Abstract
Background
Circulating plasma cells (CPCs) are defined by the presence of peripheral blood clonal plasma cells, which would contribute to the progression and dissemination of multiple myeloma (MM). An increasing number of studies have demonstrated the predictive potential of CPCs in the past few years. Therefore, there is a growing need for an updated meta-analysis to identify the specific relationship between CPCs and the prognosis of MM based on the current research status.
Methods
The PubMed, Embase, and Cochrane Library databases were screened to determine eligible studies from inception to November 5, 2023. Publications that reported the prognostic value of CPCs in MM patients were included. Hazard ratios (HRs) with 95% confidence intervals (CIs) of overall survival (OS) and progression-free survival (PFS) were extracted to pool the results. Subgroup analyses were performed based on region, sample size, cut-off value, detection time, initial treatment, and data type. The association between CPCs level and clinicopathological characteristics, including the International Staging System (ISS), Revised-ISS (R-ISS), and cytogenetic abnormalities were also evaluated. Statistical analyses were conducted using STATA 17.0 software.
Results
Twenty-two studies with a total of 5637 myeloma patients were enrolled in the current meta-analysis. The results indicated that myeloma patients with elevated CPCs were expected to have a poor OS (HR = 2.19, 95% CI: 1.81–2.66, p < 0.001) and PFS (HR = 2.45, 95% CI: 1.93–3.12, p < 0.001). Subgroup analyses did not alter the prognostic role of CPCs, regardless of region, sample size, cut-off value, detection time, initial treatment, or data type. Moreover, the increased CPCs were significantly related to advanced tumour stage (ISS III vs. ISS I–II: pooled OR = 2.89, 95% CI: 2.41–3.46, p < 0.001; R-ISS III vs. R-ISS I–II: pooled OR = 3.65, 95% CI: 2.43–5.50, p < 0.001) and high-risk cytogenetics (high-risk vs. standard-risk: OR = 2.22, 95% CI: 1.60–3.08, p < 0.001).
Conclusion
Our meta-analysis confirmed that the increased number of CPCs had a negative impact on the PFS and OS of MM patients. Therefore, CPCs could be a promising prognostic biomarker that helps with risk stratification and disease monitoring.
KEY MESSAGES
There is a growing need for an updated meta-analysis to identify the specific relationship between CPCs and the prognosis of MM based on the current research status.
Our meta-analysis revealed that a high CPCs level was significantly associated with worse OS and PFS in MM patients.
CPCs could be a promising predictive biomarker that helps with risk stratification and disease monitoring.
Introduction
Multiple myeloma (MM) is one of the most common haematological malignancies, characterized by the clonal proliferation of malignant plasma cells. Despite the improvement in survival over time, the prognosis of MM is highly heterogeneous [Citation1,Citation2]. The recognition of high-risk factors and the implementation of risk stratification for MM patients can substantially aid in treatment planning and prognosis evaluation. Several variables that affect survival outcomes have been identified and integrated into the prognostic stratification practice, such as the International Staging System (ISS) and the Revised ISS (R-ISS) [Citation3,Citation4]. However, despite the statistical difference in survival, the predictive accuracy of the current stratification systems remains unsatisfactory.
Circulating plasma cells (CPCs) are peripheral blood myeloma cells, which may originate from the bone marrow (BM) or extramedullary sites with reduced expression of integrin, adhesion molecules, and activation molecules. The presence of CPCs would contribute to the dissemination of the tumour and, hence, may indicate a more aggressive disease [Citation5,Citation6]. In 2021, the International Myeloma Working Group recommended that MM patients with 5% or more CPCs in peripheral blood smears should be diagnosed with plasma cell leukaemia (PCL), which represents the most aggressive plasma cell dyscrasia with a very dismal survival rate [Citation7]. However, there is accumulating evidence indicating that the presence of CPCs is associated with a poor outcome in myeloma patients who failed to meet the criteria of PCL [Citation8–23]. It has been reported that the high CPCs level was an independent predictive factor, which could have implications for redefining high-risk MM [Citation15,Citation17,Citation23–25]. However, the prognostic significance of CPCs in MM is still controversial because the previous studies were heterogeneous in detection methods and cut-off values.
