Abstract
Background
Daytime sleepiness is an important health problem. However, the dimensionality of the Epworth Sleepiness Scale (ESS) in older adults remains unclear. This study aimed to determine the prevalence of ESS-defined excessive daytime sleepiness in older adults. Furthermore, the dimensionality of ESS and its respective correlates were also compared.
Materials and methods
This is a community-based survey in which community-dwelling older adults aged ≥ 65 years participated. Excessive daytime sleepiness was assessed using the ESS and was defined as an ESS score of > 10. Exploratory factor analysis was performed to identify the ESS factors. Multiple logistic regression analysis was used to examine the independent correlates of the ESS-defined and factor-specific correlates of excessive daytime sleepiness.
Results
In total, 3978 older adults participated in this study. The mean age was 76.6 ± 6.7 years, with 53.8% ≥ 75 years, and 57.1% were female. The prevalence of ESS-defined excessive daytime sleepiness was 16.0%. An exploratory factor analysis revealed two factors in the ESS, which were designated as ‘passive’ and ‘active’ according to the soporific levels of ESS items loaded in each factor. Multiple logistic regression showed that male, illiteracy, depression, disability, short sleep duration and no exposure to hypnotics were risk indicators for ESS-defined excessive daytime sleepiness. However, the correlates for passive and active factor-defined excessive daytime sleepiness differ in pattern, especially in variables related to education, exercise, mental health, and sleep.
Conclusions
The prevalence of ESS-defined excessive daytime sleepiness is high, and its correlates vary among older adults. This study also suggests a dual ESS structure in community-dwelling older adults.
KEY MESSAGES
Daytime sleepiness is prevalent in older adults.
The Epworth Sleepiness Scale (ESS) has dual constructs in older adults.
Correlates for excessive daytime sleepiness vary by constructs of the ESS.
Introduction
Daytime sleepiness (DS) is a common phenomenon in the general population, defined as ‘the inability to stay awake and alert during the major waking episodes of the day, resulting in unintended lapses into drowsiness or sleep’. This unintentional dozing off distinguishes DS from insomnia, where patients feel tired or fatigue. However, excessive daytime sleepiness (EDS) is a common and deleterious health problem. Certain intrinsic sleep disorders, such as narcolepsy and primary hypersomnia, have EDS as their core symptom. Other sleep disorders that affect nighttime sleep, such as sleep apnea, and periodic limb movement disorders, are also associated with EDS [Citation1]. EDS also co-occurs with various physical and mental morbidities, including diabetes, cardiovascular disease [Citation2], and depression [Citation1]. In older adults, due to age-related changes in sleep physiology and higher susceptibility to age-dependent physical morbidities and primary sleep disorders, the prevalence and impact of EDS are higher. Specifically, EDS is associated with a higher risk of falls, depression, cognitive decline, and cardiovascular disease in old adults [Citation3]. Therefore, understanding the prevalence and identifying correlates of EDS in older adults is imperative for public health, particularly in aging societies worldwide.
Notably, the link between primary sleep disorders and EDS has also been mitigated, including insomnia [Citation4–5], disordered breathing sleep [Citation6] and sleep-related movement disorders [Citation4,Citation7]. Specifically, a few studies have failed to illustrate the adverse impact of EDS on several adverse outcomes, including arrhythmia [Citation8], coronary heart disease [Citation9], dynapenia and frailty [Citation10]. Accordingly, the increasing prevalence of EDS in older adults may be a combined effect of EDS derived from normal aging and pathological EDS. Thus, disentangling EDS symptomatology and implementing risk stratification may assist in precision medicine in the older population.
The Epworth Sleepiness Scale (ESS) is currently the most convenient and widely used tool to assess excessive daytime sleepiness. Studies in the literature that have examined the factor structure of the ESS in the non-older population have commonly found a single factor, particularly in patient groups such as those with obstructive sleep apnoea, Parkinson’s disease, narcolepsy and idiopathic hypersomnia [Citation11–12]. In contrast, multiple constructs are more commonly recognized in healthier populations, such as the general adult population or students [Citation13–16]. One study explicitly demonstrated single and dual factors in patients with disordered breathing sleep and normal controls, respectively [Citation17]. These findings suggest that dozing in highly soporific circumstances, as depicted in the ESS, may be functional in essence among non-older healthy adults and is less toxic [Citation13]. In contrast, a component of increased DS prevalence in older adults is merely a behavioural consequence secondary to decreased social demand, lower physical activity, and increased sedentary behaviours [Citation18]. Accordingly, the question of whether the ESS continues to be a single-dimensional tool in relatively healthy older populations remains unanswered. Investigating the clinical implications of these individual constructs is crucial if ESS exhibits multi-dimensionality in relatively healthy older adults.
This study had two objectives. First, it aimed to investigate the prevalence of ESS-defined EDS in a relatively healthy population of community-dwelling older adults. Second, it aimed to investigate the dimensionality of ESS and identify and compare the correlates for each dimension.
