Abstract
Background
Pertussis (Whooping Cough) is a respiratory infection caused by Bordetella pertussis. Pertussis usually occurs in childhood; severe infections are most common in infants. It can be fatal with severe complications such as pulmonary hypertension, heart failure, and encephalitis.
Objectives
We sought to synthesize the existing literature on severe pertussis in infants and inform further study.
Methods
A scoping review was performed based on the methodological framework developed by Arksey & O’Malley. Search in Pubmed and Embase databases, with no restrictions on the language and date of publication.
Results
Of the 1299 articles retrieved, 64 were finally included. The selected articles were published between 1979 and 2022, with 90.6% (58/64) of the studies in the last two decades. The studies covered epidemiology, pathology, clinical characteristics, risk factors, treatments, and burden of disease.
Conclusion
The literature reviewed suggests that studies on severe pertussis in infants covered a variety of clinical concerns. However, these studies were observational, and experimental studies are needed to provide high-quality evidence.
Keywords:
Introduction
Pertussis (Whooping Cough) is a respiratory infection caused by Bordetella pertussis and characterized by paroxysmal coughing ending in a prolonged crowing intake of breath. Pertussis usually occurs in childhood; severe infections are most common in infants. It can be fatal with severe complications such as pulmonary hypertension, heart failure, and encephalitis. Pertussis is a vaccine-preventable disease, and the implementation of pertussis vaccine immunization has led to a decline in the number of pertussis cases and deaths among children since the inception of the WHO Expanded Programme on Immunization in 1974 [Citation1]. However, even in countries with widespread pertussis vaccine coverage, such as the United States, Canada, and Australia [Citation2], the incidence of pertussis has been on the rise again after maintaining a low rate for many years, with outbreaks in some places, which is referred to as ‘re-emergence of pertussis’.
There are multiple challenges to the management of pertussis. The change in the mode of transmission, from the previous child-child epidemic mode to an adult-child epidemic mode after the introduction of the immunization program, makes infants and young children more susceptible to infection [Citation3,Citation4]. Furthermore, given the considerable side effects of whole-cell pertussis vaccine, the acellular pertussis vaccine was introduced at the end of the twentieth century and is already used in many countries and regions. Although acellular pertussis vaccination has fewer adverse events than whole-cell pertussis vaccination, many studies have shown that acellular pertussis vaccination is associated with a higher risk of pertussis morbidity over time [Citation5–7]. The acellular pertussis vaccine only protects against disease, not the infection, which could make it possible for people who were vaccinated with the acellular pertussis vaccine to act as asymptomatic or mildly symptomatic carriers and play an important role in the spread of pertussis in the population [Citation8]. In addition, since the first strain of erythromycin-resistant Bordetella pertussis isolated from a 2-month-old infant with pertussis was reported in Arizona, USA, in 1994, subsequent reports of antibiotic-resistant Bordetella pertussis have been reported in many countries, such as France, China, Iran, Viet Nam [Citation9–13].
Nowadays, pertussis remains a public health problem of great concern, with high rates of morbidity and mortality. WHO estimates that about 24.1 million cases of pertussis and 160,700 children under 5 years of age die of pertussis in a year [Citation14]. According to the United States Centers for Disease Control and Prevention, about one-third of infants with pertussis require hospitalization, and 1% of those cases die [Citation15]. Pertussis is one of the significant causes of illness and death in infants. Therefore, we sought to synthesize the existing literature on severe pertussis in infants and inform further study.
The following research questions were used to guide this evidence review:
How many studies have been published on severe pertussis in infants?
What aspects of the study have been done on severe pertussis in infants?
What study designs have been used in studies on severe pertussis in infants?
Methods
A scoping review was performed based on the methodological framework developed by Arksey & O’Malley [Citation16].
Information sources and Search strategies
The following terms were used to construct the search strategies in the Pubmed and Embase databases: (Whooping cough or Pertussis) AND (Infant or Newborn or Neonate) AND (Critical illness or Severe or Fatal or Malignant or Fulminant) (). There were no restrictions on the language and date of publication. The literature was last searched on 30 June 2023.