A prior meta-analysis showed that the presence of CPCs was associated with an aggressive disease course and short survival in MM patients [Citation26]. However, there were only 11 studies eligible at that time, and nearly all of the involved studies were conducted in Western countries, which could restrict the statistical capability of the findings. Over the last few years, a growing number of studies have revealed the prognostic role of CPCs. Thus, we performed an updated meta-analysis based on the current research status to identify the specific relationship between CPCs levels and the prognosis of MM.
Methods
Search strategy
The present meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). This study has been registered in PROSPERO (CRD42023480942). A comprehensive search was conducted to identify all relevant publications until November 5, 2023, throughout databases, including PubMed, Embase, and Cochrane Library. The search strategy included keywords and MeSH terms related to ‘CPCs’ and ‘MM’. The complete search strategies are presented in Supplementary File 1. We also manually searched the reference lists of the retrieved studies to identify additional eligible studies.
Selection criteria
Studies were included in the meta-analysis if they met all of the following criteria: (i) Involving more than 20 patients that were diagnosed with MM; (ii) Evaluating the association between the CPCs and survival outcomes of MM patients; (iii) Containing Hazard ratios (HRs) with 95% confidence intervals (95% CIs) or providing data from which HR and CI can be derived; (iv) The publication language was confined to English. Exclusion criteria were: (i) Abstracts, letters, reviews, and case reports; (ii) Studies without specific data concerning myeloma or CPCs; (iii) Studies with insufficient data for analysis; (iv) Studies focused on PCL (with a cut-off of CPCs ≥5% according to the new criteria). (v) Multiple published reports. When there were multiple reports available on the same cohort, we incorporated the most recent publication.
Data extraction
Two investigators (QL and LA) independently identified the eligible studies and performed data extraction for this meta-analysis. Any disagreement was resolved by discussion with the other researchers. The Newcastle–Ottawa Quality Assessment Scale (NOS) was used to assess the qualities of the included studies [Citation27]. NOS scores of ≥ 7 were considered high-quality studies. The following data were extracted from the included studies: first author’s name, publication year, study time, country of the population, sample size, detection time, patients’ age, follow-up period, CPCs detection method, CPCs cut-off value, staging system, high/standard risk chromosomal abnormalities (high-risk was defined as the presence of t(4,14), t(14,16), and/or del(17p); standard risk was defined as the absence of these genetic abnormalities), initial treatment, data type, and outcome data. Outcome data just included overall survival (OS) and progression-free survival (PFS). A cut-off value was used to define ‘elevated CPCs’.
Statistical analysis
HRs and 95% CIs were obtained directly from each piece of literature. If the univariate and multivariate survival analysis results were simultaneously presented, we would choose the latter. In the absence of reported HR, we estimated the data according to the methods previously proposed by Tierney et al. or Parmaret et al. [Citation28,Citation29]. The combined HRs and corresponding 95% CIs were used to evaluate the association between CPCs and prognosis parameters. A pooled HR > 1 without a 95% CI overlapping 1 indicated a significant association with poor outcomes.
Heterogeneity between studies was assessed by Cochran’s Q-test and I2 statistics. A random-effects model was used when high heterogeneity was determined based on I2 > 50% and Q-test p < 0.10; otherwise, the fixed-effect model was chosen. Subgroup analyses were carried out according to predefined variables that might affect the findings: region, sample size, cut-off value, detection time, initial treatment, and data type. Sensitivity analysis using the leave-one-out test was conducted to examine the robustness of the overall pooled results. Publication bias was evaluated by using Begg’s test. A p > 0.05 was defined as no obvious publication bias. All analyses were carried out using STATA statistical software package version 17.0 (STATA, College Station, TX, USA).
Results
Selection and characteristics of included studies
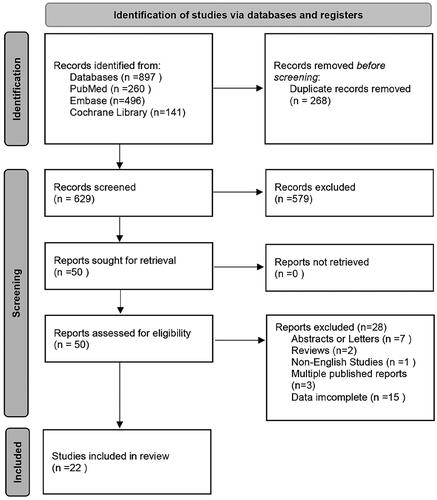
As shown in , a total of 897 studies were initially obtained. After the removal of duplicates (n = 268), 629 publications remained. After title and abstract screening, 50 publications were further reviewed by reading the full text. Additional 28 studies were then excluded because they were conference abstracts or letters (n = 7), reviews (n = 2), in other languages (n = 1), multiple published reports (n = 3), or did not report sufficient data (n = 15). Therefore, 22 studies between 1996 and 2023 with a total of 5637 myeloma patients were enrolled in our meta-analysis.
