Sri Lanka: a randomized clinical trial. JAMA. 2018;320(6):566–579. doi: 10.1001/jama.2018.10359.
- Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American heart association. Hypertension. 2018;72(5):e53–e90. Nov doi: 10.1161/HYP.0000000000000084.
- Williams B, MacDonald TM, Morant S, et al. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet. 2015;386(10008):2059–2068. doi: 10.1016/S0140-6736(15)00257-3.
- Lauder L, Mahfoud F, Azizi M, et al. Hypertension management in patients with cardiovascular comorbidities. Eur Heart J. 2023;44(23):2066–2077. doi: 10.1093/eurheartj/ehac395.
- Akbari P, Khorasani-Zadeh A. Thiazide Diuretics. [Updated 2023 Jan 23]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532918/
- Olde Engberink RH, Frenkel WJ, van den Bogaard B, et al. Effects of thiazide-type and thiazide-like diuretics on cardiovascular events and mortality: systematic review and meta-analysis. Hypertension. 2015;65(5):1033–1040. doi: 10.1161/HYPERTENSIONAHA.114.05122.
- Ishani A, Cushman WC, Leatherman SM, et al. Chlorthalidone vs. hydrochlorothiazide for hypertension-cardiovascular events. N Engl J Med. 2022;387(26):2401–2410. doi: 10.1056/NEJMoa2212270.
- Ishani A, Cushman WC, Leatherman SM. Chlorthalidone vs. hydrochlorothiazide for hypertension-cardiovascular events. Reply. N Engl J Med. 2023;388(14):1342. Apr 6 doi: 10.1056/NEJMc2301922.
- Chaaban N, Kshatriya S. Thiazide-induced pancreatitis. Kans J Med. 2022;15(2):220–221. doi: 10.17161/kjm.vol15.16534.
- Martins VM, Ziegelmann PK, Ferrari F, et al. Thiazide diuretics alone or combined with potassium-sparing diuretics to treat hypertension: a systematic review and network meta-analysis of randomized controlled trials. J Hypertens. 2023;41(7):1108–1116. doi: 10.1097/HJH.0000000000003436.
- Teles F, Peçanha de Miranda Coelho JA, Albino RM, et al. Effectiveness of thiazide and thiazide-like diuretics in advanced chronic kidney disease: a systematic review and meta-analysis. Ren Fail. 2023;45(1):2163903. doi: 10.1080/0886022X.2022.2163903.
- Agarwal R, Sinha AD, Cramer AE, et al. Chlorthalidone for hypertension in advanced chronic kidney disease. N Engl J Med. 2021;385(27):2507–2519. doi: 10.1056/NEJMoa2110730.
- Feig PU. Cellular mechanism of action of loop diuretics: implications for drug effectiveness and adverse effects. Am J Cardiol. 1986;57(2):14A–19A. doi: 10.1016/0002-9149(86)91001-5.
- Weir MR, Dzau VJ. The renin-angiotensin-aldosterone system: a specific target for hypertension management. Am J Hypertension. 1999;12(S9):205S–213S. doi: 10.1016/S0895-7061(99)00103-X.
- Chen R, Suchard MA, Krumholz HM, et al. Comparative first-line effectiveness and safety of ACE (angiotensin-converting enzyme) inhibitors and angiotensin receptor blockers: a multinational cohort study. Hypertension. 2021;78(3):591–603. doi: 10.1161/HYPERTENSIONAHA.120.16667.
- Piepho RW. Overview of the angiotensin-converting-enzyme inhibitors. Am J Health Syst Pharm. 2000;57(Suppl 1):S3–S7. doi: 10.1093/ajhp/57.suppl_1.S3.
- Burnakis TG, Mioduch HJ. Combined therapy with captopril and potassium supplementation. A potential for hyperkalemia. Arch Intern Med. 1984;144(12):2371–2372.
- Khosla S, Ahmed A, Siddiqui M, et al. Safety of angiotensin-converting enzyme inhibitors in patients with bilateral renal artery stenosis following successful renal artery stent revascularization. Am J Ther. 2006;13(4):306–308. doi: 10.1097/00045391-200607000-00005.
- Lunde H, Hedner T, Samuelsson O, et al. Dyspnoea, asthma, and bronchospasm in relation to treatment with angiotensin converting enzyme inhibitors. BMJ. 1994;308(6920):18–21. doi: 10.1136/bmj.308.6920.18.
- Israili ZH, Hall WD. Cough and angioneurotic edema associated with angiotensin-converting enzyme inhibitor therapy. A review of the literature and pathophysiology. Ann Intern Med. 1992;117(3):234–242. doi: 10.7326/0003-4819-117-3-234.