Literature selection and Inclusion criteria
Study selection took place in two stages: first, titles and abstracts were reviewed, followed by the full text. Titles and abstracts were reviewed independently by two researchers, the abstracts that did not meet the inclusion criteria or that met the exclusion criteria were discarded. Articles without an available abstract or for which abstracts did not provide sufficient information to determine their exclusion were directly included in the full-text review stage. The full-text review was performed independently by two researchers, and disagreements were resolved by a third researcher. The inclusion of articles met the following criteria: (a) The study subjects were limited to infants under one year of age; (b) The study focuses on severe pertussis infections. Severe infection was defined as infants with pertussis who had hypoxemia, pulmonary hypertension, and heart failure, required respiratory support, were admitted to the intensive care unit, or died. Exclusion of review studies. The final search results were exported to EndNote and duplicate studies were removed.
Results synthesis and Data charts
We summarize the information extracted from the included studies in the table. Two researchers developed data charts jointly and independently extracted data from articles that fit this study, and then exchanged them for validation. A third researcher solved the disagreement. Variables included author, journal, year of publication, country, language of publication, study design, sample size, sample characteristics, and purpose or topic of the study.
Table 1. Search strategy.
Results
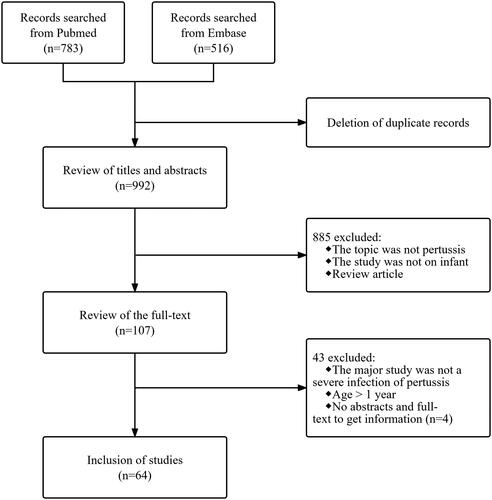
The search strategy identified 1299 articles, with 992 articles after deleted duplicates. Screening through titles and abstracts left 107 articles for full-text evaluation. The abstract and full text were not available for four articles, so sufficient information could not be obtained, and a total of 64 studies were finally included ().
The selected articles were published between 1979 and 2022, with 90.6% (58/64) of the studies in the last two decades. Including studies from 22 countries, the top three numbers were from the United States (25.0%, 16/64), China (9.3%, 6/64), and France (9.3%, 6/64). Out of six publication languages, most studies were published in English (75.0%, 48/64). All study designs were observational studies, with the most case reports (62.5%, 40/64) ().
Table 2. Characteristics of the included studies.
There are no standardized diagnostic criteria for severe pertussis. In these included studies, severe pertussis was defined as requiring respiratory support, admission to the intensive care unit, or resulting in death. The case reports () [Citation17–56] covered clinically severe manifestations and treatments; these severe manifestations are characterized by increased white blood cells, pulmonary hypertension, acute respiratory distress syndrome, encephalopathy, respiratory failure, cardiovascular collapse, and septic shock; of the treatments, most related to a reduction of white blood cells such as leukapheresis (10.0%, 4/40) and exchange transfusion (27.5%, 11/40), in addition to extracorporeal life support (10.0%, 4/40). In infants with severe pertussis and severe leukocytosis, early reduction of white blood cell therapy may be a helpful and rapid life-saving treatment before cardiopulmonary complications develop. Among other studies (except for case reports) () [Citation57–80], the major study designs were descriptive studies (62.5%, 15/24), case-control studies (20.8%, 5/24), and cohort studies (12.5%, 3/24); 45.8% (11/24) analyzed the risk factors associated with severe infection or death; Other contents involved epidemiology (12.5%, 3/24), clinical manifestations (8.3%, 2/24), pathological features (12.5%, 3/24), treatment (16.7%, 4/24), and disease burden (4.2%, 1/24). Increased white blood cell (81.8%, 9/11) is one of the risk factors associated with severe infection or death in infants with pertussis shown in most studies, as well as increased lymphocyte, apnea, co-infections, pulmonary hypertension, age, fever, cyanosis, and abnormal liver function.
Table 3. Study characteristics from case reports.
Table 4. Study characteristics from studies other than case reports.
Discussion
We conducted a scoping review for studies of severe pertussis in infants. These patients’ common critical illness characteristic was a need for intensive care treatment, with specific definitions of severe pertussis given in individual studies. Most of the studies (40/64) were case reports and gave limited information. More than half of the studies described characteristics (or risk factors) of severe course or death from pertussis. There was a significant association between elevated white blood cell counts/pulmonary hypertension and increased severity of the disease/death.