The characteristics of the 22 studies are shown in . The included studies were primarily conducted in America, Europe, and Asia. Blood samples were collected at baseline or after treatment (including before and after autologous stem cell transplantation and at the relapsed or refractory stage). The CPCs were detected by various methods, including conventional morphology (CM), immunofluorescence (IF), polymerase chain reaction (PCR), multi-parameter flow cytometry (MFC), and next-generation flow cytometry (NGF). The cut-off points for positive CPCs were heterogeneous. Multiple HRs were extracted in three studies for the following reasons: Korthal et al. [Citation11] and Chakraborty et al. [Citation14] detected CPCs at different sampling times; Jelinek et al. [Citation21] investigated CPCs in patients from transplant-ineligible and transplant-eligible groups. Overall, 24 HRs for OS were available in 21 studies. 17 HRs for PFS were available in 15 studies. The included studies were of high quality; twenty studies had a NOS score of 7 or higher.
Table 1. Characteristics of studies included in the meta-analysis.
Association between CPCs and OS of myeloma patients
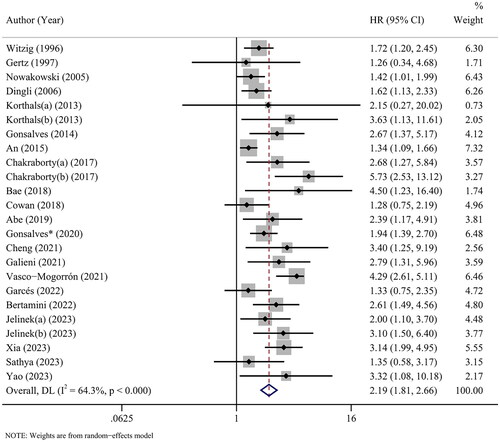
Twenty-four cohorts from 21 studies revealed the association between CPCs and OS in myeloma patients. With heterogeneity (I2 = 64.3%, p < 0.001), the pooled HR of 2.19 (95% CI: 1.81-2.66, p < 0.001) indicated that myeloma patients with elevated CPCs were expected to have a poor OS ().
Subgroup analyses were performed based on various factors. Overall, an increased CPCs level remained a significant marker for predicting OS in the subgroups of any region, sample size, cut-off value, detection time and initial treatment. Stratification by data type produced a pooled HR of 2.45 (95% CI: 1.90–3.17, p < 0.001) for studies using multivariate analysis, suggesting that CPCs was an independent prognostic factor of OS in myeloma patients. There was heterogeneity between the studies in most subgroups. The details are available in .
Table 2. Subgroup analysis of prognostic role of CPCs for OS in patients with MM.
Association between CPCs and PFS of myeloma patients
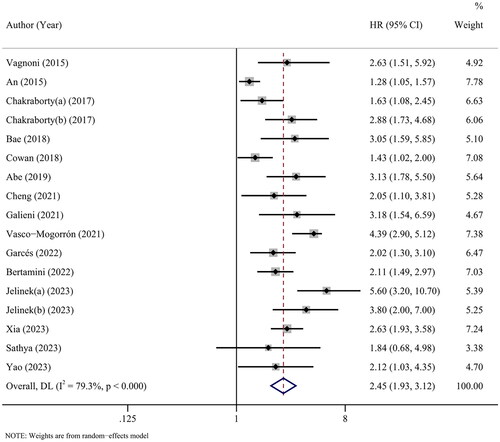
Furthermore, seventeen cohorts from 15 studies covered PFS. The results of the overall meta-analysis showed that there was a significant relationship between high CPCs and shorter PFS (HR = 2.45, 95% CI: 1.93–3.12, p < 0.001), with high heterogeneity (I2 = 79.3%, p < 0.001) ().
As shown in , the subgroup analyses did not alter the prognostic role of CPCs in PFS regardless of region, sample size, cut-off value, detection time or, initial treatment, with significant heterogeneity across studies in most subgroups. In addition, multivariate survival analysis was applied in 11 cohorts, and the combined results (HR = 2.37, 95% CI: 1.83–3.06, p < 0.001) further validated that CPCs was an independent prognostic factor of PFS in myeloma patients.