Materials and methods
Study design and participants
This community-based, cross-sectional study was part of the umbrella cohort of the Yilan study. Details of the Yilan study have been reported elsewhere [Citation19]. In brief, the cohort of the Yilan study included adults aged ≥ 65 years living in Yilan City, Taiwan. Face-to-face interviews were conducted by trained interviewers from January 2012 to November 2016. The collected data included sociodemographic characteristics, anthropometric measurements, lifestyle factors, comorbidities, and sleep-related factors. Interviews also recorded the participant’s medical history, level of anxiety or depression, degree of disability, and sleep-related disturbances. This study was reviewed and approved by the Institutional Review Boards of the National Taiwan University (IRB No. 202101095RINA) and the National Yang Ming Chiao Tung University Hospital (IRB No. 2011A016). All the participants provided written informed consent.
Evaluation of excessive daytime sleepiness
This study used the Chinese version of the ESS to assess EDS. Participants were asked to respond to, according to their own experience, the likelihood of falling asleep in the past month in the following situations: item (1) Sitting and reading; item (2) Watching TV; item (3) Sitting quietly in a public place; item (4) As a passenger in a car for an hour without a break; item (5) Lying down to rest in the afternoon when circumstances permit; item (6) Sitting and talking to someone; item (7) Sitting quietly after a lunch without alcohol; item (8) In a car, while stopped for a few minutes in the traffic. The frequency was divided into ‘would never doze,’ ‘slight chance of dozing,’ ‘moderate chance of dozing’ and ‘high chance of dozing,’ which correspond to 0 to 3 points, respectively, with a total score of 0 to 24 points. A score > 10 can efficiently screen out patients with obstructive sleep apnoea syndrome, narcolepsy and idiopathic hypersomnia [Citation20]. The Chinese version of the ESS has been validated [Citation21]. In addition, the validity of ESS has been confirmed in older adults [Citation22]. In this study, ESS > 10 was defined as the cut-off for ESS-defined EDS.
Other variables
Body mass index and sociodemographic characteristics
The height and weight of the participants were measured on-site, and their body mass index was classified as underweight (<18.5 kg/m2), normal (18.5–23.9 kg/m2), or overweight (>23.9 kg/m2) [Citation23]. Participants who were unable to measure their height or weight for physical reasons were classified as ‘unmeasured’. The participants’ educational level was categorized as ‘literate’ or ‘illiterate’. ‘Literate’ indicated participants who had ever attended schools or could read Mandarin; ‘illiterate’ indicated those who did not attend schools and could not read Mandarin. The living situations were divided into ‘living with others’ and ‘living alone’.
Lifestyle
Exercise habits were coded positive if they were > 10 min per session and more than three times per week. Smoking and drinking habits will be divided into ‘never used’, ‘quit’ or ‘current users’.
Medical history
Physical morbidities including diabetes, hypertension, cardiac disease, hyperlipidaemia, stroke, and snoring were defined as self-reported by the participants and receiving corresponding treatment [Citation22].
Evaluation of anxiety and depression
Older adults are more likely to have physical illnesses, which may mimic the somatic domain of depressive and anxiety symptomatology and lead to overestimation. The Hospital Anxiety and Depression Scale (HADS) was used to assess participants’ symptoms of anxiety and depression. Additionally, the HADS has been widely used to assess clinical and subclinical depression and anxiety symptoms in the general population, with verified validity. The validity of the Chinese version of the HADS was also verified [Citation24]. In older Chinese adults, the clinically significant cut-off points for depression and anxiety are ≥ 6 and ≥ 3 points, respectively [Citation24].
Evaluation of functional disability
The Groningen Activity Restriction Scale (GARS) was used to evaluate functional ability. The GARS comprises 18 daily activities, including basic Activities of Daily Living (ADL) and Instrumental ADL (IADL) [Citation25]. Responses for each item ranged from 1 to 4. Because the participants in this study were relatively healthy, we defined ‘disability’ as a score of ≥ 19, suggesting difficulty in any item.
Sleep-related variables
In addition to DS, other sleep-related variables were collected, including sleep duration, bedtime, insomnia, and hypnotic use. Self-reported sleep duration and bedtime were assessed. According to previous evidence in Taiwan, a self-reported sleep duration of ≥8 h in older adults was suggested as the optimal cutoff for detecting adverse health outcomes, such as elevated risk for mortality [Citation26–27], poor cardiac autonomic control [Citation28], hand grip strength [Citation29], depression [Citation30], and sarcopenia [Citation31]. Meanwhile, a sleep duration of ≤5 h is a universally accepted cutoff in older populations [Citation32]; thus, the self-reported sleep duration was categorized into short (≤5 h), mid-range (6–7 h), and long (≥8 h). Bedtime in military hours was categorized into tertiles with earlier (<21:30) and late (> 22:30) bedtimes. A five-item version of the Athens Insomnia Scale (AIS-5) was used to assess insomnia. The Chinese version of AIS-5 has also been shown to have good validity [Citation33]. Each item is scored from 0 to 3, and a score of ≥ 5 is defined as clinically significant insomnia. Hypnotic use was defined as exposure to sedatives or sleeping pills in the past month.