Pulmonary histopathology [Citation74,Citation75,Citation80] demonstrated abundant leukocyte aggregates in small pulmonary arteries, veins, and lymphatics; it was supposed that vascular infiltration and blood hyperviscosity by elevated leukocyte counts may be a factor for pulmonary hypertension and heart failure. Virulence factors, the key pathogenicity determinants of B. pertussis, mediate different stages of disease pathology. The postmortem was pathologically characterized by necrotizing bronchitis, bronchiolitis, pulmonary hemorrhage, edema, and extensive areas of alveolar epithelial necrosis, indicating that toxin-mediated processes or invasive infections damage the bronchi and alveolar epithelium, which could also be responsible for the secondary increased pulmonary vascular resistance, pulmonary hypertension, and subsequent fatal cardiac failure. In the first few months of life, especially in neonates, there is still a prominent muscular component in the arteries, and these vessels are very reactive to constrictive stimuli [Citation81]. Hypoxemia, or coexistent acidosis, can trigger pulmonary vasoconstriction, and result in an acute increase in pulmonary vascular resistance. Thus, critical hypoxemia and heart failure may be due to a combination of factors, and further studies are needed to clarify the pathogenesis of refractory cardiopulmonary failure caused by severe pertussis.
Although the association between leukocyte counts and disease severity is well known, as well as strategies such as leukapheresis and exchange transfusion to reduce high leukocyte counts have been used clinically, there are still no standardized leukocyte cut-off values for initiating the treatment. A total of 18 articles (15 case reports, 3 case series studies) reported the use of leukoreduction therapy in severe pertussis with increased leukocytes, which ranged from 45–204.9 × 109/L. Only one study had proposed a specific quantified leukodepletion strategy, patients were given a suitable treatment respectively based on a leukocyte count greater than 50/70/100 × 109/L with or without cardiopulmonary dysfunction, which made a marked decrease in mortality [Citation72]. Five articles (four case reports and one case series study) reported extracorporeal membrane oxygenation (ECMO). There is no optimal management method for pertussis infants treated with ECMO. Some researchers consider the early use of ECMO as the key to obtaining a good treatment effect and improving survival.
There are some limitations to our review. Due to the strict criteria, that is, the age of infants was younger than 1 year, studies that had a portion of the subjects meet the age criteria may have been missed. We minimized this shortcoming by including studies that had the majority of subjects meet the age criteria. Furthermore, 62.5% of the articles were case reports, which lacked a comprehensive description of the topic they covered. It was difficult to unify the variables in the data charts of the 64 articles, so we characterized case reports by developing the data chart separately.
In summary, the 64 articles included studies on epidemiology, pathology, clinical characteristics, risk factors, treatments, and burden of disease. Although these studies covered a variety of clinical concerns, most of them are descriptive, and targeted studies are still insufficient. Experimental studies are needed to provide high-quality evidence.
Authors contributions
GS and WCM conceptualized the review. ZY and GQ conducted the title/abstract and full-text screening. GS extracted and synthesized data with inputs from all authors. GS drafted the manuscript. ZY, GQ, and WCM revised for critical content. All authors contributed to the interpretation of findings and approved the version for submission.
Ethical approval
This study was a summary and analysis of the existing literature and ethical approval was not applicable.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Additional information
Funding
References
- Pertussis vaccines: WHO position paper - September 2015. Wkly Epidemiol Rec. 2015;90:1–10.
- World Health Organization. Pertussis reported cases and incidence. [cited 30 June 2023]. https://immunizationdata.who.int/pages/incidence/PERTUSSIS.html.
- Kara EO, Campbell H, Ribeiro S, et al. Survey of household contacts of infants with laboratory-confirmed pertussis infection during a national pertussis outbreak in England and Wales. Pediatr Infect Dis J. 2017;36(2):140–145. doi:10.1097/INF.0000000000001378.
- Wiley KE, Zuo Y, Macartney KK, et al. Sources of pertussis infection in young infants: a review of key evidence informing targeting of the cocoon strategy. Vaccine. 2013;31(4):618–625. doi:10.1016/j.vaccine.2012.11.052.
- Klein NP, Bartlett J, Rowhani-Rahbar A, et al. Waning protection after fifth dose of acellular pertussis vaccine in children. N Engl J Med. 2012;367(11):1012–1019. doi:10.1056/NEJMoa1200850.