Table 3. Subgroup analysis of prognostic role of CPCs for PFS in patients with MM.
Association between CPCs and clinicopathological features
We next analysed the correlation between CPCs and clinicopathological factors. Fourteen cohorts included data on ISS and eight cohorts included R-ISS; the pooled estimates showed the elevated number of CPCs was significantly related to advanced stage (ISS III vs. ISS I-II: pooled OR = 2.89, 95% CI: 2.41–3.46, p < 0.001; R-ISS III vs. R-ISS I-II: pooled OR = 3.65, 95% CI: 2.43–5.50, p < 0.001) (). Moreover, five cohorts reported chromosomal abnormalities; a combined OR of 2.22 (95% CI: 1.60–3.08, p < 0.001) revealed a positive relationship between increased CPCs and high-risk cytogenetics ().
Table 4. Association between CPCs and clinicopathological features in patients with MM.
Sensitivity analyses
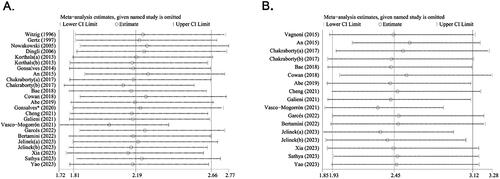
A single study involved in the meta-analysis was deleted each time to assess the robustness of the meta-analysis. The results showed that no single study influenced the pooled HRs and 95% CIs, indicating that the results of the meta-analysis were robust for both OS () and PFS ().
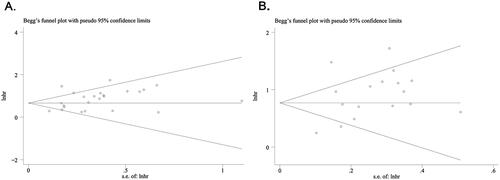
Publication bias
We used Begg’s test to analyse the publication bias. The funnel plot for OS () and PFS () was symmetric, and the result of Begg’s (OS: p = 0.107; PFS: p = 0.773) test indicated the absence of significant publication bias in the present study.
Discussion
An increasing number of studies have demonstrated the predictive potential of CPCs. In the present study, an updated systematic review and meta-analysis was conducted to confirm the correlation between CPCs and the prognosis of patients with MM. The meta-analytic results showed that the increased number of CPCs had a negative prognostic impact on PFS and OS. Significant heterogeneity was observed among the included studies; however, consistent results on sensitivity and subgroup analyses further support the robustness of our results. Additionally, CPCs positivity was significantly associated with advanced ISS/R-ISS stages and high-risk cytogenetics, indicating that higher numbers of CPCs may reflect more malignant clinical behaviours.
A prior meta-analysis has explored the effects of CPCs status on the outcomes of MM patients [Citation26]. However, the limited number of involved studies and the relatively small sample size for most cohorts may result in relatively insufficient statistical significance of the findings. Moreover, over ninety percent of the involved studies were conducted in Western countries; it remains unclear whether the conclusion can be extrapolated to Asian populations. In the present study, twice as many studies as in the previous meta-analysis were incorporated; the included studies had larger sample sizes and covered more Asian populations. In addition, extensive subgroup analyses were performed according to region, sample size, cut-off value, detection time, initial treatment and data type, lending more comprehensive and persuasive support to our conclusions.