Statistical analysis
Data were analysed using IBM SPSS Statistics version 20. Univariate analyses were performed using chi-square and analysis of variance. Exploratory factor analysis (EFA) was conducted to determine the latent dimensions of ESS. The Kaiser–Meyer–Olkin measure of sampling adequacy (KMO) and Bartlett’s test of sphericity were used to ensure the suitability of the factor analysis. Principal component analysis and varimax rotation were used for factor extraction. Factor numbers were determined using eigenvalues greater than 1, and items were loaded onto a specific factor if their factor loadings were above 0.3. Regression analysis was used to estimate the factor scores, and the top 15 percentile of factor scores for each factor was defined as the cutoff for factor-specific DS because this cutoff is akin to the prevalence rate of ESS-defined DS in this study. Logistic regression analyses were used to examine the correlation between ESS-defined and factor-specific DS scores. Statistical significance was set at p < 0.05.
Results
shows the sociodemographic, lifestyle, and clinical characteristics of the participants. In total, 3978 older adults participated in this study. The mean age was 76.6 ± 6.7 years, with 53.8% ≥ 75 years, and 57.1% were female. The prevalence of ESS-defined DS was 16.0% in the entire cohort, 17.3% in males, and 15.0% in females. Supplementary Table 1 provides the number and proportion of individuals with EDS, measured by the Epworth Sleepiness Scale, across various variables in univariate analyses.
Table 1. Sociodemographic and clinical characteristics of participants (n = 3978).
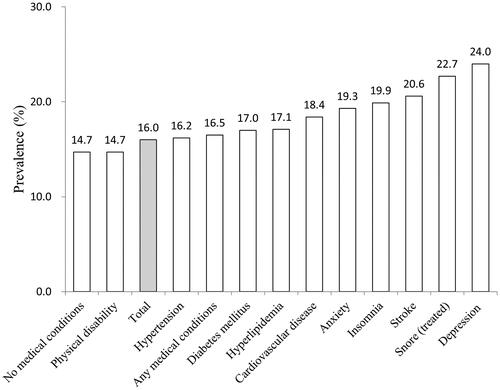
illustrates the prevalence of ESS-defined EDS according to the various physical and mental health conditions. The prevalence of EDS was higher in those with any medical condition (16.5%) than in those without (14.7%). Among all physical and mental health conditions, the prevalence of EDS ranges from 16.2% to 24.0%, with the highest being depression (24.0%). Among all physical morbidities, the highest prevalence of EDS was found in treated snore (22.7%), followed by stroke (20.6%).
In terms of the dimensionality of the ESS, KMO (0.86) and Bartlett’s test of sphericity (df = 22367.93, p < 0.001) justified the use of EFA. Eventually, EFA identified two factors (Supplementary Table 2) that together explain 74.9% of the variance. Factor 1, designated as the passive factor, includes ‘Watching TV,’ ‘Sitting, inactive in a public place’, ‘As a passenger in a car for an hour without a break’, and ‘Sitting quietly after a lunch without alcohol’ (factor loading: 0.87–0.90), while Factor 2, designated as the active factor, includes ‘Sitting and reading’, ‘Lying down to rest in the afternoon when circumstances permit’, ‘Sitting and talking to someone’, and ‘In a car, while stopped for a few minutes in the traffic’ (factor loading: 0.54–0.91). According to the prevalence of ESS-defined EDS reported in this study, the highest 15.0 percentile was used as the cutoff to define factor-specific EDS, including Passive Factor-defined and Active Factor-defined EDS.
Supplementary Table 3 summarizes the crude effect size of correlates for the ESS-defined EDS. Among sociodemographic and lifestyle factors, overweight (OR: 1.22, 95% CI: 1.01–1.46), unmeasured BMI (OR: 1.44, 95% CI:1.01-2.05), illiterate (OR: 1.43, 95% CI: 1.17–1.73), ex-drinker (OR: 1.72, 95% CI: 1.24–2.39), and disability (1.77, 95% CI: 1.40–2.25) correlated with EDS. In medical morbidities, most diseases are not significantly associated with EDS, except for heart disease (OR: 1.29, 95% CI: 1.08–1.54) and stroke (OR: 1.40, 95% CI: 1.00–1.95). Regarding mental morbidities, depression (OR: 1.83, 95% CI: 1.47–2.28) and anxiety (OR: 1.46, 95% CI: 1.23–1.74) correlate with EDS. Most of the sleep-related variables are associated with EDS, including insomnia (OR: 1.42, 95% CI: 1.17–1.72), long sleep duration (≥ 8 h) (OR: 1.28, 95% CI: 1.03–1.59) or short sleep duration (≤ 5 hr) (OR: 1.37, 95% CI: 1.12–1.67). Notably, those who took hypnotics are less likely to have EDS (OR: 0.51, 95% CI: 0.39–0.68).