- Gustafsson L, Hessel L, Storsaeter J, et al. Long-term follow-up of Swedish children vaccinated with acellular pertussis vaccines at 3, 5, and 12 months of age indicates the need for a booster dose at 5 to 7 years of age. Pediatrics. 2006;118(3):978–984. doi:10.1542/peds.2005-2746.
- Schwartz KL, Kwong JC, Deeks SL, et al. Effectiveness of pertussis vaccination and duration of immunity. CMAJ. 2016;188(16):E399–E406. doi:10.1503/cmaj.160193.
- Warfel JM, Zimmerman LI, Merkel TJ. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proc Natl Acad Sci U S A. 2014;111(2):787–792. doi:10.1073/pnas.1314688110.
- Centers for Disease Control and Prevention (CDC). Erythromycin-resistant Bordetella pertussis–Yuma County, Arizona, May-October 1994. MMWR Morb Mortal Wkly Rep. 1994;43(44):807–810.
- Guillot S, Descours G, Gillet Y, et al. Macrolide-resistant Bordetella pertussis infection in newborn girl, France. Emerg Infect Dis. 2012;18(6):966–968. doi:10.3201/eid1806.120091.
- Zhang Q, Li M, Wang L, et al. High-resolution melting analysis for the detection of two erythromycin-resistant Bordetella pertussis strains carried by healthy schoolchildren in China. Clin Microbiol Infect. 2013;19(6):E260–262. doi:10.1111/1469-0691.12161.
- Shahcheraghi F, Nakhost Lotfi M, Nikbin VS, et al. The first macrolide-resistant Bordetella pertussis strains isolated from Iranian patients. Jundishapur J Microbiol. 2014;7(6):e10880. doi:10.5812/jjm.10880.
- Kamachi K, Duong HT, Dang AD, et al. Macrolide-resistant Bordetella pertussis, Vietnam, 2016-2017. Emerg Infect Dis. 2020;26(10):2511–2513. doi:10.3201/eid2610.201035.
- Yeung KHT, Duclos P, Nelson EAS, et al. An update of the global burden of pertussis in children younger than 5 years: a modelling study. Lancet Infect Dis. 2017;17(9):974–980. doi:10.1016/S1473-3099(17)30390-0.
- Centers for Disease Control and Prevention. Pertussis (whooping cough). [cited 30 June 2023]. https://www.cdc.gov/pertussis/about/complications.html
- Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32. doi:10.1080/1364557032000119616.
- Long S, Lowe RB. Severe pertussis infection with hyperleukocytosis in a 10-month-old unvaccinated Amish female: a case report. Cureus. 2022;14(7):e26885. doi:10.7759/cureus.26885.
- Liao Y, Li WR, Zhu Y, et al. Invasive Bordetella pertussis infection in infants: a case report. Open Forum Infect Dis. 2022;9(10):ofac478. doi:10.1093/ofid/ofac478.
- Son PT, Reda A, Viet DC, et al. Exchange transfusion in the management of critical pertussis in young infants: a case series. Vox Sang. 2021;116(9):976–982. doi:10.1111/vox.13085.
- Kolind RS, Jensen AB, von Linstow ML. Malignant pertussis in a three-week-old girl. Ugeskr Laeger. 2021;183:V09200691.
- Fueta PO, Eyituoyo HO, Igbinoba O, et al. Cardiopulmonary arrest and pulmonary hypertension in an infant with pertussis case report. Case Rep Infect Dis. 2021;2021:6686185. doi:10.1155/2021/6686185.
- Rossetti E, Appierto L, Meschini A, et al. Early leukapheresis depletion in an ex-premature with severe acute respiratory distress syndrome due to Bordetella Pertussis and coronavirus infection. Blood Purif. 2020;49(6):758–760. doi:10.1159/000507873.
- Chen X, Jiang S. Case report of infant severe pertussis with encephalopathy treated by leukapheresis. Chinese J Appl Clin Pediatr. 2020;35:1908–1910.
- Wei XM, Yang H, Lei M, et al. Blood exchange transfusion for treatment of severe pertussis in an infant. Zhongguo Dang Dai Er Ke Za Zhi. 2019;21:214–217.
- Alkan G, Keser EM. Severe pertussis pneumonia in an infant: treated with exchange transfusion: case report. Turkiye Klinikleri J Pediatr. 2017;26(1):32–34. doi:10.5336/pediatr.2016-52637.