Subgroup analysis showed that the elevated level of CPCs was highly predictive of the OS and PFS in MM patients, both at diagnosis and post-treatment. The overwhelming complexity and ambiguity of prognosis in MM require a comprehensive risk stratification at diagnosis. Recent studies have shown that the integration of CPCs could help with the accurate risk stratification of R-ISS [Citation36]. Galieni et al. revealed that the quantification of CPCs was able to better sub-stratify the large and heterogeneous group of R-ISS II [Citation18]. Gonsalves et al. showed that the presence of ≥5 CPCs/μL can potentially upstage the newly diagnosed MM patients with R-ISS I and II to a similar outcome as expected in R-ISS III disease [Citation24]. Yao et al. draw a similar conclusion, showing that patients with CPCs ≥0.165% in the R-ISS II stage had a comparable survival rate to those in the III stage [Citation25]. It’s worth noting that sequencing of CPCs was able to detect all reported genetic abnormalities, including t(4;14), t(14;16), 1q gain, del(13q), and del(17)p, demonstrating the viability of enumeration and genomic assessment of CPCs to replace BM biopsy for refining MM classification [Citation37]. In addition to the value of risk stratification at diagnosis, CPCs show potential advantages for real-time monitoring of the disease, as blood draws are minimally invasive, repeatable, and free of haemodilution. Thus, one of the most promising applications of CPCs is to replace the BM in the evaluation of measurable residual disease (MRD). Sanoja-Flores et al. have reported that among 137 newly diagnosed MM patients after active treatment, every CPCs MRD-positive case was BM MRD-positive [Citation38]. Tembhare et al. performed CPCs detection in MM patients treated without autologous transplant at the end of 3-cycles and 6-cycles of chemotherapy. They found that detectable CPCs MRD was strongly associated with event-free survival (EFS) and OS; early CPCs MRD clearance predicted better EFS and persistence of CPCs MRD predicted poor OS [Citation39]. Moreover, the presence of CPCs before or after autologous stem cell transplantation is also a predictor of inferior outcomes [Citation11,Citation14,Citation31]. In conclusion, future studies focusing on the evaluation of CPCs for myeloma patients at multiple time points are encouraged to demonstrate the value of CPCs for longitudinal monitoring.
To date, several techniques have been applied to detect CPCs. Conventional cytology was first used for the identification of CPCs in blood smears; it remains the basis for the diagnosis of PCL. However, the limited sensitivity of the technique indicates that patients with <10−2 CPCs in blood may not be recognized. Immunocytochemistry-based approaches were subsequently adopted based on restricted light chain expression by myeloma cells [Citation40]. Although the technique improved the detection sensitivity of CPCs to 10−4, many laboratories found it difficult to carry out regularly due to the tedious and time-consuming procedures [40]. In order to further increase the sensitivity and specificity of the above methods, molecular techniques, together with flow cytometry-based procedures, were subsequently developed. Molecular techniques do not require fresh samples and could detect the presence of CPCs with a sensitivity of 10−5-10−6. However, they have limited availability and applicability [Citation40]. Compared with the previous meta-analysis conducted in 2017, our study included more recent publications adopting flow cytometry-based techniques for CPCs evaluation. MFC is an easy, fast, and affordable approach with a sensitivity of 10−5-10−4. It is currently the most widely used technique for CPCs detection. Nevertheless, the reproducibility of results has been restricted by the widely varying sensitivity levels and the absence of standardized protocols [Citation40]. More recently, the EuroFlow-NGF approach with an extremely high sensitivity (≤10−6) has been applied for the CPCs quantification [Citation41]. This technique provides a standardized procedure and reproducible results, but it requires a larger blood sample and is more expensive. The various detection techniques implied disparities in the threshold values. Therefore, we conducted stratified analysis according to the range of CPCs thresholds, categorizing studies as ≥1%, 0.01%–1%, and ≤0.01%. The result showed a consistent prognostic value of CPCs across subgroups, indicating that CPCs positivity was highly related to worse outcomes regardless of the cut-off levels. This finding highlights the significance of CPCs quantification in medical management. Overall, it is still challenging to define a consensus cut-off value for CPCs that confer poor prognosis in MM patients. It is suggested that a medical center can adopt an appropriate cut-off value according to the EuroFlow-NGF studies or establish its own standardized detection procedure and CPCs-positive definition for clinical management.
Our meta-analysis reviewed publications from 1996 to 2023. During this time period, traditional chemotherapy was replaced by novel agents, including proteasome inhibitors and immunomodulatory. Considering the potential impact of treatment change on study outcomes, we conducted a stratified analysis based on initial treatment. The results suggested that the elevated number of CPCs was a stable predictor for different treatment contexts. Unfortunately, none of the studies included in our analysis treated patients with immunotherapies; thus, it is currently unclear whether CPCs can serve as an excellent prognostic indicator in the era of novel immunotherapies. It’s worth noting that the administration of antibody drugs, such as daratumumab, may prevent CPCs detection by flow cytometry [Citation42]. Therefore, an alternative biomarker, such as CLIMP-63 on the rough endoplasmic reticulum, is recommended for CPCs detection in patients treated with such regimes [Citation42].