Supplementary Table 3 also compared correlates for the ESS-defined, Passive Factor-defined, and Active Factor-defined EDS in univariate analyses. Overall, the correlations of Passive Factor-defined EDS were similar to those of the original ESS-defined EDS, while the correlations of Active Factor-defined EDS differed. Socioeconomic factors, lifestyle, and medical morbidities (excluding stroke) were not significantly related to Active Factor-defined EDS. Notably, a few variables that were not correlated with ESS-defined EDS, correlated with factor-specific EDS. For example, older adults who exercise less frequently or have diabetes are more likely to have Passive Factor-defined EDS, while those with earlier bedtimes are associated with a lower risk for Active Factor-defined EDS.
summarizes the independent relationships between various variables and different EDS definitions. Disability and hypnotic use consistently correlated with three different definitions of EDS. Notably, taking hypnotic use was less likely to correlate with EDS across the three different definitions. Male, illiterate, depression, disability, and short sleep duration were independently associated with a higher risk of ESS-defined EDS. All these correlates of ESS-defined EDS also correlated with a higher risk of Passive Factor-defined EDS, except for depression. Additionally, hypertension, hyperlipidaemia, and anxiety were specifically related to Passive Factor-defined EDS. On the other hand, the patterns of correlates for Active Factor-defined EDS were quite different. Depression, disability, and long sleep duration were associated with a higher risk of Active Factor-defined EDS, while older adults with low education, low physical activity, and earlier bedtime had a lower likelihood of having Active Factor-defined EDS.
Table 2. Multiple logistic regression analyses for factors associated with daytime sleepiness.
Discussion
This study used a large sample to examine the prevalence of EDS and its correlates. As a result, the prevalence of ESS-defined EDS in community-dwelling older adults is high, and the associates are diverse, including sociodemographic characteristics, physical and mental comorbidities, disability, and sleep-related variables. This study also identified the dual dimensions of ESS and demonstrated factor-specific correlates. The candidate variables used in this study are comprehensively compared with those in the literature. Additionally, this study used a standardized assessment tool to evaluate EDS, rendering it comparable to previous studies.
Prevalence of the ESS-defined EDS
In the present study, the prevalence of ESS-defined EDS was 16.0%. The prevalence rates of various definitions of EDS in older adults vary greatly, ranging from 7.0% to 34.0% [Citation34–47]. In general, studies that used non-standardized, single global questions, such as ‘feel sleepy in the daytime’ tend to have higher prevalence rates, ranging from 13.6% to 25.2% [Citation34–36,Citation47]. In contrast, studies that used ESS tended to yield relatively low prevalence rates. Specifically, if the cutoffs for EDS are ≥ 10, the prevalence of EDS in older adults ranges from 14.9% to 23.8% [Citation37,Citation40–42]. In contrast, if the cut-off values were > 10, the prevalence rate ranged from 7.0% to 21.0% [Citation38,Citation39,Citation43–46]. Conceivably, using a more standardized instrument and stringent definition of EDS may provide a more precise estimation of the prevalence rate; however, the range of prevalence rates remains wide even when using similar tools and cutoffs, such as ESS >10.
In addition to the different definitions of EDS, the participants’ health status is a key factor that affects the prevalence rates. The present study found that prevalence rates varied significantly depending on mental and physical morbidities. This finding corroborates with the literature [Citation48]. In addition, the cognitive function [Citation49] and physical mobility [Citation38] determine the appearance of EDS. The participants in this study were community-dwelling older adults. Regardless of having various chronic morbidities, they should have a certain level of mobility and cognitive function to participate in and complete the Yilan study questionnaire. Accordingly, we believe that the participants of this study should be healthier than the demented or institutionalized individuals. Thus, the characteristics of participants may also partially explain the medium level of the prevalence rate in this study when compared with the literature with the same cutoffs (7.0–21.0%).
Factor structures of the ESS in older adults
To the best of our knowledge, this is the first study to examine the dimensionality of ESS in older adults. According to the literature on the non-older population, the number of embedded factors in the ESS seems to depend on the health status of the participants [Citation11–17]. Considering the participant characteristics of this study, it is expected that a dual-factor structure was identified.