- Ganeshalingham A, Anderson BJ, Zuccollo J, et al. Porcelain lung: calcification in severe Bordetella pertussis infection. Arch Dis Child. 2016;101(5):421–421. doi:10.1136/archdischild-2015-310204.
- Liko J, Koenig WJ, Cieslak PR. Suffer the infants: a severe case of pertussis in Oregon, 2012. Public Health Rep. 2015;130(5):435–439. doi:10.1177/003335491513000505.
- Chantreuil J, Fakhri N, Labarthe F, et al. Malignant pertussis and exchange transfusion. Arch Pediatr. 2015;22(1):84–87. doi:10.1016/j.arcped.2014.10.010.
- Assy J, Séguéla PE, Guillet E, et al. Severe neonatal pertussis treated by leukodepletion and early extra corporeal membrane oxygenation. Pediatr Infect Dis J. 2015;34(9):1029–1030. doi:10.1097/INF.0000000000000781.
- Kurvers RA, Westra D, van Heijst AF, et al. Severe infantile Bordetella pertussis pneumonia in monozygotic twins with a congenital C3 deficiency. Eur J Pediatr. 2014;173(12):1591–1594. doi:10.1007/s00431-013-2107-3.
- Al Hanshi S, Al Ghafri M, Al Ismaili S. Severe pertussis pneumonia managed with exchange transfusion. Oman Med J. 2014;29(3):e074. doi:10.5001/omj.2014.65.
- Nataprawira HM, Somasetia DH, Sudarwati S, et al. Critical pertussis in a young infant requiring mechanical ventilation. Case Rep Emerg Med. 2013;2013:125043. doi:10.1155/2013/125043.
- Mata AF, Sarnaik AA. Bronchoscopy with N-acetylcysteine lavage in severe respiratory failure from pertussis infection. Pediatrics. 2013;132(5):e1418–e1423. doi:10.1542/peds.2013-0912.
- Martinez M, Rochat I, Corbelli R, et al. Early blood exchange transfusion in malignant pertussis: a case report. Pediatr Crit Care Med. 2011;12(2):e107–e109. doi:10.1097/PCC.0b013e3181f3a189.
- Menif K, Bouziri A, Khaldi A, et al. Pertussis infection and fatal pulmonary hypertension. Arch Pediatr. 2010;17(11):1550–1552. doi:10.1016/j.arcped.2010.08.016.
- Kundrat SL, Wolek TL, Rowe-Telow M. Malignant pertussis in the pediatric intensive care unit. Dimens Crit Care Nurs. 2010;29(1):1–5. doi:10.1097/DCC.0b013e3181be489c.
- Freitas J, de Sousa SG, Miguel C, et al. Pertussis keeps on killing. Rev Port Pneumol. 2010;16(2):315–320. doi:10.1016/s0873-2159(15)30029-5.
- Berthomieu L, Boumahni B, Jamal Bey K, et al. Malignant pertussis: 3 case reports. Arch Pediatr. 2010;17(2):144–148. doi:10.1016/j.arcped.2009.10.020.
- Couchot E, Paut O, Ghez O, et al. Extracorporeal membranous oxygenation in severe infant pertussis: a case report. Ann Fr Anesth Reanim. 2009;28(1):74–77. doi:10.1016/j.annfar.2008.11.007.
- Theilen U, Johnston ED, Robinson PA. Rapidly fatal invasive pertussis in young infants–how can we change the outcome? BMJ. 2008;337(v27 2):a343–a343. doi:10.1136/bmj.39575.715787.80.
- Soares S, Rocha G, Pissarra S, et al. Pertussis with severe pulmonary hypertension in a newborn with good outcome - case report. Rev Port Pneumol. 2008;14(5):687–692. doi:10.1016/S0873-2159(15)30277-4.
- Vaessen S, Anthopoulou A, Bricteux G. Fatal pertussis infection in a 2 month old infant. Rev Med Liege. 2006;61:145–148.
- Grzeszczak MJ, Churchwell KB, Edwards KM, et al. Leukopheresis therapy for severe infantile pertussis with myocardial and pulmonary failure. Pediatr Crit Care Med. 2006;7(6):580–582. doi:10.1097/01.PCC.0000235253.19315.56.
- Gonzalez-Escudero R, Llorente Otones L, Gonzalez-Tome MI, et al. D. Malignant pertussis in a 25-day-old newborn. Acta Pediatr Esp. 2006;64:297–299.