In addition to showing prognostic and monitoring value in MM, CPCs have the potential to aid in the identification of smoldering multiple myeloma (SMM) patients who are at risk of progression. A Mayo study demonstrated that the presence of CPCs (≥150/150000 cells) in patients with SMM predicted a higher 2-year probability of progression to MM compared to those without detectable CPCs (58% vs. 9%) [Citation43]. An iMMunocell study revealed that CPCs >0.015% was associated with a 4.3-fold increment in the risk of progression [Citation44]. Of further importance, this study suggested that the CPCs-based 20/2/0.015 model (sFLC ratio > 20, M-protein > 2g/dL, CPCs > 0.015%) outperformed the BM-based 20/2/20(sFLC ratio > 20, M-protein > 2g/dL, BM plasma cell > 20%) IMWG model [Citation44]. These findings suggested CPCs detection may replace BM biopsy in the serial assessment of SMM in the future.
Some limitations existed in our meta-analysis. Firstly, heterogeneity was still noticeable among the included studies, even though we performed subgroup analyses. The persistent heterogeneity could be attributed to clinical variables such as patient characteristics, different detection methods and thresholds, and changes in anti-MM treatment. In addition, some HRs and 95% CIs were calculated from Kaplan–Meier survival curves, which could be somewhat at odds with reality. Furthermore, since the majority of the included studies had positive findings, our meta-analysis may overestimate the predictive value of CPCs to some degree.
Conclusion
Our meta-analysis revealed that the increased number of CPCs was significantly associated with poor OS and PFS in patients with MM. Therefore, CPCs could be a valuable prognostic factor that helps with risk stratification and disease monitoring. More rigorously designed studies with a standard flow cytometry methodology and a consensus cut-off should be pursued to gain a thorough understanding of the role of CPCs in plasma cell disease in the future.
Author contributions
CS, QL and AL conceived and designed the study; QL and AL conducted the literature search, data extraction and analysis; LZ, JL prepared figures and tables; FZ, and AX advised on data collection and methodology; QL and AL wrote the manuscript; BZ and LC performed the language editing; YH and CS reviewed the manuscript and revised it. All authors read and approved the final manuscript.
Supplemental Material
Download MS Word (12.8 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Pulte D, Jansen L, Brenner H. Changes in long term survival after diagnosis with common hematologic malignancies in the early 21st century. Blood Cancer J. 2020;10(5):56. doi: 10.1038/s41408-020-0323-4.
- Kumar SK, Dispenzieri A, Lacy MQ, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2014;28(5):1122–1128. doi: 10.1038/leu.2013.313.
- Greipp PR, San Miguel J, Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23(15):3412–3420. doi: 10.1200/jco.2005.04.242.
- Palumbo A, Avet-Loiseau H, Oliva S, et al. Revised international staging system for multiple myeloma: a report from international myeloma working group. J Clin Oncol. 2015;33(26):2863–2869. doi: 10.1200/jco.2015.61.2267.
- Paiva B, Paino T, Sayagues J-M, et al. Detailed characterization of multiple myeloma circulating tumor cells shows unique phenotypic, cytogenetic, functional, and circadian distribution profile. Blood. 2013;122(22):3591–3598. doi: 10.1182/blood-2013-06-510453.
- van de Donk N. How We manage newly diagnosed multiple myeloma with circulating tumor cells. J Clin Oncol. 2023;41(7):1342–1349. doi: 10.1200/jco.22.02114.
- Fernández de Larrea C, Kyle R, Rosiñol L, et al. Primary plasma cell leukemia: consensus definition by the international myeloma working group according to peripheral blood plasma cell percentage. Blood Cancer J. 2021;11(12):192. doi: 10.1038/s41408-021-00587-0.
- An G, Qin X, Acharya C, et al. Multiple myeloma patients with low proportion of circulating plasma cells had similar survival with primary plasma cell leukemia patients. Ann Hematol. 2015;94(2):257–264. doi: 10.1007/s00277-014-2211-0.
- Witzig TE, Gertz MA, Lust JA, et al. Peripheral blood monoclonal plasma cells as a predictor of survival in patients with multiple myeloma. Blood. 1996;88(5):1780–1787. doi: 10.1182/blood.V88.5.1780.1780.
- Nowakowski GS, Witzig TE, Dingli D, et al. Circulating plasma cells detected by flow cytometry as a predictor of survival in 302 patients with newly diagnosed multiple myeloma. Blood. 2005;106(7):2276–2279. doi: 10.1182/blood-2005-05-1858.
- Korthals M, Sehnke N, Kronenwett R, et al. Molecular monitoring of minimal residual disease in the peripheral blood of patients with multiple myeloma. Biol Blood Marrow Transplant. 2013;19(7):1109–1115. doi: 10.1016/j.bbmt.2013.04.025.