In this study, according to the items’ general features, we imitated and designated two factors as ‘passive’ and ‘active’ [Citation13]. Compared with studies that reported a dual structure in non-older populations, the pattern of item loading slightly differed from that in our study. Akin to our finding, item 6 (sitting and talking to someone) and item 8 (in a car, while stopped for a few minutes in the traffic) used to load in one factor [Citation13–16], since they are regarded as the least soporific circumstances. Interestingly, Item 5 (lying down to rest in the afternoon when circumstances permit), which is considered to have the highest somnificity [Citation20], is loaded together with Items 6 and 8 in this study. The underlying mechanism of this unexpected finding is unclear. However, this finding, at least partially, disagrees with the argument that dual factors of the ESS are just reflecting ‘difficulty factor (level of severity)’, not genuinely different constructs [Citation11].
Correlates for original ESS-defined EDS
In this study, male, low education level, depression, disability, short sleep duration, and “not” using hypnotics were independent risk indicators for ESS-defined EDS, which is in line with the literature [Citation7,Citation50,Citation51]. Notably, this study failed to link insomnia and treated snoring to ESS-defined EDS. It is conceivable that snoring in older adults did not manifest as significant DS because they had been treated. Moreover, the daytime impact of disordered breathing sleep [Citation52] and insomnia is alleviated in older adults [Citation53]. In terms of hypnotic use, similar findings have been reported in older females [Citation50]. Hypnotics use may be a proxy of severe insomnia that manifests ‘hyperarousal,’ the core physiopathology of insomnia, and leads to less propensity of DS [Citation54].
Factor-specific correlates for EDS
This study uniquely revealed some ‘masked’ correlates that were disclosed only in factor-specific analyses of EDS. This finding supports the argument that the phenotype of the ESS-defined EDS may be heterogeneous. In general, the independent correlates of Passive Factor-defined EDS were similar to those of ESS-defined EDS. Notably, hypertension, hyperlipidaemia and anxiety were found to be Passive Factor-specific correlates. Older adults with hypertension and hyperlipidaemia may have sedentary lifestyles [Citation3,Citation55]. As a result, they are more likely to be exposed to highly soporific circumstances and doze off. Additionally, an anxious mood may exhaust individuals in the must-be awake period and make them intentionally rest in high somnificity situations.
By contrast, the risk indicators for Active Factor-defined EDS are intriguing and surprising. Higher education, more exercise, longer sleep duration, and earlier bedtime indicate DS in the context of active circumstances. This finding may be partially explained by the capacity to satisfy social demands. Older adults who are literate and exercise frequently are more likely to remain active in social activities, which may necessitate talking with others or driving longer distances. A previous study also showed that current drivers in old adults had few physical limitations and tended to be married [Citation56]. Thus, they may have a greater opportunity to doze off in situations with low somnificity. By contrast, less socially active older adults, such as those illiterate or those who exercise less frequently, may have limited experience in talking with others or driving after retirement. They could only imagine the likelihood of falling asleep when talking to others or driving a car. Thus, they responded to items of Active Factor under social desirability rather than true experience. However, long sleep duration and earlier bedtime seem to implicate an additional latent mechanism underlying Active Factor. Long sleep duration and advanced sleep phase [Citation57] have been found to indicate subclinical health status deterioration. Thus, these two correlates may be an epiphenomenon of excessive aging in the central nervous system, and a higher risk of dozing in an Active Factor-related context is a consequence of failure to maintain wakefulness. In summary, although the implications of Active Factor remain unclear, it seems to be related to non-clinical conditions and significantly differs from Passive Factor.
Limitation
This study has a few limitations. First, this was a cross-sectional study in which causal relationships could not be inferred. Second, because Yilan City is an agricultural suburban area in Taiwan, our results cannot be generalized to older adults who are institutionalized or reside in a metropolitan area. Finally, this study did not control for the confounding effects of intrinsic sleep disorders. However, many primary sleep disorders, such as sleep-related breathing disorders, restless leg syndrome and periodic limb movement disorders, have been found to have a mitigated impact on EDS in older adults [Citation22]. Accordingly, our findings would be biased to a lesser extent by endogenous sleep disorders.
Implications
The findings in this study suggest that aggregating of item scores related to the active and passive factors of ESS respectively may enhance the precision and clinical relevance of EDS assessment in older population. Our findings also indicate that the contradictory findings regarding the adverse impact of EDS in the literature may attribute to the multidimensional structures of EDS that show differential clinical significance of in older adults. However, follow-ups and randomized controlled trials that use mortality or incident diseases as outcome variables are necessary to confirm the clinical validity of specific EDS circumstances in older adults.
Conclusions
In addition to sleep disturbance, a high prevalence of EDS in this study also raises attention regarding the difficulty to maintain wakefulness in community-dwelling older adults. Non-unitary constructs of ESS in older adults indicate heterogenous phenotypes of EDS that may have different underlying etiologies, clinical significance, and interventions. If a sensitive and effective instrument that can recognize EDS with clinical implications is developed, we would have opportunities to implement precision public health in older populations.
Author’s contribution
CJH and HCC prepared the first draft. HCC, and NWH designed the research. HCC performed statistical analysis. All authors participated in drafting and revision of the manuscript. All authors have read and approved the manuscript.