- Donoso AF, Cruces PI, Camacho JF, et al. Exchange transfusion to reverse severe pertussis-induced cardiogenic shock. Pediatr Infect Dis J. 2006;25(9):846–848. doi:10.1097/01.inf.0000232630.70138.a2.
- Donoso A, León J, Ramírez M, et al. Pertussis and fatal pulmonary hypertension: a discouraged entity. Scand J Infect Dis. 2005;37(2):145–148. doi:10.1080/00365540510026436.
- De Berry BB, Lynch JE, Chung DH, et al. Pertussis with severe pulmonary hypertension and leukocytosis treated with extracorporeal membrane oxygenation. Pediatr Surg Int. 2005;21(8):692–694. doi:10.1007/s00383-005-1458-x.
- Cruces RP, Gonzalez MM, Maldonado VB, et al. Severe pertussis with pulmonary hypertension: cardiorespiratory improvement after exchange transfusion. Rev Chil Pediatr. 2005;76:513–517.
- Wauters O, Brumioul D, Sacré JP, et al. Malignant whooping cough in an infant. Rev Med Liege. 2004;59:555–556.
- Romano MJ, Weber MD, Weisse ME, et al. Pertussis pneumonia, hypoxemia, hyperleukocytosis, and pulmonary hypertension: improvement in oxygenation after a double volume exchange transfusion. Pediatrics. 2004;114(2):e264–e266. doi:10.1542/peds.114.2.e264.
- McEniery JA, Delbridge RG, Reith DM. Infant pertussis deaths and the management of cardiovascular compromise. J Paediatr Child Health. 2004;40(4):230–232. doi:10.1111/j.1440-1754.2004.00344.x.
- Pilorget H, Montbrun A, Attali T, et al. Malignant pertussis in the young infant. Arch Pediatr. 2003;10(9):787–790. doi:10.1016/s0929-693x(03)00411-1.
- Sreenan CD, Osiovich H. Neonatal pertussis requiring extracorporeal membrane oxygenation. Pediatr Surg Int. 2001;17(2–3):201–203. doi:10.1007/s003830000429.
- Corkins M, Grose C, Halbur T. Fatal pertussis in an Iowa infant. Iowa Med. 1991;81:383–384.
- Tam AY, Yeung CY. Severe neonatal pertussis treated by salbutamol. Arch Dis Child. 1986;61(6):600–602. doi:10.1136/adc.61.6.600.
- Theilade D. Nasal continuous positive airway pressure in the treatment of whooping cough. Anaesthesia. 1979;34(10):1028–1031. doi:10.1111/j.1365-2044.1979.tb06253.x.
- Zhang C, Zong Y, Wang Z, et al. Risk factors and prediction model of severe pertussis in infants < 12 months of age in Tianjin, China. BMC Infect Dis. 2022;22(1):24. doi:10.1186/s12879-021-07001-x.
- Thuy Nga DT, Thi Bich Thuy P, Ainai A, et al. Association between real-time polymerase chain reaction cycle threshold value and clinical severity in neonates and infants infected with Bordetella pertussis. Pediatr Infect Dis J. 2022;41(5):388–393. doi:10.1097/INF.0000000000003471.
- Coquaz-Garoudet M, Ploin D, Pouyau R, et al. Malignant pertussis in infants: factors associated with mortality in a multicenter cohort study. Ann Intensive Care. 2021;11(1):70. doi:10.1186/s13613-021-00856-y.
- Şık G, Demirbuğa A, Annayev A, et al. The clinical characteristics and prognosis of pertussis among unvaccinated infants in the pediatric intensive care unit. Turk Pediatri Ars. 2020;55(1):54–59. doi:10.14744/TurkPediatriArs.2020.82435.
- Liu C, Yang L, Cheng Y, et al. Risk factors associated with death in infants <120 days old with severe pertussis: a case-control study. BMC Infect Dis. 2020;20(1):852. doi:10.1186/s12879-020-05535-0.
- Kavitha TK, Samprathi M, Jayashree M, et al. Clinical profile of critical pertussis in children at a pediatric intensive care unit in Northern India. Indian Pediatr. 2020;57(3):228–231. doi:10.1007/s13312-020-1756-3.