- Overlack A. ACE inhibitor-induced cough and bronchospasm. Incidence, mechanisms and management. Drug Saf. 1996;15(1):72–78. doi: 10.2165/00002018-199615010-00006.
- Kronish IM, Woodward M, Sergie Z, et al. Meta-analysis: impact of drug class on adherence to antihypertensives. Circulation. 2011;123(15):1611–1621. doi: 10.1161/CIRCULATIONAHA.110.983874.
- Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358(15):1547–1559. doi: 10.1056/NEJMoa0801317.
- Helmer A, Slater N, Smithgall S. A review of ACE inhibitors and ARBs in black patients with hypertension. Ann Pharmacother. 2018;52(11):1143–1151. doi: 10.1177/1060028018779082.
- Williams SF, Nicholas SB, Vaziri ND, et al. African Americans, hypertension and the renin angiotensin system. World J Cardiol. 2014;6(9):878–889. doi: 10.4330/wjc.v6.i9.878.
- Palla M, Ando T, Androulakis E, et al. Renin-Angiotensin system inhibitors vs other antihypertensives in hypertensive blacks: a meta-analysis. J Clin Hypertens 2017;19(4):344–350. doi: 10.1111/jch.12867.
- Triggle DJ. The physiological and pharmacological significance of cardiovascular T-type, voltage-gated calcium channels. Am J Hypertens. 1998;11(4):80S–87S. doi: 10.1016/s0895-7061(98)00004-1.
- Nathan S, Pepine CJ, Bakris GL. Calcium antagonists: effects on cardio-renal risk in hypertensive patients. Hypertension. 2005;46(4):637–642. doi: 10.1161/01.HYP.0000184541.24700.c7.
- Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387(10022):957–967. Mar 5 doi: 10.1016/S0140-6736(15)01225-8.
- Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure-lowering on outcome incidence in hypertension: 5. Head-to-head comparisons of various classes of antihypertensive drugs - overview and meta-analyses. J Hypertens. 2015;33(7):1321–1341. doi: 10.1097/HJH.0000000000000614.
- Chaugai S, Sherpa LY, Sepehry AA, et al. Effects of long- and Intermediate-Acting dihydropyridine calcium channel blockers in hypertension: a systematic review and meta-analysis of 18 prospective, randomized, actively controlled trials. J Cardiovasc Pharmacol Ther. 2018;23(5):433–445. doi: 10.1177/1074248418771341.
- Kubota K, Pearce GL, Inman WH. Vasodilation-related adverse events in diltiazem and dihydropyridine calcium antagonists studied by prescription-event monitoring. Eur J Clin Pharmacol. 1995;48(1):1–7. doi: 10.1007/BF00202163.
- Kusumoto FM, Schoenfeld MH, Barrett C, et al. 2018 ACC/AHA/HRS guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart rhythm society. Circulation. 2019;140(8):e382–e482. doi: 10.1161/CIR.0000000000000628.
- Opie LH. Calcium channel antagonists. Part IV: side effects and contraindications drug interactions and combinations. Cardiovasc Drugs Ther. 1988;2(2):177–189. doi: 10.1007/BF00051233.
- Farzam K, Jan A. Beta Blockers. [Updated 2023 Aug 22]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532906
- Wiysonge CS, Bradley HA, Volmink J, et al. Beta-blockers for hypertension. Cochrane Database Syst Rev. 2017;1(1):CD002003. doi: 10.1002/14651858.CD002003.pub5.
- Khan N, McAlister FA. Re-examining the efficacy of beta-blockers for the treatment of hypertension: a meta-analysis. CMAJ. 2006;174(12):1737–1742. doi: 10.1503/cmaj.060110.
- Podymow T, August P. Update on the use of antihypertensive drugs in pregnancy. Hypertension. 2008;51(4):960–969. doi: 10.1161/HYPERTENSIONAHA.106.075895.
- Brown CM, Garovic VD. Drug treatment of hypertension in pregnancy. Drugs. 2014;74(3):283–296. doi: 10.1007/s40265-014-0187-7.
- Park E. Inhibition of the renin-angiotensin system during fetal kidney development. Clin Exp Pediatr. 2021;64(3):121–122. doi: 10.3345/cep.2020.01228.
- Bullo M, Tschumi S, Bucher BS, et al. Pregnancy outcome following exposure to angiotensin-converting enzyme inhibitors or angiotensin receptor antagonists: a systematic review. Hypertension. 2012;60(2):444–450. doi: 10.1161/HYPERTENSIONAHA.112.196352.
- Wiciński M, Malinowski B, Puk O, et al. Methyldopa as an inductor of postpartum depression and maternal blues: a review. Biomed Pharmacother. 2020;127:110196. doi: 10.1016/j.biopha.2020.110196.