Supplemental Material
Download MS Word (47.1 KB)Acknowledgements
The authors would also like to thank Dr. Pesus Chou for her help with the study design and Yang-Ming Crusaders, Mr. Da-Wei Lin, Ms. Yu-Hui Lin, Mr. Chia-Hsiang Lin, and Ms. Tzu-Chun Lo for their help with the data collection.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets generated during and analysed during the current study are not publicly available due to the risk of compromising participant confidentiality but are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Pérez-Carbonell L, Mignot E, Leschziner G, et al. Understanding and approaching excessive daytime sleepiness. Lancet. 2022;400(10357):1–11. doi: 10.1016/S0140-6736(22)01018-2.
- Asplund R. Daytime sleepiness and napping amongst the elderly in relation to somatic health and medical treatment. J Intern Med. 1996;239(3):261–267. doi: 10.1046/j.1365-2796.1996.453806000.x.
- Basta M, Lin HM, Pejovic S, et al. Lack of regular exercise, depression, and degree of apnea are predictors of excessive daytime sleepiness in patients with sleep apnea: sex differences. J Clin Sleep Med. 2008;4(1):19–25. doi: 10.5664/jcsm.27074.
- Spira AP, Beaudreau SA, Stone KL, et al. Reliability and validity of the pittsburgh sleep quality index and the epworth sleepiness scale in older men. J Gerontol A Biol Sci Med Sci. 2012;67(4):433–439. doi: 10.1093/gerona/glr172.
- Thorarinsdottir EH, Bjornsdottir E, Benediktsdottir B, et al. Definition of excessive daytime sleepiness in the general population: feeling sleepy relates better to sleep-related symptoms and quality of life than the epworth sleepiness scale score. Results from an Epidemiological Study. J Sleep Res. 2019;28(6):e12852.
- Iannella G, Vicini C, Colizza A, et al. Aging effect on sleepiness and apneas severity in patients with obstructive sleep apnea syndrome: a meta-analysis study. Eur Arch Otorhinolaryngol. 2019;276(12):3549–3556. doi: 10.1007/s00405-019-05616-0.
- Pack AI, Dinges DF, Gehrman PR, et al. Risk factors for excessive sleepiness in older adults. Ann Neurol. 2006;59(6):893–904. doi: 10.1002/ana.20863.
- Full KM, Lutsey PL, Norby FL, et al. Association between excessive daytime sleepiness and measures of supraventricular arrhythmia burden: evidence from the atherosclerosis risk in communities (ARIC) study. Sleep Breath. 2020;24(3):1223–1227. doi: 10.1007/s11325-020-02046-9.
- Blachier M, Dauvilliers Y, Jaussent I, et al. Excessive daytime sleepiness and vascular events: the three city study. Ann Neurol. 2012;71(5):661–667. doi: 10.1002/ana.22656.
- Soysal P, Smith L, Tan SG, et al. Excessive daytime sleepiness is associated with an increased frequency of falls and sarcopenia. Exp Gerontol. 2021;150:111364. doi: 10.1016/j.exger.2021.111364.
- Hagell P, Broman JE. Measurement properties and hierarchical item structure of the epworth sleepiness scale in parkinson’s disease. J Sleep Res. 2007;16(1):102–109. doi: 10.1111/j.1365-2869.2007.00570.x.
- Takegami M, Suzukamo Y, Wakita T, et al. Development of a japanese version of the epworth sleepiness scale (JESS) based on item response theory. Sleep Med. 2009;10(5):556–565. doi: 10.1016/j.sleep.2008.04.015.
- Kim H, Young T. Subjective daytime sleepiness: dimensions and correlates in the general population. Sleep. 2005;28(5):625–634. doi: 10.1093/sleep/28.5.625.
- Baumgartel KL, Terhorst L, Conley YP, et al. Psychometric evaluation of the epworth sleepiness scale in an obstetric population. Sleep Med. 2013;14(1):116–121. doi: 10.1016/j.sleep.2012.10.007.
- Gelaye B, Lohsoonthorn V, Lertmeharit S, et al. Construct validity and factor structure of the pittsburgh sleep quality index and epworth sleepiness scale in a multi-national study of african, South East asian and South American college students. PLoS One. 2014;9(12):e116383-e116383. doi: 10.1371/journal.pone.0116383.
- Pilcher JJ, Switzer FS, 3rd, Munc A, et al. Psychometric properties of the epworth sleepiness scale: a factor analysis and item-response theory approach. Chronobiol Int. 2018;35(4):533–545. doi: 10.1080/07420528.2017.1420075.
- Zhang JN, Peng B, Zhao TT, et al. Modification of the epworth sleepiness scale in Central China. Qual Life Res. 2011;20(10):1721–1726. doi: 10.1007/s11136-011-9898-3.