- Gonsalves WI, Rajkumar SV, Gupta V, et al. Quantification of clonal circulating plasma cells in newly diagnosed multiple myeloma: implications for redefining high-risk myeloma. Leukemia. 2014;28(10):2060–2065. doi: 10.1038/leu.2014.98.
- Vagnoni D, Travaglini F, Pezzoni V, et al. Circulating plasma cells in newly diagnosed symptomatic multiple myeloma as a possible prognostic marker for patients with standard-risk cytogenetics. Br J Haematol. 2015;170(4):523–531. doi: 10.1111/bjh.13484.
- Chakraborty R, Muchtar E, Kumar SK, et al. Serial measurements of circulating plasma cells before and after induction therapy have an independent prognostic impact in patients with multiple myeloma undergoing upfront autologous transplantation. Haematologica. 2017;102(8):1439–1445. doi: 10.3324/haematol.2017.166629.
- Abe Y, Narita K, Kobayashi H, et al. Pretreatment (18)F-FDG PET/CT combined with quantification of clonal circulating plasma cells as a potential risk model in patients with newly diagnosed multiple myeloma. Eur J Nucl Med Mol Imaging. 2019;46(6):1325–1333. doi: 10.1007/s00259-019-4275-5.
- Han W, Jin Y, Xu M, et al. Prognostic value of circulating clonal plasma cells in newly diagnosed multiple myeloma. Hematology. 2021;26(1):510–517. doi: 10.1080/16078454.2021.1948208.
- Cheng Q, Cai L, Zhang Y, et al. Circulating plasma cells as a biomarker to predict newly diagnosed multiple myeloma prognosis: developing nomogram prognostic models. Front Oncol. 2021;11:639528. doi: 10.3389/fonc.2021.639528.
- Galieni P, Travaglini F, Vagnoni D, et al. The detection of circulating plasma cells may improve the revised international staging system (R-ISS) risk stratification of patients with newly diagnosed multiple myeloma. Br J Haematol. 2021;193(3):542–550. doi: 10.1111/bjh.17118.
- Vasco-Mogorrón MA, Campillo JA, Periago A, et al. Blood-based risk stratification for pre-malignant and symptomatic plasma cell neoplasms to improve patient management. Am J Cancer Res. 2021;11:2736–2753.
- Bertamini L, Oliva S, Rota-Scalabrini D, et al. High levels of circulating tumor plasma cells as a key hallmark of aggressive disease in transplant-eligible patients with newly diagnosed multiple myeloma. J Clin Oncol. 2022;40(27):3120–3131. doi: 10.1200/jco.21.01393.
- Jelinek T, Bezdekova R, Zihala D, et al. More than 2% of circulating tumor plasma cells defines plasma cell Leukemia-Like multiple myeloma. J Clin Oncol. 2023;41(7):1383–1392. doi: 10.1200/jco.22.01226.
- Xia Y, Shen N, Zhang R, et al. High-risk multiple myeloma predicted by circulating plasma cells and its genetic characteristics. Front Oncol. 2023;13:1083053. doi: 10.3389/fonc.2023.1083053.
- Garcés JJ, Cedena MT, Puig N, et al. Circulating tumor cells for the staging of patients with newly diagnosed transplant-eligible multiple myeloma. J Clin Oncol. 2022;40(27):3151–3161. doi: 10.1200/jco.21.01365.
- Gonsalves WI, Jevremovic D, Nandakumar B, et al. Enhancing the R-ISS classification of newly diagnosed multiple myeloma by quantifying circulating clonal plasma cells. Am J Hematol. 2020;95(3):310–315. doi: 10.1002/ajh.25709.
- Yao W, Yang H, You H, et al. The prognostic significance of circulating plasma cells in newly diagnosed multiple myeloma patients. Front Oncol. 2023;13:1266868. doi: 10.3389/fonc.2023.1266868.
- Li J, Wang N, Tesfaluul N, et al. Prognostic value of circulating plasma cells in patients with multiple myeloma: a meta-analysis. PLoS One. 2017;12(7):e0181447. doi: 10.1371/journal.pone.0181447.
- Gláucia FC, Marcos RDS, Tatiani OF, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies 2013.
- Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8(1):16. doi: 10.1186/1745-6215-8-16.
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statist Med. 1998;17(24):2815–2834. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8.