- Fernández-Ruiz I. Promising novel siRNA for the treatment of hypertension. Nat Rev Cardiol. 2023;20(10):647–647. doi: 10.1038/s41569-023-00916-9.
- Desai AS, Webb DJ, Taubel J, et al. Zilebesiran, an RNA interference therapeutic agent for hypertension. N Engl J Med. 2023;389(3):228–238. doi: 10.1056/NEJMoa2208391.
- Malik AH, Aronow WS. Efficacy of sacubitril/valsartan in hypertension. Am J Ther. 2022;29(3):e322–e333. doi: 10.1097/MJT.0000000000000925.
- Freeman MW, Halvorsen YD, Marshall W, et al. Phase 2 trial of baxdrostat for treatment-resistant hypertension. N Engl J Med. 2023;388(5):395–405. doi: 10.1056/NEJMoa2213169.
- Schlaich MP, Bellet M, Weber MA, et al. Dual endothelin antagonist aprocitentan for resistant hypertension (PRECISION): a multicentre, blinded, randomised, parallel-group, phase 3 trial. Lancet. 2022;400(10367):1927–1937. doi: 10.1016/S0140-6736(22)02034-7.
- Garvey WT, Batterham RL, Bhatta M, et al. Two-year effects of semaglutide in adults with overweight or obesity: the STEP 5 trial. Nat Med. 2022;28(10):2083–2091. doi: 10.1038/s41591-022-02026-4.
- Jastreboff AM, Aronne LJ, Ahmad NN, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387(3):205–216. doi: 10.1056/NEJMoa2206038.
- Mehta A, Marso SP, Neeland IJ. Liraglutide for weight management: a critical review of the evidence. Obes Sci Pract. 2017;3(1):3–14. doi: 10.1002/osp4.84.
- Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989–1002. doi: 10.1056/NEJMoa2032183.
- Gao X, Hua X, Wang X, et al. Efficacy and safety of semaglutide on weight loss in obese or overweight patients without diabetes: a systematic review and meta-analysis of randomized controlled trials. Front Pharmacol. 2022;13:935823. doi: 10.3389/fphar.2022.935823.
- Zhang Q, Zhou S, Liu L. Efficacy and safety evaluation of SGLT2i on blood pressure control in patients with type 2 diabetes and hypertension: a new meta-analysis. Diabetol Metab Syndr. 2023;15(1):118. doi: 10.1186/s13098-023-01092-z.
- Ahmad Y, Francis DP, Bhatt DL, et al. Renal denervation for hypertension: a systematic review and meta-analysis of randomized, blinded, placebo-controlled trials. JACC Cardiovasc Interv. 2021;14(23):2614–2624. doi: 10.1016/j.jcin.2021.09.020.
- Sesa-Ashton G, Nolde JM, Muente I, et al. Catheter-based renal denervation: 9-year follow-up data on safety and blood pressure reduction in patients with resistant hypertension. Hypertension. 2023;80(4):811–819. doi: 10.1161/HYPERTENSIONAHA.122.20853.
- Wallbach M, Koziolek MJ. Baroreceptors in the carotid and hypertension-systematic review and meta-analysis of the effects of baroreflex activation therapy on blood pressure. Nephrol Dial Transplant. 2018;33(9):1485–1493. doi: 10.1093/ndt/gfx279.
- Birtwhistle RV, Godwin MS, Delva MD, et al. Randomised equivalence trial comparing three month and six month follow up of patients with hypertension by family practitioners. BMJ. 2004;328(7433):204–200. doi: 10.1136/bmj.37967.374063.EE.
- Rave K, Bender R, Heise T, et al. Value of blood pressure self-monitoring as a predictor of progression of diabetic nephropathy. J Hypertens. 1999;17(5):597–601. doi: 10.1097/00004872-199917050-00002.
- Johnson AL, Taylor DW, Sackett DL, et al. Self-recording of blood pressure in the management of hypertension. Can Med Assoc J. 1978;119(9):1034–1039.
- Omboni S, McManus RJ, Bosworth HB, et al. Evidence and recommendations on the use of telemedicine for the management of arterial hypertension: an international expert position paper. Hypertension. 2020;76(5):1368–1383. doi: 10.1161/HYPERTENSIONAHA.120.15873.
- Omboni S, Panzeri E, Campolo L. E-health in hypertension management: an insight into the current and future role of blood pressure telemonitoring. Curr Hypertens Rep. 2020;22(6):42. doi: 10.1007/s11906-020-01056-y.
- Wang JG, Li Y, Chia YC, et al. Telemedicine in the management of hypertension: evolving technological platforms for blood pressure telemonitoring. J Clin Hypertens 2021;23(3):435–439. doi: 10.1111/jch.14194.
- Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16(4):223–237. doi: 10.1038/s41581-019-0244-2.