- Hoyos CM, Gordon C, Terpening Z, et al. Circadian rhythm and sleep alterations in older people with lifetime depression: a case-control study. BMC Psychiatry. 2020;20(1):192. doi: 10.1186/s12888-020-02606-z.
- Hsu NW, Tsao HM, Chen HC, et al. Anxiety and depression mediate the health-related quality of life differently in patients with cardiovascular disease and stroke-preliminary report of the yilan study: a population-based community health survey. PLoS One. 2014;9(9):e107609. doi: 10.1371/journal.pone.0107609.
- Johns MW. A new method for measuring daytime sleepiness: the epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540.
- Chen NH, Johns MW, Li HY, et al. Validation of a chinese version of the epworth sleepiness scale. Qual Life Res. 2002;11(8):817–821. doi: 10.1023/a:1020818417949.
- Beaudreau SA, Spira AP, Stewart A, et al. Validation of the pittsburgh sleep quality index and the epworth sleepiness scale in older black and white women. Sleep Med. 2012;13(1):36–42. doi: 10.1016/j.sleep.2011.04.005.
- Taiwan Ministry of Health and Welfare. Standards of Healthy Body Weight for Adult. 2018. Available from: https://www.hpa.gov.tw/Pages/Detail.aspx?nodeid=542&pid=9737.
- Leung CM, Ho S, Kan CS, et al. Evaluation of the chinese version of the hospital anxiety and depression scale. A cross-cultural perspective. Int J Psychosom. 1993;40(1-4):29–34.
- Suurmeijer TP, Doeglas DM, Moum T, et al. The Groningen activity restriction scale for measuring disability: its utility in international comparisons. Am J Public Health. 1994;84(8):1270–1273. doi: 10.2105/ajph.84.8.1270.
- Lan TY, Lan TH, Wen CP, et al. Nighttime sleep, chinese afternoon nap, and mortality in the elderly. Sleep. 2007;30(9):1105–1110. doi: 10.1093/sleep/30.9.1105.
- Chen HC, Su TP, Chou P. A nine-year follow-up study of sleep patterns and mortality in community-dwelling older adults in Taiwan. Sleep. 2013;36(8):1187–1198. doi: 10.5665/sleep.2884.
- Chen HC, Hsu NW, Chou P. The association between extreme sleep duration and cardiac autonomic control in community-dwelling older adults: the yilan study, Taiwan. J Gerontol A Biol Sci Med Sci. 2017;72(7):929–936. doi: 10.1093/gerona/glx045.
- Chen HC, Hsu NW, Chou P. The association between sleep duration and hand grip strength in community-dwelling older adults: the yilan study, Taiwan. Sleep. 2017;40(4):ZSX021. doi: 10.1093/sleep/zsx021.
- Lai HC, Hsu NW, Chou P, et al. The associations between various sleep-wake disturbances and depression in community-dwelling older adults- the yilan study, Taiwan. Aging Ment Health. 2020;24(5):717–724. doi: 10.1080/13607863.2019.1582006.
- Chien MY, Wang LY, Chen HC. The relationship of sleep duration with obesity and sarcopenia in community-dwelling older adults. Gerontology. 2015;61(5):399–406. doi: 10.1159/000371847.
- Watson NF, Badr MS, Belenky G, et al. Joint consensus statement of the American academy of sleep medicine and sleep research society on the recommended amount of sleep for a healthy adult: methodology and discussion. Sleep. 2015;38(8):1161–1183. doi: 10.5665/sleep.4886.
- Sun JL, Chiou JF, Lin CC. Validation of the taiwanese version of the athens insomnia scale and assessment of insomnia in taiwanese cancer patients. J Pain Symptom Manage. 2011;41(5):904–914. doi: 10.1016/j.jpainsymman.2010.07.021.
- Hays JC, Blazer DG, Foley DJ. Risk of napping: excessive daytime sleepiness and mortality in an older community population. J Am Geriatr Soc. 1996;44(6):693–698. doi: 10.1111/j.1532-5415.1996.tb01834.x.
- Newman AB, Spiekerman CF, Enright P, et al. Daytime sleepiness predicts mortality and cardiovascular disease in older adults. The cardiovascular health study research group. J Am Geriatr Soc. 2000;48(2):115–123. doi: 10.1111/j.1532-5415.2000.tb03901.x.
- Ohayon MM, Vecchierini MF. Daytime sleepiness and cognitive impairment in the elderly population. Arch Intern Med. 2002;162(2):201–208. doi: 10.1001/archinte.162.2.201.
- Goldstein IB, Ancoli-Israel S, Shapiro D. Relationship between daytime sleepiness and blood pressure in healthy older adults. Am J Hypertens. 2004;17(9):787–792. doi: 10.1016/j.amjhyper.2004.05.009.