- Gertz MA, Witzig TE, Pineda AA, et al. Monoclonal plasma cells in the blood stem cell harvest from patients with multiple myeloma are associated with shortened relapse-free survival after transplantation. Bone Marrow Transplant. 1997;19(4):337–342. doi: 10.1038/sj.bmt.1700670.
- Dingli D, Nowakowski GS, Dispenzieri A, et al. Flow cytometric detection of circulating myeloma cells before transplantation in patients with multiple myeloma: a simple risk stratification system. Blood. 2006;107(8):3384–3388. doi: 10.1182/blood-2005-08-3398.
- Gonsalves WI, Morice WG, Rajkumar V, et al. Quantification of clonal circulating plasma cells in relapsed multiple myeloma. Br J Haematol. 2014;167(4):500–505. doi: 10.1111/bjh.13067.
- Bae MH, Park CJ, Kim BH, et al. Increased circulating plasma cells detected by flow cytometry predicts poor prognosis in patients with plasma cell myeloma. Cytometry B Clin Cytom. 2018;94(3):493–499. doi: 10.1002/cyto.b.21606.
- Cowan AJ, Stevenson PA, Libby EN, et al. Circulating plasma cells at the time of collection of autologous PBSC for transplant in multiple myeloma patients is a negative prognostic factor even in the age of Post-Transplant maintenance therapy. Biol Blood Marrow Transplant. 2018;24(7):1386–1391. doi: 10.1016/j.bbmt.2018.02.017.
- Sathya P, Kayal S, Srinivas BH, et al. Quantification of circulating clonal plasma cells by multiparametric flow cytometry as a prognostic marker in patients with newly diagnosed multiple myeloma. Int J Lab Hematol. 2023;45(6):917–926. doi: 10.1111/ijlh.14156.
- Garcés JJ, San-Miguel J, Paiva B. Biological characterization and clinical relevance of circulating tumor cells: opening the pandora’s box of multiple myeloma. Cancers (Basel). 2022;14(6):1430. doi: 10.3390/cancers14061430.
- Dutta AK, Alberge JB, Lightbody ED, et al. MinimuMM-seq: genome sequencing of circulating tumor cells for minimally invasive molecular characterization of multiple myeloma pathology. Cancer Discov. 2023;13(2):348–363. doi: 10.1158/2159-8290.Cd-22-0482.
- Sanoja-Flores L, Flores-Montero J, Puig N, et al. Blood monitoring of circulating tumor plasma cells by next generation flow in multiple myeloma after therapy. Blood. 2019;134(24):2218–2222. doi: 10.1182/blood.2019002610.
- Tembhare PR, Sriram H, Khanka T, et al. Circulating clonal plasma cells at diagnosis and peripheral blood measurable residual disease assessment provide powerful prognostication biomarkers in newly-diagnosed multiple myeloma patients treated without autologous transplant. Blood. 2022;140(Supplement 1):1135–1136. doi: 10.1182/blood-2022-165715.
- Sanoja-Flores L, Flores-Montero J, Pérez-Andrés M, et al. Detection of circulating tumor plasma cells in monoclonal gammopathies: methods, pathogenic role, and clinical implications. Cancers (Basel). 2020;12(6):12. doi: 10.3390/cancers12061499.
- Sanoja-Flores L, Flores-Montero J, Garcés JJ, et al. Next generation flow for minimally-invasive blood characterization of MGUS and multiple myeloma at diagnosis based on circulating tumor plasma cells (CTPC). Blood Cancer J. 2018;8(12):117. doi: 10.1038/s41408-018-0153-9.
- Mizuta S, Kawata T, Kawabata H, et al. VS38 as a promising CD38 substitute antibody for flow cytometric detection of plasma cells in the daratumumab era. Int J Hematol. 2019;110(3):322–330. doi: 10.1007/s12185-019-02685-z.
- Gonsalves WI, Rajkumar SV, Dispenzieri A, et al. Quantification of circulating clonal plasma cells via multiparametric flow cytometry identifies patients with smoldering multiple myeloma at high risk of progression. Leukemia. 2017;31(1):130–135. doi: 10.1038/leu.2016.205.
- Termini R, Žihala D, Terpos E, et al. Circulating tumor and immune cells for minimally invasive risk stratification of smoldering multiple myeloma. Clin Cancer Res. 2022;28(21):4771–4781. doi: 10.1158/1078-0432.Ccr-22-1594.