- Cherry JD, Wendorf K, Bregman B, et al. An observational study of severe pertussis in 100 infants ≤120 days of age. Pediatr Infect Dis J. 2018;37(3):202–205. doi:10.1097/INF.0000000000001710.
- Macdonald-Laurs E, Ganeshalingham A, Lillie J, et al. Increasing incidence of life-threatening pertussis: a retrospective cohort study in New Zealand. Pediatr Infect Dis J. 2017;36(3):282–289. doi:10.1097/INF.0000000000001441.
- Straney L, Schibler A, Ganeshalingham A, et al. Burden and outcomes of severe pertussis infection in critically ill infants. Pediatr Crit Care Med. 2016;17(8):735–742. doi:10.1097/PCC.0000000000000851.
- Winter K, Zipprich J, Harriman K, et al. Risk factors associated with infant deaths from pertussis: a case-Control study. Clin Infect Dis. 2015;61(7):1099–1106. doi:10.1093/cid/civ472.
- Tiwari TS, Baughman AL, Clark TA. First pertussis vaccine dose and prevention of infant mortality. Pediatrics. 2015;135(6):990–999. doi:10.1542/peds.2014-2291.
- Rocha G, Flôr-de-Lima F, Soares P, et al. Severe pertussis in newborns and young vulnerable infants. Pediatr Infect Dis J. 2013;32(10):1152–1154. doi:10.1097/INF.0b013e31829f0b1a.
- Nieves D, Bradley JS, Gargas J, et al. Exchange blood transfusion in the management of severe pertussis in young infants. Pediatr Infect Dis J. 2013;32(6):698–699. doi:10.1097/INF.0b013e31828c3bb3.
- Murray EL, Nieves D, Bradley JS, et al. Characteristics of severe Bordetella pertussis infection among infants ≤90 days of age admitted to pediatric intensive care Units - Southern California, September 2009-June 2011. J Pediatric Infect Dis Soc. 2013;2(1):1–6. doi:10.1093/jpids/pis105.
- Taffarel P, Bonetto G, Haimovich A. Severe pertussis, progression and exchange transfusion as an alternative treatment. Arch Argent Pediatr. 2012;110(4):327–330. doi:10.5546/aap.2012.327.
- Rowlands HE, Goldman AP, Harrington K, et al. Impact of rapid leukodepletion on the outcome of severe clinical pertussis in young infants. Pediatrics. 2010;126(4):e816–e827. doi:10.1542/peds.2009-2860.
- Bouziri A, Hamdi A, Khaldi A, et al. Malignant pertussis: an underdiagnosed illness. Med Trop (Mars). 2010;70:245–248.
- Sawal M, Cohen M, Irazuzta JE, et al. Fulminant pertussis: a multi-center study with new insights into the clinico-pathological mechanisms. Pediatr Pulmonol. 2009;44(10):970–980. doi:10.1002/ppul.21082.
- Paddock CD, Sanden GN, Cherry JD, et al. Pathology and pathogenesis of fatal Bordetella pertussis infection in infants. Clin Infect Dis. 2008;47(3):328–338. doi:10.1086/589753.
- Vitek CR, Pascual FB, Baughman AL, et al. Increase in deaths from pertussis among young infants in the United States in the 1990s. Pediatr Infect Dis J. 2003;22(7):628–634. doi:10.1097/01.inf.0000073266.30728.0e.
- Pooboni S, Roberts N, Westrope C, et al. Extracorporeal life support in pertussis. Pediatr Pulmonol. 2003;36(4):310–315. doi:10.1002/ppul.10351.
- Mikelova LK, Halperin SA, Scheifele D, et al. Predictors of death in infants hospitalized with pertussis: a case-control study of 16 pertussis deaths in Canada. J Pediatr. 2003;143(5):576–581. doi:10.1067/S0022-3476(03)00365-2.
- Pierce C, Klein N, Peters M. Is leukocytosis a predictor of mortality in severe pertussis infection? Intensive Care Med. 2000;26(10):1512–1514. doi:10.1007/s001340000587.
- Hackman R, Perrin DG, Karmali M, et al. Fatal Bordetella pertussis infection: report of two cases with novel pathologic findings. Pediatr Pathol Lab Med. 1996;16(4):643–653. doi:10.1080/15513819609168700.
- Rudolph AM. High pulmonary vascular resistance after birth: I. Pathophysiologic considerations and etiologic classification. Clin Pediatr (Phila). 1980;19(9):585–590. doi:10.1177/000992288001900902.