- Tsuno N, Jaussent I, Dauvilliers Y, et al. Determinants of excessive daytime sleepiness in a french community-dwelling elderly population. J Sleep Res. 2007;16(4):364–371. doi: 10.1111/j.1365-2869.2007.00606.x.
- Empana JP, Dauvilliers Y, Dartigues JF, et al. Excessive daytime sleepiness is an independent risk indicator for cardiovascular mortality in community-dwelling elderly: the three city study. Stroke. 2009;40(4):1219–1224. doi: 10.1161/STROKEAHA.108.530824.
- Vaz Fragoso CA, Gahbauer EA, Van Ness PH, et al. Sleep-wake disturbances and frailty in community-living older persons. J Am Geriatr Soc. 2009;57(11):2094–2100. doi: 10.1111/j.1532-5415.2009.02522.x.
- Vaz Fragoso CA, Miller ME, Fielding RA, et al. Sleep-wake disturbances in sedentary community-dwelling elderly adults with functional limitations. J Am Geriatr Soc. 2014;62(6):1064–1072. doi: 10.1111/jgs.12845.
- Hayley AC, Williams LJ, Kennedy GA, et al. Excessive daytime sleepiness and falls among older men and women: cross-sectional examination of a population-based sample. BMC Geriatr. 2015;15(1):74. (doi: 10.1186/s12877-015-0068-2.
- Lima CA, Soares W, Bilton TL, et al. Correlates of excessive daytime sleepiness in community-dwelling older adults: an exploratory study. Rev Bras Epidemiol. 2015;18(3):607–617. doi: 10.1590/1980-5497201500030007.
- Vashum KP, McEvoy MA, Hancock SJ, et al. Prevalence of and associations with excessive daytime sleepiness in an Australian older population. Asia Pac J Public Health. 2015;27(2):Np2275–2284. doi: 10.1177/1010539513497783.
- Okamura T, Ura C, Miyamae F, et al. Excessive daytime sleepiness is related to subjective memory impairment in late life: a cross-sectional community-based study. Psychogeriatrics. 2016;16(3):196–201. doi: 10.1111/psyg.12139.
- Brewster GS, Hirschman KB, Riegel BJ, et al. Association of health related quality of life domains with daytime sleepiness among elderly recipients of long-term services and supports. Geriatr Nurs. 2019;40(4):417–423. doi: 10.1016/j.gerinurse.2019.01.006.
- Hara C, Stewart R, Lima-Costa MF, et al. Insomnia subtypes and their relationship to excessive daytime sleepiness in Brazilian community-dwelling older adults. Sleep. 2011;34(8):1111–1117. doi: 10.5665/SLEEP.1172.
- Stroe AF, Roth T, Jefferson C, et al. Comparative levels of excessive daytime sleepiness in common medical disorders. Sleep Med. 2010;11(9):890–896. doi: 10.1016/j.sleep.2010.04.010.
- Okudur SK, Soysal P. Excessive daytime sleepiness is associated with malnutrition, dysphagia, and vitamin D deficiency in older adults. J Am Med Dir Assoc. 2021;22(10):2134–2139. doi: 10.1016/j.jamda.2021.05.035.
- Whitney CW, Enright PL, Newman AB, et al. Correlates of daytime sleepiness in 4578 elderly persons: the cardiovascular health study. Sleep. 1998;21(1):27–36. doi: 10.1093/sleep/21.1.27.
- Miner B, Gill TM, Yaggi HK, et al. The epidemiology of Patient-Reported hypersomnia in persons with advanced age. J Am Geriatr Soc. 2019;67(12):2545–2552. doi: 10.1111/jgs.16107.
- Lader M. Benzodiazepine harm: how can it be reduced? Br J Clin Pharmacol. 2014;77(2):295–301. doi: 10.1111/j.1365-2125.2012.04418.x.
- Reynolds CF, 3rd, Jennings JR, Hoch CC, et al. Daytime sleepiness in the healthy "old old": a comparison with young adults. J Am Geriatr Soc. 1991;39(10):957–962. doi: 10.1111/j.1532-5415.1991.tb04041.x.
- Ong JC, Crawford MR. Insomnia and obstructive sleep apnea. Sleep Med Clin. 2013;8(3):389–398. doi: 10.1016/j.jsmc.2013.04.004.
- Lowden A, Holmbäck U, Akerstedt T, et al. Performance and sleepiness during a 24 h wake in constant conditions are affected by diet. Biol Psychol. 2004;65(3):251–263. doi: 10.1016/s0301-0511(03)00114-5.
- Brayne C, Dufouil C, Ahmed A, et al. Very old drivers: findings from a population cohort of people aged 84 and over. Int J Epidemiol. 2000;29(4):704–707. doi: 10.1093/ije/29.4.704.
- Morewitz JH. Evaluation of excessive daytime sleepiness in the elderly. J Am Geriatr Soc. 1988;36(4):324–330. doi: 10.1111/j.1532-5415.1988.tb02359.x.